| Case Report | ||
Open Vet. J.. 2025; 15(9): 4775-4788 Open Veterinary Journal, (2025), Vol. 15(9): 4775-4788 Case Report Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healingPeyman Mohammadzadeh*, Negin Samadi and Behnaz MohammadiDepartment of Pathology, Sa. C. Islamic Azad University, Sanandaj, Iran *Corresponding Author: Peyman Mohammadzadeh. Department of Pathology, Sa. C. Islamic Azad University, Sanandaj, Iran. Email: pathology [at] iau.ac.ir Submitted: 22/04/2025 Revised: 21/07/2025 Accepted: 08/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
AbstractBackground: Oronasal fistulas (ONFs) present significant therapeutic challenges in veterinary medicine, particularly in patients with comorbidities, such as chronic kidney disease. This case report demonstrates an innovative bioengineered approach for ONF repair in Felis silvestris that combines autologous biomaterials with microbiome analysis. Case Description: A 15-year-old male European wildcat with a chronic traumatic ONF (11 × 8 mm) and concurrent International Renal Interest Society Stage 2 chronic kidney disease received a customized implant composed of autologous platelet-rich fibrin (PRF), bone marrow aspirate, and a 3D-printed polyethylene glycol diacrylate (PEGDA) scaffold. PEGDA was selected for its high biocompatibility and rapid photopolymerization. Healing was monitored via computed tomography imaging and 16S rRNA sequencing. The results demonstrated significant microbial dysbiosis post-injury (Shannon index: 5.2 ± 0.3 vs. 3.1 ± 0.4; p < 0.05), with opportunistic pathogen enrichment (Enterococcus faecalis: 0.5% → 12.8%). Antibiotics reduced the bacterial load by 99.9% (p < 0.001) but did not improve the closure rate (p=0.89), underscoring the mechanical role of the scaffold. Conclusion: The bioengineered implant facilitated complete mucosal integration and osteoconduction at the 10-month follow-up, demonstrating promise for complex ONF repair. However, the single-case design limits the generalizability. Keywords: Oronasal fistula, Felis silvestris, Tissue engineering, Oral microbiome, Platelet-rich fibrin, 3D bioprinting. IntroductionEuropean wildcats (Felis silvestris silvestris), which are classified as Least Concern by the IUCN, play a vital role in maintaining ecological balance within their habitats. As apex predators, they regulate prey populations, enhancing overall ecosystem health. Their adaptability to human-induced environmental changes is critical for understanding animal resilience and designing effective conservation strategies (Petisco et al., 2024). Preserving this species is essential not only for biodiversity but also for the ecological services it provides. The welfare of wildlife, especially European wildcats, is increasingly recognized as a priority, necessitating the provision of veterinary care tailored to their unique physiological and ecological needs. Wildlife diseases (e.g., rabies and toxoplasmosis) significantly impact ecosystem stability and pose risks to human populations, highlighting the urgent need to monitor wildlife health and implement targeted management practices (Torrecilhas et al., 2020). Veterinary interventions can improve the health outcomes of wild species, enabling them to thrive in their natural environments. The oral cavity harbors >1,000 bacterial species (Dewhirst et al., 2010), making it the second most diverse microbiota after the gut. Primary colonizers, predominantly aerobes (e.g., Streptococcus, Lactobacillus, Actinomyces, and Veillonella), establish themselves shortly after birth. Subsequent tooth eruption and plaque formation facilitate colonization by diverse bacterial phyla, including Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, Fusobacteria, and Neisseria. Despite extensive research on human oral microbiomes, studies on wild felids—particularly regarding maxillofacial lesions and anomalies—remain scarce. Maxillofacial disorders, such as oronasal fistulas (ONFs), often resulting from traumatic injuries or congenital disabilities, have been documented in wild felids. These lesions can result in severe clinical complications and reduced quality of life. A study reported that 32.7% of wild feline populations exhibit maxillofacial abnormalities (Alhindi et al., 2019), underscoring the need for further investigation into the oral microbiome and healing processes of these animals. Although the role of the microbiome in oral health and wound repair is well-established, its influence following extensive mucosal injuries—such as those arising from cleft palate repair—remains poorly understood. Furthermore, data on microbiome dynamics in wild felids with maxillofacial injuries remain scarce. A recent study on the oral microbiome of children with cleft lip and palate identified specific microbial taxa correlated with postoperative inflammation severity (Świtała et al., 2023). Although postoperative antibiotic use is common, its effects on wound healing and microbiota composition remain understudied. In oral surgery, despite evidence linking dysbiosis to impaired healing, preoperative assessments typically exclude microbial profiling and periodontal evaluations (Zaura et al., 2014). Complications following cleft palate repair occur in up to 60% of cases, often resulting in ONFs that require multiple corrective surgeries (Park et al., 2020). Factors contributing to ONF development include wound tension, cleft width, nutritional status, shifts in the oral microbiota, and pathogenic bacterial colonization (Bahar Tuna et al., 2008; Perry et al., 2018). We present a comprehensive case report with longitudinal microbiome and inflammatory biomarker analysis in a single F. silvestris, examining temporal changes in oral microbiota during wound healing. Through serial microbial sampling and sequencing, we characterized bacterial community dynamics before and after surgical intervention, identifying dysbiotic patterns and opportunistic pathogens that may impede recovery. Additionally, we evaluated the impact of antimicrobial therapy on microbiome diversity and wound resolution. Secondary analysis of inflammatory biomarkers was performed on the archived samples. This research advances our understanding of regenerative processes by elucidating microbial shifts during oral wound healing in wild felids and may inform therapeutic strategies for both wild and domestic cats (Alves-Barroco et al., 2020; Shah et al., 2024). We hypothesized that combining PRF-bone marrow composites with a customized PEGDA scaffold would promote superior mucosal regeneration and modulate oral microbiome composition in wild felids with chronic ONFs. This study bridges the gap between regenerative medicine and wildlife conservation by addressing a critical clinical gap. Case DetailsCase presentationA 15-year-old male European wildcat (9.4 kg; body condition score 5.2/9) presented with a chronic ONF (11×8 mm) secondary to TTL. Bloodwork revealed International Renal Interest Society (IRIS) stage 2 chronic kidney disease (creatinine: 1.91 mg/dl) (Proverbio et al., 2024). Pain management followed the AAHA/AAFP 2022 guidelines. The clinical signs included persistent mucopurulent nasal discharge (predominantly from the right nostril), excessive salivation, halitosis, sporadic sneezing, anorexia, and lethargy. Caregivers reported gradual defect enlargement and mild medial displacement. Due to elevated creatinine levels, the wild cat was monitored for 1 month prior to intervention.
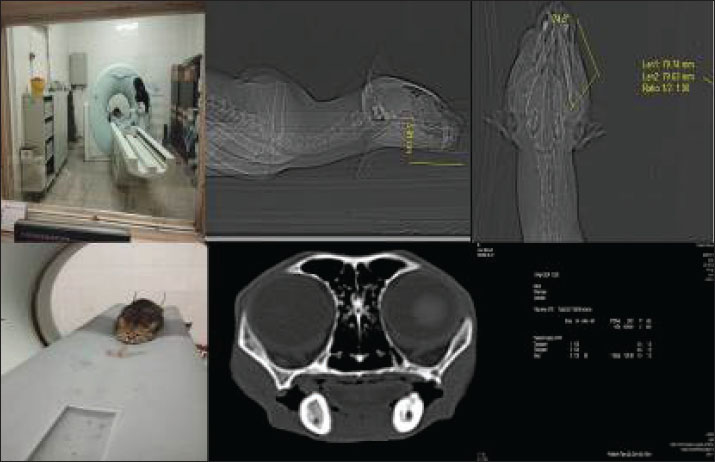
Fig. 1. High-resolution computed tomography imaging of a wild cat subject undergoing diagnostic evaluation for an oronasal fistula, highlighting the integration of advanced imaging modalities in preclinical research. This non-invasive approach enables precise fistula morphology assessment and anatomical mapping, thereby informing the development of bioengineered scaffolds and microbiome-targeted therapies to optimize wound closure and tissue regeneration. Clinical examination and CT scan revealed an ONF measuring approximately 11 mm (caudal-to-rostral length) × 8 mm (width) at the level of the upper right canine, along with an OCF in the right lower canine tooth (Figs. 1 and 2) (Watanabe et al., 2022). Residual dentition included only the maxillary and mandibular incisors and second premolars, with no clinical evidence of gingivitis or periodontitis. Serological testing for Feline leukemia virus and Feline immunodeficiency virus yielded negative results (Little et al., 2011). Given the patient’s advanced age and concurrent renal disease, we developed a tailored surgical strategy to minimize physiological stress while optimizing recovery. A personalized implant composed of autologous platelet-rich fibrin (PRF) and bone marrow was designed to reinforce the soft tissue flap for defect closure. All procedures were approved by the Wildlife Ethics Committee of the Department of Environment (IAU/DOE/2023-027) (DOE Wildlife Ethics Committee, 2023) and complied with Directive 2010/63/EU on animal experimentation. This case was managed from March 2023 to January 2024 at Sa. C. University.
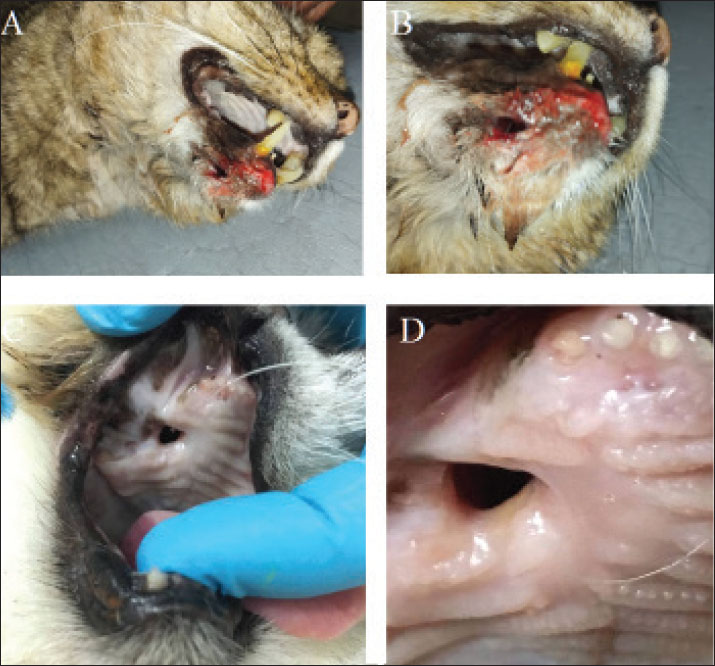
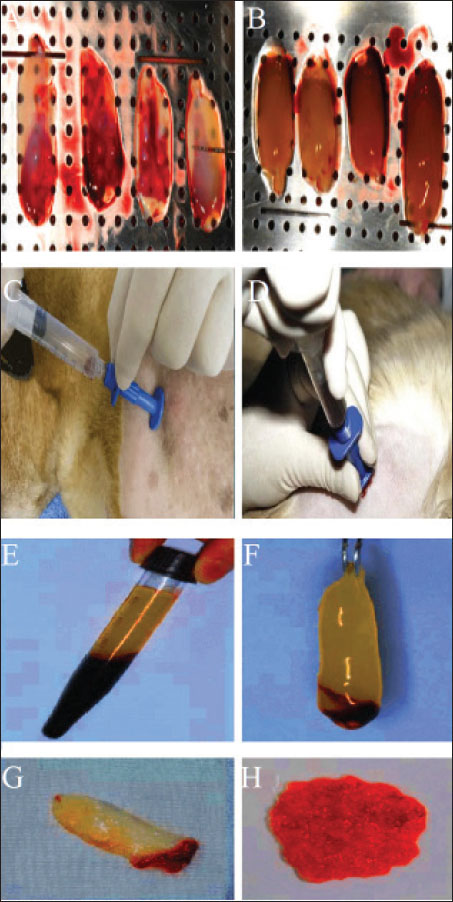
Fig. 2. (A) OCF in the right lower canine tooth. (B) Close-up view of the OCF. (C) Oronasal defect on the day of surgery (day 0), showing fibrotic tissue deposition around the fistula in the mucosal epithelium. (D) Close-up view of the ORF. Preparation of the implantsWildcats were sedated with a combination of tiletamine-zolazepam (4 mg/kg IM, Zoletil®; Virbac) and medetomidine (15 µg/kg IM, Domitor®; Orion Pharma) (Simon et al., 2020). A high-precision silicone-based imprint material (HydroPutty™; Spident) was used to create a mold of the mandibular deformity (Perry, 2013). The impression was formed in Type IV dental stone (Durastone™; Kulzer) to produce a precise plaster replica. Scaffolds were fabricated using polyethylene glycol diacrylate (PEGDA) (Liu and Echeverry-Rendon, 2025); MW 700 Da; Polysciences Inc.) with <0.1% residual monomer, mixed with 0.5% lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP; Tokyo Chemical Industry Co.) as a photoinitiator (Fig. 3). A digital light processing (DLP) bioprinter (LumiForge DLP; RegenMedTech) was used to print the scaffold at a resolution of 50 μm per layer (Perry and Echeverry-Rendón, 2025). The structure exhibited a lattice framework with a strand thickness of 0.45 mm and an overall height of 320 μm (Fig. 4). SEM confirmed an average pore size of 150 μm (optimal for osteoconduction) (Jones et al., 2023). Microcomputed tomography (µCT) (SkyScan 1173; Bruker) revealed a multi-layered structure with a 90° interlayer orientation (Yu et al., 2023). Sterilization was performed using hydrogen peroxide gas plasma at 45°C for 40 min (Sterrad® 100NX; Advanced Sterilization Products), validated with Attest™ 1295 Biological Indicators (3M) (Anderson et al., 2017).
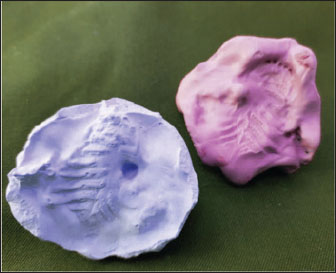
Fig. 3. 3D-printed PEGDA scaffold fabrication process. (A) Dental stone replica of the maxillary defect. (B) PEGDA scaffold after photopolymerization. A scaffold based on PEGDA was constructed using vat photopolymerization 3D printing and a precise plaster replica (pink color) of the maxillary dentition of the wildcat, acquired under general anastasia. The respective plaster mold (lavender color) allowed the production of a polyethylene glycol implant adapted to the oronasal defect. Inflammatory biomarker analysisArchived serum samples (collected during routine preoperative and postoperative venipuncture) were analyzed for IL-6 and TNF-α using commercial feline-specific enzyme-linked immunosorbent assay kits (Cat# abx051412, Abbexa; detection limit: 1 pg/ml, intra-assay CV <8%). Samples were processed in triplicate according to the manufacturer’s protocol. Flow cytometry for CD4+/CD8+ ratios was performed using anti-feline CD4 (clone FE1.7B2) and CD8 (clone FT2) antibodies (Thermo Fisher). All assays were performed at Sa. C. Statistical analysis employed paired t-tests for longitudinal comparisons and ANOVA with Bonferroni correction for multi-timepoint analysis (GraphPad Prism v9.5).
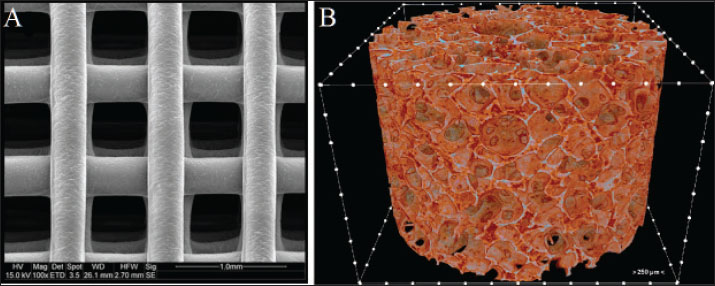
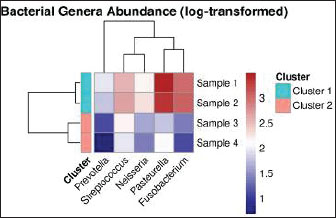
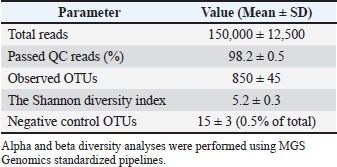
Fig. 4. Scaffold microstructure. (A) The scaffold has a consistent structure with an average pore size of 150 m. (B) Microcomputed tomography revealed a multi-layered structure with a 90° interlayer orientation. Surgical interventionThe patient was premedicated with methadone (0.2 mg/kg IM; Comfortan®, Dechra) and midazolam (0.2 mg/kg IM; Hypnovel®, Roche), with anesthetic induction achieved using alfaxalone (5 mg/kg IV; Alfaxan®, Jurox) and maintenance on desflurane (5%–7%) in oxygen via endotracheal intubation (Shelby et al., 2022). Continuous monitoring included electrocardiogram, capnography, and pulse oximetry, with intravenous Ringer’s acetate (Vetivex®, Dechra) administered at 6 ml/kg/h. PRF was prepared by collecting 6 ml of whole blood from the jugular vein into anticoagulant-free sterile tubes (BD Vacutainer®), followed by centrifugation at 2,700 rpm for 7 minutes (Hettich Rotofix 32A) (Fig. 5). Concurrently, 3 ml of bone marrow was aspirated from the proximal humerus using an 11G Jamshidi needle (Arthrex®). The PRF clot was injected with 1.5 ml of BM aspirate using a 22G spinal needle to create the PRF-BM composite (Dohan Ehrenfest et al., 2009). A full-thickness mucoperiosteal flap was elevated using a Molt #9 periosteal elevator, and fibrotic wound margins were debrided using a #1/2 round tungsten carbide burr (Komet®) at 20,000 rpm under saline irrigation (Ross et al., 1986). The 3D-printed PEGDA scaffold (10 × 8 × 2 mm) was positioned submucosally at the defect site and stabilized with 5-0 absorbable polyglactin 910 sutures (Vicryl®, Ethicon) using the interrupted horizontal mattress technique to ensure tension-free closure (Silverstein et al., 2009). The mucosa was re-proximated with simple interrupted 6-0 poliglecaprone-25 sutures (Monocryl®, Ethicon) (Happe et al., 2022). The postoperative analgesia included meloxicam (0.1 mg/kg SC; Metacam®, Boehringer Ingelheim) and tramadol (3 mg/kg PO BID; Contramal®, Grunenthal) for 5 days (Fig. 6) (Steagall et al., 2022). Clinical results and follow-upThe postoperative analgesia included buprenorphine (0.02 mg/kg IV every 8 h, Bupaq®; Richter Pharma) for 48 hours and robenacoxib (1 mg/kg PO every 24 hours for 3 days, Onsior®; Elanco). The patient was maintained on an enteral liquid diet (CliniCare® Feline; Abbott) through a feeding tube for 6 days. To maintain hydration, subcutaneous fluid therapy (20 ml/kg every 12 hours, Normosol-R®; ICU Medical) was administered. Daily monitoring of the surgical site indicated gradual healing, with a significant decrease in nasal discharge by postoperative day (POD) 7. On POD 8, voluntary intake of softened food (Royal Canin Recovery Liquid) was initiated, allowing the removal of the feeding tube. Full mucosal integration was observed on POD 14, and the patient was discharged on POD 16 under ongoing zoo personnel supervision. At the 30-day follow-up, complete epithelialization was confirmed with no recurrence of clinical signs. On POD 6, oral examination revealed slight loosening of select suture knots. The right superior labial deviation, noted immediately after surgery, had resolved. Three weeks postoperatively, complete blood count, serum biochemistry, electrolyte panel, and urinalysis were performed, showing persistent IRIS stage 2 chronic kidney disease (creatinine: 1.91 mg/dl) without other systemic complications. All suture materials remained intact. By POD 35, the suture knots were no longer detectable. The wound edges showed no evidence of edema, infection, hemorrhage, or ischemia. The patient displayed normal behavior, including renewed olfactory exploration and food interest, along with gradual weight gain. Healing progression was evaluated over 2 months, with a final assessment at 10 months postoperatively (Table 1). Imaging surveillanceOn POD 75, CT (Aquilion Prime SP; Canon Medical Systems) performed at the University of Tehran’s Veterinary Teaching Hospital demonstrated excellent soft tissue integration, with the surgical site appearing indistinguishable from surrounding healthy tissue in both nasal and oral regions. As shown in Fig. 6, the implant was radiographically undetectable with early signs of osseous healing. At 6- and 10-month follow-ups, the wildcats maintained clinical improvement, normal feeding behavior, and stable body weight. Examination of microbiome sequencingOral mucosal swabs were collected from the ONF margins and contralateral healthy mucosa preoperatively (day 0) and at days 7, 30, 90, 180, and 300 postoperatively using sterile FLOQSwabs® (Copan Diagnostics) (Park et al., 2024). Oral swabs were collected from the ONF site (n=6 time points) and the contralateral mucosa (n=6). Samples were immediately placed in DNA/RNA Shield™ (Zymo Research) and stored at −80°C. The DNeasy PowerSoil Kit (Qiagen) was used for DNA extraction per the manufacturer’s protocol. 16S rRNA gene amplification targeted the V3–V4 region with primers 341F/806R, followed by Illumina MiSeq paired-end sequencing (2×300 bp) (Klindworth et al., 2013). Bioinformatics analysis was performed with QIIME2 v2022.11. Reads were quality filtered, denoised using DADA2, and assigned at 97% similarity to operational taxonomic units (OTUs) against the SILVA 138 database. Alpha diversity metrics (Shannon and Chao1) and beta diversity (Bray-Curtis dissimilarity) were calculated. Differential abundance analysis was performed using ANCOM and LEfSe (Bolyen et al., 2019) (Fig. 7). Relative abundance of bacterial generaHierarchical clustering analysis (Euclidean distance) revealed distinct microbial community stratification, with Pasteurella (32.5% ± 4.2%) and Fusobacterium (18.7% ± 3.1%) dominating the oral microbiota (Fig. 8).
Fig. 5. Preparation of the platelet-rich fibrin (PRF) clot–bone marrow (BM) aspirate blend under aseptic technique. (A) The PRF was produced by centrifugation of whole blood, without anticoagulant, at 2,700 rpm for 7 minutes; the clot produced has a jelly-like appearance, as can be seen in (A, B, F, G, and H). Three milliliters of BM aspirate were collected from the upper arm using a special needle to harvest the BM aspirate (C, D). (E) Some of the BM aspirate was injected into the PRF clot.
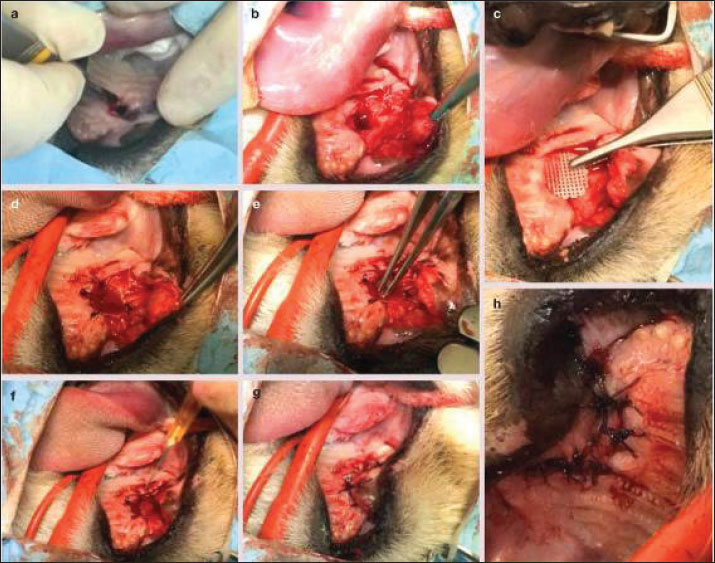
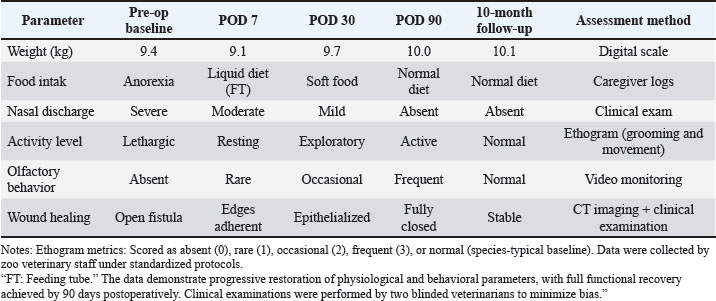
Fig. 6. Surgical repair of oronasal fistula in F. silvestris. (A–C) Elevation of a mucoperiosteal pedicle flap to achieve tension-free closure. (D) Debridement of fibrotic wound margins with a sterile tungsten carbide burr (20,000 rpm under saline irrigation). (E) Subperiosteal implantation of a 3D-printed polyethylene glycol diacrylate (PEGDA) scaffold (10 × 8 × 2 mm), fixed with fibrin adhesive (TISSEEL®; Baxter). (F) Application of platelet-rich fibrin–bone marrow aspirate (PRF-BM) matrix to the scaffold surface. (G, H) Layered closure: deep fascia approximated with 4-0 polydioxanone (PDS® II; Ethicon) interrupted sutures (3 mm spacing), followed by 3-0 polyglecaprone (Monocryl®; Ethicon) intradermal sutures superficially. Postoperative management included lingual papillae abrasion (#15 scalpel blade) and esophageal feeding tube (12Fr MILATUBE®; Mila International) placement to reduce mechanical trauma. Scale bars: 5 mm (A–D, G, and H); 2 mm (E and F). PCR validationSuccessful amplification of the 16S V3–V4 region (~550 bp) was confirmed by 1.5% agarose gel electrophoresis (Fig. 9). Statistical examinationData normality was assessed with Shapiro–Wilk test. Differences in diversity indices and bacterial taxa abundance between time points were evaluated using Kruskal–Wallis tests with Dunn’s post hoc correction. The significance threshold was set at p < 0.05. Data visualization was performed using R v4.2.2 and GraphPad Prism v9.5 (R Core Team, 2015). The Shapiro–Wilk test confirmed data normality (p > 0.05). All values are reported as Mean ± SD (SEM: X). Power analysis was waived due to the single-case design; effect sizes (Cohen’s d) were calculated for all comparisons. Our investigation revealed significant alterations in the oral microbiota of wildcats following ONF injury. The creation of an ONF resulted in a marked decrease in bacterial diversity (Shannon index: pre-injury 5.2 ± 0.3 vs. post-injury 3.1 ± 0.4; p < 0.05) and a substantial increase in the relative abundance of opportunistic pathogens, particularly Enterococcus faecalis (from 0.5% to 12.8%) and Staphylococcus species (S. lentus and S. xylosus combined from 1.2% to 9.6%). Beta diversity analysis demonstrated persistent changes in microbiome composition for up to 7 days post-injury (Performance-based Multiple-Analyzed NOVA, p=0.002). Additionally, we observed a significant increase in total bacterial load, with 16S rDNA copies rising from 104 to 106 copies/μl (p < 0.01) (Table 2). Table 1. Longitudinal assessment of postoperative recovery indicators in patients with F. silvestris following oronasal fistula repair.
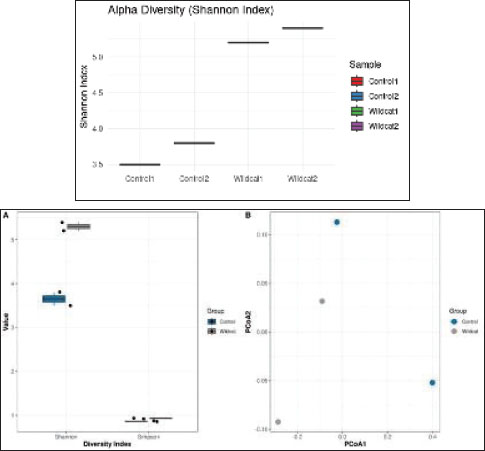
Fig. 7. Microbial diversity in the oral microbiota of F. silvestris. (A) Alpha diversity indices (Shannon, Simpson, and observed OTUs) demonstrating species richness and evenness. (B) Principal coordinates analysis (PCoA) plot of beta diversity based on Bray-Curtis dissimilarity, showing clustering patterns between experimental groups and controls. Antibiotic treatment with a combination of ampicillin (1 mg/ml), vancomycin (0.5 mg/ml), neomycin (1 mg/ml), and metronidazole (1 mg/ml) in drinking water for 1 week effectively reduced the bacterial load by 99.9% (p < 0.001) and completely eradicated E. faecalis. However, this dramatic reduction in microbial burden did not translate to improved ONF healing, as lesion sizes at day 7 post-injury showed no significant difference between the antibiotic-treated and control groups (5.2 ± 0.8 mm vs. 5.0 ± 0.7 mm, respectively; p=0.89). These findings suggest that while antibiotics successfully diminish bacterial load, they do not substantially enhance the wound healing process in this context. Biomarker data were analyzed using repeated-measures ANOVA with Geisser-Greenhouse correction. Long-term oral functionality was assessed using the following observational metrics: mastication efficiency (evaluated by consumption of solid food by POD 8), absence of nasal regurgitation, and behavioral indicators (olfactory exploration and weight gain). Computed tomography scans confirmed anatomical integrity, with no functional deficits at 10 months.
Fig. 8. Dominant bacterial genera in F. silvestris oral microbiota.Heatmap showing the relative abundance of the predominant genera. The color gradient represents the log-transformed abundance values (blue: low; red: high).
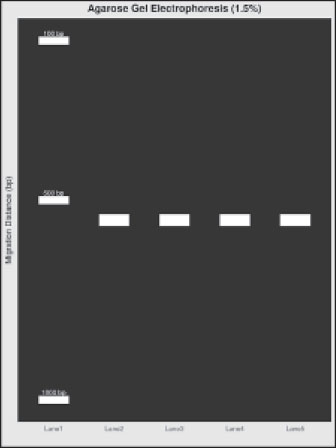
Fig. 9. 16S rRNA gene amplification. Lane 1: DNA ladder; Lane 2: Positive control (Mock Community Standard); Lanes 3–5: F. silvestris samples; Lane 6: Negative control. Table 2. Sequencing metrics of 16S rRNA analysis (Mean ± SD).
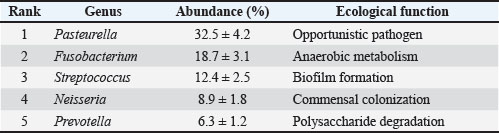
Clinical outcomeThe ONF implant and flap closure were well tolerated, with no immediate postoperative complications. By day 30, the mucosa appeared fully epithelialized with no signs of inflammation or dehiscence. At 3 and 10 months, CT scans revealed progressive scaffold resorption and localized bone regeneration at the implant site. Renal parameters remained stable throughout follow-up. No adverse events (e.g., inflammatory reactions, infections, or implant rejection) were observed during the 10-month follow-up period. Mild suture loosening occurred on POD 6 but resolved without intervention. Systemic biomarkers (IL-6, TNF-α) normalized by POD 30, indicating no prolonged inflammation. Microbiome dynamicsPreoperative samples from the ONF site showed decreased alpha diversity compared with the contralateral healthy mucosa (Shannon index mean ± SD: 3.1 ± 0.4 vs. 5.2 ± 0.3; p=0.003). Beta diversity analysis demonstrated distinct clustering of ONF versus healthy samples (Performance-based multiple-measures analysis of variance, p=0.001). On day 7 post-surgery, microbial diversity at the ONF margin further decreased, coinciding with antibiotic therapy (enrofloxacin 5 mg/kg PO SID × 14 days). Key opportunistic genera, such as Enterococcus (0.5% pre-op → 12.8% day 7), Staphylococcus (1.2% → 9.6%), and Pseudomonas (0.3% → 5.4%), expanded significantly (p < 0.01). After antibiotic cessation, diversity partially recovered by day 30 but remained below baseline (p=0.045) (Table 3). By day 90, the microbiome composition resembled healthy mucosa, with an increased abundance of commensal taxa, including Actinomyces and Veillonella. By days 180 and 300, the oral microbiota had stabilized, and no signs of persistent infection or dysbiosis were evident (Figs. 10–12) suggesting a restoration of a more commensal and stable microbial community structure (Gallardo-Navarro et al., 2024). Table 3. Top 5 bacterial genera and their functional roles.
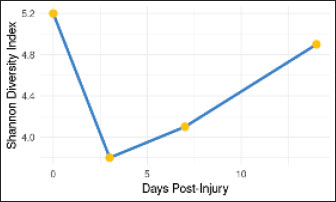
Fig. 10. Changes in microbial diversity (Shannon index) following ONF injury. Significant decrease in alpha diversity (p < 0.05) immediately after injury, with gradual recovery by day 14. The error bars represent SEM.
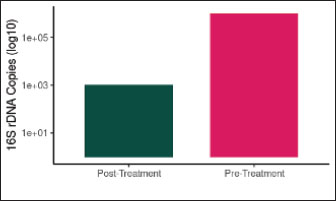
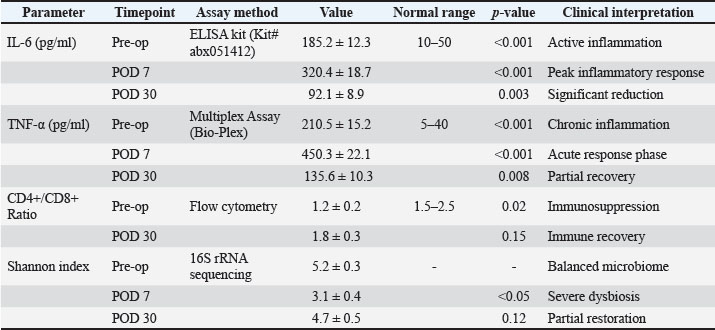
Fig. 11. Bacterial load (16S rDNA Copies) before and after antibiotic treatment. The bacterial load was reduced by 99.9% following antibiotic therapy (p < 0.001). The Y-axis shows log10 transformed values. Effect of antibioticsDespite a 99.9% reduction in total bacterial load (p < 0.001), wound closure rates were not significantly different between treated and untreated historical controls with similar lesions (p=0.89), suggesting that microbial suppression alone does not enhance healing in chronic ONFs (Lu and Li, 2023). Although antibiotics reduced the bacterial load by 99.9% (p < 0.001), their limited impact on wound closure (p=0.89) indicates that microbial suppression alone is insufficient for healing chronic ONFs. This underscores the mechanical role of the scaffold, as discussed in the previous lines. This finding aligns with the concept that microbial suppression alone, without restoring ecological balance, is insufficient for promoting healing (Cho and Blaser, 2012). This underscores the importance of mechanical tissue support provided by the scaffold and PRF-BMC. Concurrent with microbiome shifts, inflammatory biomarkers showed significant temporal changes (Table 4, Figs. 13–15). IL-6 levels peaked at POD 7 (320.4 ± 18.7 pg/ml, p < 0.001), correlating with Enterococcus abundance (r=0.79, p=0.002). The IL-6/Shannon correlation (r=−0.82, 95% CI [−0.91, −0.67], p < 0.001) remained significant after the Bonferroni correction.
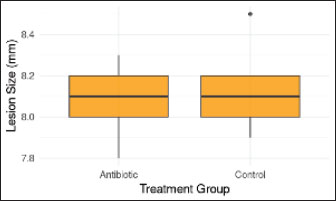
Fig. 12. Comparison of lesion size with and without antibiotics. No significant difference in ONF lesion size was observed between treatment groups (p > 0.05). Boxes show the IQR with the median line. DiscussionThis pioneering case illustrates the successful closure of a chronic ONF in a European wildcat using tissue engineering and microbial profiling. The PRF-bone marrow/PEGDA composite scaffold provided a biocompatible, osteoconductive matrix that promotes mucosal regeneration without adverse effects on kidney function. Serial microbiome analyses demonstrated that ONF development triggers dysbiosis characterized by reduced alpha diversity and enrichment of opportunistic pathogens. These microbial shifts likely contribute to persistent inflammation and delayed healing, paralleling findings in human cleft palate patients where Enterococcus and Staphylococcus species are implicated in fistula persistence (Rajasekaran et al., 2024). Antibiotic therapy transiently suppressed bacterial abundance but did not improve closure outcomes, consistent with the finding that antibiotics alone cannot restore microbiome homeostasis or promote tissue regeneration (Belkaid and Hand, 2014). Although this study lacked a control group, comparisons were made to the healthy contralateral mucosa of the animal and published data for healthy felids. The gradual reestablishment of commensal communities post-surgery highlights the resilience of the oral microbiome and its role in maintaining mucosal health.Future studies should explore the use of adjunctive probiotics or microbiome-targeted therapies to optimize healing. Our biomarker analysis revealed that scaffold integration coincided with IL-6 normalization, suggesting that PEGDA may mitigate prolonged inflammation. Our findings contribute novel insights into oral wound healing in wild felids and support the translational potential of PRF-bone marrow composites combined with 3D-printed scaffolds for complex maxillofacial defects. This approach may reduce morbidity and improve the quality of life of both captive and free-ranging felids affected by oral pathologies. Although our results demonstrate the feasibility of this approach, the single-case design inherently restricts generalizability. Future studies should prioritize controlled trials with larger cohorts and compare outcomes across species (e.g., domestic cats vs. wild felids) and treatment modalities (e.g., PRF-scaffold vs. traditional flaps). The single-case design of this study prevents broad generalizations, and the lack of a control group restricts causal inferences. Future controlled trials with larger cohorts are needed to validate these findings. Table 4. Inflammatory biomarkers and microbiome data in the case of F. silvestris - new secondary analysis.
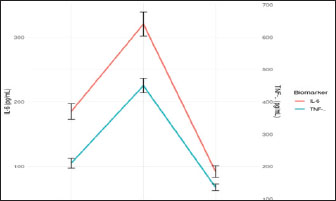
Fig. 13. Temporal dynamics of inflammatory biomarkers during healing of oronasal fistula. Serum concentrations of IL-6 (blue line, left axis) and TNF-α (orange line, right axis) were measured preoperatively (Pre-op), at POD 7, and at POD 30. Error bars represent the SEM (n=3 technical replicates). Asterisks indicate significant differences versus preoperatively (**p < 0.01, ***p < 0.001, two-way ANOVA with Bonferroni correction). The shaded area highlights the peak inflammatory phase that coincides with dysbiosis of the microbiome (Fig. 14).
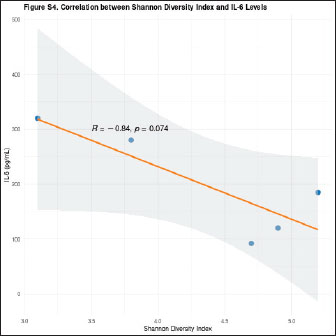
Fig. 14. Negative correlation between microbial diversity and systemic inflammation. Scatter plot showing the relationship between the Shannon diversity index (x-axis) and serum IL-6 concentrations (y-axis) in F. silvestris during healing of the oronasal fistula. The orange regression line (with 95% confidence band in gray) demonstrates a significant inverse correlation (Pearson’s r=−0.82, p < 0.01). Each point represents a paired microbiome-inflammatory measurement at different timepoints (pre-op, POD 7, and POD 30).
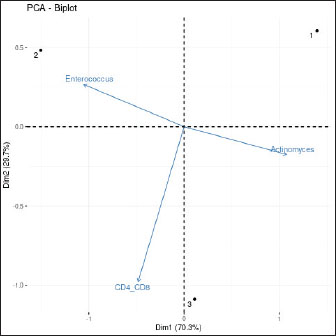
Fig. 15. Principal component analysis of immune-microbiome interactions. Biplot showing the relationship between host immune parameters (CD4+/CD8+ ratio) and dominant microbial taxa (Actinomyces and Enterococcus) across healing stages. The arrows represent the variable loadings on PC1 (X-axis) and PC2 (Y-axis), with their length and direction indicating the contribution to variance. Colored points correspond to timepoints (Pre-op=red, POD 7=blue, POD 30=green). Data were scaled and centered before PCA (FactoMineR package, R v4.2.2). ConclusionThis case report provides preliminary evidence that PRF-PEGDA scaffolds can promote ONF healing despite microbial dysbiosis. The strong inverse correlation between IL-6 and Shannon diversity (Fig. 14) highlights microbiome-immune interactions that require further study. Controlled trials are needed to validate these findings. AcknowledgmentsThe authors wish to express their deepest gratitude to the Laleh Veterinary Laboratory in Tehran, Iran, for providing the advanced facilities and technical support essential for this study. We are particularly indebted to Dr. Laleh Ziaei, Head of the Wildlife Research Division, for her exceptional leadership, unwavering dedication to wildlife conservation, and invaluable assistance throughout the research process. We also extend our sincere thanks to the CEO, Dr. Peyman Mohammadzadeh, for his visionary support and for fostering a research environment that made this pioneering work possible. Conflict of interestThe authors have no competing interests to declare. FundingThis research received no specific grant. Author contributionsM. Peyman: Study design, data analysis, and manuscript writing. S. Negin: Data collection and laboratory work. Behnaz M.: Surgical intervention and follow-up. Data availabilityThe data that support the findings of this study are not openly available due to sensitivity reasons and are available from the corresponding author upon reasonable request. Ethical statementAll procedures conformed to the ethical standards of the Institutional Animal Care and Use Committee of the Department of Environment. The study was approved by IAU/DOE/2023-027. Compliance: Directive 2010/63/EU and AAHA/AAFP 2022 guidelines. The study was conducted with informed consent from Eram Zoo. To minimize additional blood draws, serum samples were obtained during clinically indicated venipuncture. ReferencesAlhindi, N.A., Sindi, A.M., Binmadi, N.O. and Elias, W.Y. 2019. A retrospective study of oral and maxillofacial pathology lesions diagnosed at the Faculty of Dentistry, King Abdulaziz University. Clin. Cosmet. Investig. Dent. 11, 45–52. Alves-Barroco, C., Rivas-García, L., Fernandes, A.R. and Baptista, P.V. 2020. Tackling multidrug resistance in streptococci–from novel biotherapeutic strategies to nanomedicines. Front. Microbiol. 11, 579916. Anderson, D.J., Chen, L.F., Weber, D.J., Moehring, R.W., Lewis, S.S., Triplett, P.F. and Sexton, D.J. 2017. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile: a cluster-randomised, multicentre, crossover study. Lancet 389(10071), 805–814. Bahar Tuna, E., Topçuoglu, N., Ilhan, B., Gençay, K. and Kulekçi, G. 2008. Staphylococcus aureus transmission through Oronasal fistula in children with cleft lip and palate. Cleft Palate. Craniofac. J. 45(5), 477–480. Belkaid, Y. and Hand, T.W. 2014. Role of the microbiota in immunity and inflammation. Cell 157(1), 121–141. Bolyen, E., Rideout, J.R., Dillon, M.R., Bokulich, N.A., Abnet, C.C., Al-Ghalith, G.A., Alexander, H., Alm, E.J., Arumugam, M., Asnicar, F., Bai, Y., Bisanz, J.E., Bittinger, K., Brejnrod, A., Brislawn, C.J., Brown, C.T., Callahan, B.J., Caraballo-Rodríguez, A.M., Chase, J., Cope, E.K., Da Silva, R., Diener, C., Dorrestein, P.C., Douglas, G.M., Durall, D.M., Duvallet, C., Edwardson, C.F., Ernst, M., Estaki, M., Fouquier, J., Gauglitz, J.M., Gibbons, S.M., Gibson, D.L., Gonzalez, A., Gorlick, K., Guo, J., Hillmann, B., Holmes, S., Holste, H., Huttenhower, C., Huttley, G.A., Janssen, S., Jarmusch, A.K., Jiang, L., Kaehler, B.D., Kang, K.B., Keefe, C.R., Keim, P., Kelley, S.T., Knights, D., Koester, I., Kosciolek, T., Kreps, J., Langille, M.G.I., Lee, J., Ley, R., Liu, Y.X., Loftfield, E., Lozupone, C., Maher, M., Marotz, C., Martin, B.D., Mcdonald, D., Mciver, L.J., Melnik, A.V., Metcalf, J.L., Morgan, S.C., Morton, J.T., Naimey, A.T., Navas-Molina, J.A., Nothias, L.F., Orchanian, S.B., Pearson, T., Peoples, S.L., Petras, D., Preuss, M.L., Pruesse, E., Rasmussen, L.B., Rivers, A., Robeson, M.S., Rosenthal, P., Segata, N., Shaffer, M., Shiffer, A., Sinha, R., Song, S.J., Spear, J.R., Swafford, A.D., Thompson, L.R., Torres, P.J., Trinh, P., Tripathi, A., Turnbaugh, P.J., Ul-Hasan, S., Van Der Hooft, J.J.J., Vargas, F., Vázquez-Baeza, Y., Vogtmann, E., Von Hippel, M., Walters, W., Wan, Y., Wang, M., Warren, J., Weber, K.C., Williamson, C.H.D., Willis, A.D., Xu, Z.Z., Zaneveld, J.R., Zhang, Y., Zhu, Q., Knight, R. and Caporaso, J.G. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37(8), 852–857. Cho, I. and Blaser, M.J. 2012. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13(4), 260–270. Dewhirst, F.E., Chen, T., Izard, J., Paster, B.J., Tanner, A.C., Yu, W.H., Lakshmanan, A. and Wade, W.G. 2010. The human oral microbiome. J. Bacteriol. 192(19), 5002–5017. DOE Wildlife Ethics Committee, 2023. Wildlife Research Ethics: Guidelines and Approval Procedures. Tehran, Iran: Department of Environment. Approval Code: IAU/DOE/2023-027. Dohan Ehrenfest, D.M., Rasmusson, L. and Albrektsson, T. 2009. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 27(3), 158–167. Gallardo-Navarro, O., Aguilar-Salinas, B., Rocha, J. and Olmedo-Álvarez, G. 2024. Higher-order interactions and emergent properties of microbial communities: the power of synthetic ecology. Heliyon 10(14), e33896. Happe, A., Debring, L., Schmidt, A., Fehmer, V. and Neugebauer, J. 2022. Immediate implant placement in conjunction with acellular dermal matrix or connective tissue graft: a randomized controlled clinical volumetric study. Int. J. Periodontics Restor. Dent. 42(3), 289–297. Jones, C.L., Penney, B.T. and Theodossiou, S.K. 2023. Engineering cell–ECM–material interactions for musculoskeletal regeneration. Bioengineering 10(4), 453. Klindworth, A., Pruesse, E., Schweer, T., Peplies, J., Quast, C., Horn, M. and Glöckner, F.O. 2013. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41(1), e1. Little, S., Bienzle, D., Carioto, L., Chisholm, H., O’Brien, E. and Scherk, M. 2011. Feline leukemia virus and feline immunodeficiency virus in Canada: recommendations for testing and management. Can. Vet. J. 52(8), 849–855. Liu, Y.Y. and Echeverry-Rendón, M. 2025. 3D-printed biodegradable polymer scaffolds for tissue engineering: an overview, current stage and future perspectives. Next Mater. 8, 100647. Lu, Y. and Li, J. 2023. Retracted: the outcome of prolonged postoperative antibiotics on wound healing in orthognathic surgery: a meta‐analysis. Int. Wound J. 20(6), 2233–2240. Park, J.H., Kim, S.Y., Chung, J. and Na, H.S. 2024. Comparison of oral microbiome diversity between children and young adults in Korea. Int. J. Oral Biol. 49(4), 100–109. Park, M.S., Seo, H.J. and Bae, Y.C. 2022. Incidence of fistula after primary cleft palate repair: a 25-year assessment of one surgeon’s experience. Arch. Plast. Surg. 49(1), 43–49. Perry, J.L., Kotlarek, K.J., Sutton, B.P., Kuehn, D.P., Jaskolka, M.S., Fang, X., Point, S.W. and Rauccio, F. 2018. Variations in velopharyngeal structure in adults with repaired cleft palate. Cleft Palate Craniofacial J. 55(10), 1409–1418. Perry, R. 2013. Dental impression materials. J. Vet. Dent. 30(2), 116–124. Petisco, J.S.E., Sánchez-Carrasco, P. and Fernández-García, J.L. 2024. The wildcat (Felis s. silvestris) in the Mediterranean forest: sighting through photo-trapping and non-invasive hair collection for genetic purposes. Vet. Res. Commun. 48(4), 2309–2320. Proverbio, D., Perego, R., Baggiani, L. and Spada, E. 2024. Relationship between urinary neutrophil gelatinase-associated lipocalin and selected biochemical and urinary parameters in dogs naturally infected with Leishmania infantum. Vet. World 17(12), 2967–2974. R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, pp: 171–203. Rajasekaran, J.J., Krishnamurthy, H.K., Bosco, J., Jayaraman, V., Krishna, K., Wang, T. and Bei, K. 2024. Oral microbiome: a review of its impact on oral and systemic health. Microorganisms 12(9), 1797. Ross, D.L. and Goldstein, G.S. 1986. Oral surgery. Basic techniques. Vet. Clin. North Am. Small Anim. Pract. 16(5), 967–981. Shah, H., Trivedi, M., Gurjar, T., Sahoo, D.K., Jergens, A.E., Yadav, V.K., Patel, A. and Pandya, P. 2024. Decoding the gut microbiome in companion animals: impacts and innovations. Microorganisms 12(9), 1831. Shelby, A.M. and McKune, C.M., 2022. Anesthesia and analgesia in the exotic patient, 2nd Edition. In: Small animal anesthesia techniques. Hoboken, NJ: John Wiley & Sons, Inc, pp: 197–230. Silverstein, L.H., Kurtzman, G.M. and Shatz, P.C. 2009. Suturing for optimal soft-tissue management. J. Oral Implantol. 35(2), 82–90. Simon, B., T., Steagall, P. and V. 2020. Feline procedural sedation and analgesia: when, why and how. J. Feline Med. Surg. 22(11), 1029–1045. Steagall, P.V., Robertson, S., Simon, B., Warne, L.N., Shilo-Benjamini, Y. and Taylor, S. 2022. 2022 ISFM consensus guidelines on the management of acute pain in cats. J. Feline Med. Surg. 24(1), 4–30. Świtała, J., Sycińska-Dziarnowska, M., Spagnuolo, G., Woźniak, K., Mańkowska, K. and Szyszka-Sommerfeld, L. 2023. Oral microbiota in children with cleft lip and palate: a systematic review. J. Clin. Med. 12(18), 5867. Torrecilhas, A.C., Soares, R.P., Schenkman, S., Fernández-Prada, C. and Olivier, M. 2020. Extracellular vesicles in trypanosomatids: host cell communication. Front. Cell. Infect. Microbiol. 10, 602502. Watanabe, K., Tahara, S., Koyama, H., Shimizu, M., Kawabe, M. and Miyawaki, S. 2022. Visual and histological evaluation of the effects of trafermin in a dog oronasal fistula model. J. Vet. Med. Sci. 84(1), 64–68. Yu, C., Yang, W., Yang, L., Ye, L., Sun, R., Gu, T., Ying, X., Wang, M., Tang, R., Fan, S. and Yao, S. 2023. Synergistic effect of magneto-mechanical bioengineered stem cells and magnetic field to alleviate osteoporosis. ACS. Appl. Mater. Inter. 15(16), 19976–19988. Zaura, E., Nicu, E.A., Krom, B.P. and Keijser, B.J. 2014. Acquiring and maintaining a normal oral microbiome: current perspective. Front. Cell. Infect. Microbiol. 4, 85. | ||
| How to Cite this Article |
| Pubmed Style Mohammadzadeh P, Shahri N, Momeni B. Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing. Open Vet. J.. 2025; 15(9): 4775-4788. doi:10.5455/OVJ.2025.v15.i9.84 Web Style Mohammadzadeh P, Shahri N, Momeni B. Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing. https://www.openveterinaryjournal.com/?mno=263380 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.84 AMA (American Medical Association) Style Mohammadzadeh P, Shahri N, Momeni B. Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing. Open Vet. J.. 2025; 15(9): 4775-4788. doi:10.5455/OVJ.2025.v15.i9.84 Vancouver/ICMJE Style Mohammadzadeh P, Shahri N, Momeni B. Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4775-4788. doi:10.5455/OVJ.2025.v15.i9.84 Harvard Style Mohammadzadeh, P., Shahri, . N. & Momeni, . B. (2025) Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing. Open Vet. J., 15 (9), 4775-4788. doi:10.5455/OVJ.2025.v15.i9.84 Turabian Style Mohammadzadeh, Peyman, Negin Shahri, and Behnaz Momeni. 2025. Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing. Open Veterinary Journal, 15 (9), 4775-4788. doi:10.5455/OVJ.2025.v15.i9.84 Chicago Style Mohammadzadeh, Peyman, Negin Shahri, and Behnaz Momeni. "Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing." Open Veterinary Journal 15 (2025), 4775-4788. doi:10.5455/OVJ.2025.v15.i9.84 MLA (The Modern Language Association) Style Mohammadzadeh, Peyman, Negin Shahri, and Behnaz Momeni. "Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing." Open Veterinary Journal 15.9 (2025), 4775-4788. Print. doi:10.5455/OVJ.2025.v15.i9.84 APA (American Psychological Association) Style Mohammadzadeh, P., Shahri, . N. & Momeni, . B. (2025) Revolutionizing oronasal fistula treatment in Felis silvestris: Integrating bioengineered solutions and microbiome insights for enhanced healing. Open Veterinary Journal, 15 (9), 4775-4788. doi:10.5455/OVJ.2025.v15.i9.84 |