| Case Report | ||
Open Vet. J.. 2025; 15(8): 3899-3903 Open Veterinary Journal, (2025), Vol. 15(8): 3899-3903 Case Report First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical managementDonghwi Shin and Kang-hyo Park*Bundang Leaders Animal Medical Center 45 Seongnam-daero, Bundang-gu, Seongnam, Gyeonggi-do 13558, Republic of Korea *Corresponding Author: Kang-hyo Park. Bundang Leaders Animal Medical Center 45 Seongnam-daero, Bundang-gu, Seongnam, Gyeonggi-do 13558, Republic of Korea. Email: schuna99 [at] naver.com Submitted: 08/06/2025 Revised: 24/07/2025 Accepted: 31/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: An 11-year-old male Siberian Husky presented with acute hypovolemic shock on initial physical examination. Serum biochemistry revealed hyperamylasemia and hyperlipasemia. Abdominal ultrasonography identified a well-defined, round, lobulated 10.0 cm mass caudal to the stomach. A large volume of hyperechoic peritoneal fluid was detected in the abdominal cavity. The peritoneal fluid packed cell volume (PCV) was similar to the peripheral PCV, confirming hemoperitoneum. Case Description: Surgical resection of the mass and damaged pancreatic vessels was performed. Adherent mesenteric blood vessels and a segment of the jejunum were also removed due to their association with the mass, followed by jejunal anastomosis. No abnormal clinical signs were observed postoperatively. A definitive diagnosis of pancreatic acinar cell tumor was made based on histopathological findings. The patient remained clinically stable without evidence of recurrence for over 11 months after surgery. Conclusion: This report is the first documented case describing successful surgical management of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma in a dog. Histopathological evaluation confirmed a moderately well-differentiated pancreatic acinar cell carcinoma. This case suggests that timely diagnosis and intervention may improve outcomes in patients with hemorrhagic presentations of pancreatic neoplasia. Keywords: Pancreatic acinar cell carcinoma, Hemoperitoneum, Surgical management, Dog. IntroductionNontraumatic hemoperitoneum refers to blood accumulation within the abdominal cavity that occurs without any evident traumatic event (Pintar et al., 2003; Aronsohn et al., 2009; Stefano et al., 2011). This condition can arise from multiple underlying factors, such as organ injury, torsion, coagulation disorders, or disseminated intravascular coagulation associated with benign or malignant tumors (Pintar et al., 2003). Acute nontraumatic hemoperitoneum in dogs has been described in only a limited number of reports (Pintar et al., 2003; Aronsohn et al., 2009). In a study involving 60 dogs diagnosed with tumor-associated nontraumatic hemoperitoneum, hemangiosarcoma accounted for 38 cases (63.3%), splenic hematoma for 16 (26.6%), splenic torsion for three (5%), hepatocellular carcinoma for two (3.3%), and carcinomatosis for one (1.6%) (Aronsohn et al., 2009). Common clinical signs observed in acute hemoperitoneum cases include hypovolemic shock, prolonged capillary refill time, dyspnea, tachypnea, and tachycardia (Pintar et al., 2003; Aronsohn et al., 2009). Due to the life-threatening nature of this condition, early diagnosis and treatment are critical (Stefano et al., 2011). Exocrine pancreatic tumors are considered uncommon in dogs and cats, accounting for less than 0.5% of all tumors (Bennett et al., 2001; Seaman, 2004; Withrow, 2013). Although some cases of pancreatic carcinoma have been reported, only one case of pancreatic acinar cell carcinoma has been described in a dog (Chang et al., 2007). Recent histopathological research highlighted the diversity of exocrine pancreatic neoplasms in dogs and the frequent overlap of features with inflammatory lesions, underscoring diagnostic challenges (Aupperle-Lellbach et al., 2020). Hematologic and biochemical abnormalities are generally nonspecific, though findings such as anemia, neutrophilia, hyperglycemia, or bilirubinemia can be observed (Withrow, 2013; Aupperle-Lellbach et al., 2020). At diagnosis, exocrine pancreatic tumors are frequently metastatic or locally advanced, often showing extensive invasion into surrounding tissues (Withrow, 2013). Hemoperitoneum secondary to pancreatic acinar cell carcinoma has not been previously reported in dogs. This appears to be the first documented case describing successful surgical management of hemoperitoneum caused by pancreatic acinar cell carcinoma in a dog. Case DetailsAn 11-year-old intact male Siberian Husky was presented in acute hypovolemic shock. On physical examination, the patient was lethargic and exhibited tachycardia, tachypnea, and hypotension, with a recorded systolic/diastolic blood pressure of 58/31 mmHg. Abdominal radiographs were not diagnostic due to the loss of serosal detail secondary to intra-abdominal fluid accumulation. Hematologic evaluation revealed microcytic (mean cell volume, 58.4 fL; reference range, 61.6–73.5 fL) and normochromic (mean corpuscular hemoglobin concentration, 30 g/dl; reference range, 32–37.9 g/dl) anemia, with a packed cell volume (PCV) of 28.5% (reference range, 37.3%–61.7%). Additional abnormalities included hyperglycemia (231 mg/dl; reference range, 70–143 mg/dl), hyperamylasemia (4543 U/L; reference range, 500–1,500 U/L), hyperlipasemia (5,683 U/L; reference range, 200–1,800), and elevated D-dimer concentration (2 mg/dl; reference range, 0–0.3 mg/dl). Abdominal ultrasonography identified a well-circumscribed, round, lobulated mass measuring approximately 10 cm in diameter, located caudal to the stomach (Fig. 1). A large volume of peritoneal fluid was also present within the abdominal cavity (Fig. 2). Compared to the anechoic urine within the bladder, the peritoneal fluid appeared hyperechoic. Fluid collected via abdominocentesis showed a PCV of 28% (reference range, 37.3%–61.7%). Thoracic radiographs showed no evidence of pulmonary metastases or thoracic abnormalities. To correct hypovolemic shock prior to surgery, lactated Ringer’s solution was administered intravenously (IV) at a rate of 90 ml/kg/hour. Additionally, one unit of whole blood was transfused to restore circulating volume. Premedication consisted of butorphanol (0.2 mg/kg IV; Butophan Inj., Myungmoon Pharm. Co., Ltd., Seoul, Republic of Korea) and cefazolin (30 mg/kg IV; Safdin, Daehan New Pharm Co., Ltd, Republic of Korea). Anesthesia was induced with propofol (6 mg/kg IV; Provive 1%, Myungmoon Pharm. Co., Ltd., Seoul, Republic of Korea) and maintained with 1.2%–1.4% isoflurane (Forane sol., Choongwae. Co., Ltd., Seoul, Republic of Korea) delivered in 100% oxygen. Lactated Ringer’s solution was administered at 10 ml/kg/hour IV throughout the surgical procedure. Exploratory laparotomy via a ventral midline incision confirmed the presence of hemoperitoneum (Fig. 3). A firm, round, lobulated mass measuring 6 cm in height, 7 cm in width, and 8.5 cm in length was identified in the right pancreatic lobe. The mass was firmly adhered to the jejunal mesentery and adjacent mesenteric vessels (Fig. 4), consistent with its location in the caudal portion of the right pancreatic limb. Active bleeding was observed from a pancreatic vessel adjacent to the mass (Fig. 5). Blunt dissection with moistened cotton swabs was used to separate the right lobe from surrounding vessels. The pancreatic vessels were ligated using a bipolar vessel-sealing device (LigaSure; Valleylab, Tyco Healthcare Group, Boulder, CO, USA). A ligature was placed approximately 2 cm proximal to the tumor within the pancreatic parenchyma, and a portion of normal pancreas was excised. Approximately 13 cm of jejunum and the associated mesentery were also resected, followed by an end-to-end jejunal anastomosis. No gross metastatic lesions were observed in the liver or spleen. All excised tissues were fixed in 10% buffered formalin for histopathological examination.
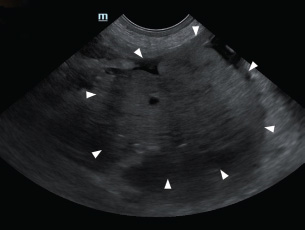
Fig. 1. Abdominal ultrasonography showed a well-circumscribed, round, and lobulated mass approximately 10 cm large (white arrowhead), located caudal to the stomach.
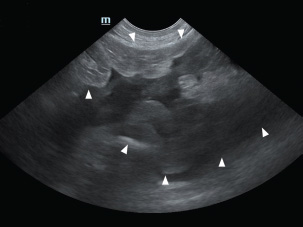
Fig. 2. A large amount of echogenic peritoneal fluid (white arrowhead), indicative of bleeding, was detected in the abdominal cavity.
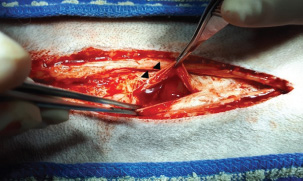
Fig. 3. Exploratory laparotomy, using a ventral midline incision, confirmed hemoperitoneum (black arrowhead).
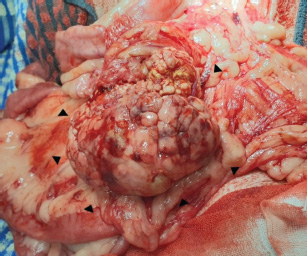
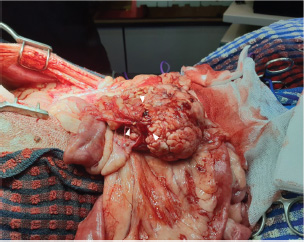
Fig. 4. During exploratory laparotomy, a firm, round, and lobulated mass approximately 6 cm in height, 7 cm in width, and 8.5 cm in length (black arrowhead) was found in the right lobe of the pancreas. This mass strongly adhered to the jejunal mesentery and mesenteric vessels.
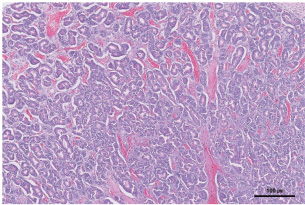
Fig. 5. Pancreatic vessels (white arrowhead), found adhered to the tumor, were bleeding. Histopathological examination of the pancreas revealed moderately well-differentiated pancreatic acinar cell carcinoma. Neoplastic cells were arranged in numerous variably sized interconnecting acini surrounded by and supported by a fine fibrovascular stroma. The acini contained intraluminal, pale, eosinophilic, and homogenous material (Fig. 6).
Fig. 6. Histopathological examination of the pancreas revealed a large pancreatic acinar cell carcinoma that was moderately well-differentiated. The neoplastic cells were arranged in numerous variably sized, interconnecting acini surrounded by and supported by fine fibrovascular stroma. The acini contained intraluminal, pale, eosinophilic, homogenous material (H&E stain, × 200 magnification, bar=100 μm). No surgery-related complications were noted. PCV was 24.5% at 2 hours postoperatively, increased to 32.5% by day 10, and normalized to 38% by day 15. Serum lipase (619 U/L) and amylase (889 U/L) returned to normal by day 10. At the follow-up conducted more than 11 months after surgery, the patient showed no clinical signs of recurrence. Follow-up thoracic radiographs and abdominal ultrasonography confirmed the absence of metastases. DiscussionNontraumatic hemoperitoneum in dogs has been associated with a range of intra-abdominal neoplasms, both benign and malignant (Brockman et al., 2000). Among these, malignant tumors represent the most frequent cause of spontaneous, nontraumatic hemoperitoneum (Pintar et al., 2003). Reported malignant tumors that have been associated with hemoperitoneum in dogs include hemangiosarcoma, mesothelioma, pheochromocytoma, hepatocellular carcinoma, and hepatic metastases from oral malignant melanoma (Pintar et al., 2003). In general, any condition that damages vascular structures adjacent to the peritoneum may lead to hemoperitoneum (Levinson et al., 2009). In one retrospective study, hemangiosarcoma was responsible for 70% of tumor-related acute nontraumatic hemoperitoneum cases in dogs (Pintar et al., 2003). Other tumors such as melanoma, mesothelioma, lymphoma, sarcoma, splenic hematoma, hepatocellular carcinoma, malignant splenic carcinoma, adrenocortical carcinoma, and pheochromocytoma have also been implicated, although these occur far less frequently than hemangiosarcoma (Pintar et al., 2003; Aronsohn et al., 2009). In addition to neoplastic causes, non-neoplastic conditions such as severe pancreatitis, hepatic rupture, coagulopathies (e.g., anticoagulant rodenticide toxicity), and vascular malformations should also be considered in the differential diagnosis for spontaneous hemoperitoneum (Brockman et al., 2000; Aronsohn et al., 2009). Notably, there have been no reports of hemoperitoneum in dogs caused by pancreatic carcinoma or other tumors of the digestive tract. In contrast, pancreatic tumors have occasionally been reported as a source of hemoperitoneum in human medicine. However, acinar cell carcinoma accounts for only 1%–2% of cases (Huang et al., 2005; Takamatsu et al., 2013). To date, there have been no prior reports of hemoperitoneum secondary to acinar cell carcinoma. The underlying mechanism of tumor rupture remains unclear. One proposed explanation suggests that thrombosis of the draining veins causes a sharp rise in intratumoral pressure and the development of fragile neovascular channels. These changes may accelerate tumor growth and increase the likelihood of trauma due to mechanical stress on surrounding tissues (Daskalopoulos et al., 2004). Another possible cause is acute torsion, particularly in organs or tumors, which can result in abrupt devascularization of the tumor and increase the likelihood of hemorrhage and rupture (Tirumani et al., 2013). Although torsion has been primarily described in humans, particularly with ovarian teratomas, it is known to cause venous and lymphatic obstruction, progressive tumor edema, arterial compromise, and ultimately hemorrhagic infarction and rupture (Park et al., 2008). In the present case, the increasing size and weight of the tumor located in the caudal portion of the right pancreatic lobe may have contributed to increased mobility. This mechanical instability might have predisposed the lesion to repetitive trauma or stretching of vessels entering or adhering to the tumor. Although speculative, one plausible explanation is that this vascular stress, potentially compounded by undetected torsion, contributed to hemorrhage and eventual hemoperitoneum. Additionally, the presence of concurrent inflammatory changes, as reported in some canine pancreatic neoplasms, may have contributed to increased vascular fragility and risk of rupture (Aupperle-Lellbach et al., 2020). Serum amylase and lipase levels can be elevated in dogs with pancreatic or hepatic tumors (Quigley et al., 2001). In one study on abnormal tumor lipase production, five of six dogs demonstrated lipase production in tumor tissues, including pancreatic adenocarcinoma, endocrine cancer, and hepatocellular carcinoma (Quigley et al., 2001). These findings suggest that unexplained hyperamylasemia and hyperlipasemia may serve as a useful biochemical indicator of pancreatic or hepatic tumors (Quigley et al., 2001). However, interpretation should be made cautiously, as pancreatic neoplasms, including acinar cell carcinoma, may exhibit concurrent inflammatory changes that can complicate the diagnostic evaluation (Aupperle-Lellbach et al., 2020). In this case, elevated lipase and amylase levels preoperatively decreased to normal postoperatively, indicating successful surgical intervention. The median survival time for dogs with nontraumatic hemoperitoneum is significantly longer in those treated surgically compared to those managed medically. Surgical management is indicated once the underlying cause of hemoperitoneum is identified, with the primary goal being to control hemorrhage and stabilize the patient (Cassie et al., 2022). Such interventions have been associated with a significant improvement in quality of life and owner satisfaction (Crawford et al., 2012). Postoperative monitoring is essential for early detection of potential coagulopathies and other complications (Cassie et al., 2022). Despite the high perioperative mortality rate, surgical treatment of nontraumatic hemoperitoneum associated with tumors often results in a short hospitalization and rapid recovery (Crawford et al., 2012). Therefore, if the underlying cause can be promptly identified and appropriate monitoring is ensured, surgical intervention may offer meaningful time and improved outcomes for both the patient and the owner (Crawford et al., 2012). To the best of our knowledge, this is the first report of pancreatic acinar cell carcinoma presenting with hemoperitoneum in a dog, successfully managed surgically. The presented patient recovered without clinical signs associated with pancreatic acinar cell carcinoma, and there was no evidence of further complications related to hemoperitoneum. These findings indicate that timely diagnosis and surgical management may improve quality of life even in cases of hemoperitoneum secondary to pancreatic tumors. Therefore, surgical management should be considered in such cases. A large case series using a large number of dogs is needed to better determine the overall success and complication rates associated with surgical management of pancreatic acinar cell carcinoma with hemoperitoneum in dogs. In addition, although the histopathological diagnosis supported a moderately well-differentiated acinar cell carcinoma, the lack of immunohistochemical analysis represents a diagnostic limitation. Immunohistochemical analysis markers such as cytokeratin and pancreatic lipase are useful in confirming acinar differentiation and excluding neuroendocrine tumors, which typically stain positively for chromogranin A or synaptophysin (Belshaw et al., 2005; Aupperle-Lellbach et al., 2020). Without these markers, a minor neuroendocrine component cannot be entirely excluded. Future studies incorporating immunohistochemical analysis during diagnostic evaluation may help facilitate more accurate classification and more meaningful clinical management. AcknowledgmentsThe authors would like to express their gratitude to the clinical staff at Bundang Leaders Animal Medical Center for their invaluable assistance and support in this study. Owner consent was obtained for all diagnostic and therapeutic procedures described in this report. Conflict of interestThe authors declare no conflict of interest. FundingNo specific grant was received. Authors’ contributionsDr. Donghwi Shin: execution of the project, preparation of the manuscript. Dr. Kang-hyo Park: project design, supervision of the report, revision of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAronsohn, M.G., Dubiel, B., Roberts, B. and Powers, B.E. 2009. Prognosis for acute nontraumatic hemoperitoneum in the dog: a retrospective analysis of 60 cases (2003-2006). J. Am. Anim. Hosp. Assoc. 45(2), 72–77; doi:10.5326/0450072 Aupperle-Lellbach, H., Törner, K., Staudacher, M., Stadler, C., Tress, U., Grassinger, J.M., Müller, E. and Weber, C.N. 2020. Histopathological findings and canine pancreatic lipase immunoreactivity in normal dogs and dogs with inflammatory and neoplastic disease of the pancreas. J. Vet. Intern. Med. 34, 1127–1134; doi:10.1111/jvim.15779 Belshaw, Z., Bacon, N.J., Foale, R.D., Mannion, P.M. and Reuter, R. 2005. Pancreatic mixed acinar-endocrine carcinoma in a dog. Vet. Comparative Oncol. 3(3), 145–148; doi:10.1111/j.1476-5810.2005.00073.x Bennett, P.F., Hahn, K.A., Toal, R.L. and Legendre, A.M. 2001. Ultrasonographic and cytopathological diagnosis of exocrine pancreatic carcinoma in the dog and cat. J. Am. Anim. Hosp. Assoc. 37(5), 466–473; doi:10.5326/15473317-37-5-466 Brockman, D.J., Mongil, C.M., Aronson, L.R. and Cimino Brown, D. 2000. A practical approach to hemoperitoneum in the dog and cat. Vet. Clin. North Am. Small Anim. Pract. 30(3), 657–668; doi:10.1016/s0195-5616(00)50044-8 Cassie, N.L., William, T.N.C. and Steven, E.E. 2022. Hemoperitoneum. 2nd. In Small animal surgical emergencies. Ed., Aronson, L.R. Hoboken, NJ: John Wiley & Sons, Inc, pp: 164–177; doi:10.1002/9781119658634.ch134 Chang, S.C., Liao, J.W., Lin, Y.C., Liu, C.I. and Wong, M.L. 2007. Pancreatic acinar cell carcinoma with intracranial metastasis in a dog. J. Vet. Med. Sci. 69(1), 91–93; doi:10.1292/jvms.69.91 Crawford, A.H., Tivers, M.S., Adamantos, S.E. and Crawford, A.H. 2012. Owner assessment of dogs’ quality of life following treatment of neoplastic haemoperitoneum. Vet. Rec. 170(22), 566–566; doi:10.1136/vr.100595 Daskalopoulos, G., Karyotis, I., Heretis, I., Anezinis, P., Mavromanolakis, E. and Delakas, D. 2004. Spontaneous perirenal hemorrhage: a 10-year experience at our institution. Int. Urol. Nephrol. 36(1), 15–19; doi:10.1023/b:urol.0000032680.65742.9a Huang, H.L., Shih, S.C., Chang, W.H., Wang, T.E., Chen, M.J. and Chan, Y.J. 2005. Solid-pseudopapillary tumor of the pancreas: clinical experience and literature review. World J. Gastroenterol. 11(9), 1403–1409; doi:10.3748/wjg.v11.i9.1403 Levinson, J.G., Bouma, J.L., Althouse, G.C. and Rieser, T.M. 2009. Prevalence of malignancy when solitary versus multiple lesions are detected during abdominal ultrasonographic examination of dogs with spontaneous hemoperitoneum: 31 cases (2003–2008). J. Vet. Emergency Crit. Care 19(5), 496–500; doi:10.1111/j.1476-4431.2009.00466.x Park, S.B., Kim, J.K., Kim, K.R. and Cho, K.S. 2008. Imaging findings of complications and unusual manifestations of ovarian teratomas. RadioGraphics 28(4), 969–983; doi:10.1148/rg.284075069 Pintar, J., Breitschwerdt, E.B., Hardie, E.M. and Spaulding, K.A. 2003. Acute nontraumatic hemoabdomen in the dog: a retrospective analysis of 39 cases (1987-2001). J. Am. Anim. Hosp. Assoc. 39(6), 518–522; doi:10.5326/0390518 Quigley, K.A., Jackson, M.L. and Haines, D.M. 2001. Hyperlipasemia in 6 dogs with pancreatic or hepatic neoplasia: evidence for tumor lipase production. Vet. Clin. Pathol. 30(3), 114–120; doi:10.1111/j.1939-165x.2001.tb00418.x Seaman, R. and L. 2004. Exocrine pancreatic neoplasia in the cat: a case series. J. Am. Anim. Hosp. Assoc. 40(3), 238–245; doi:10.5326/0400238 Stefano, P., Maria, C., Mauri, M. and Carmignani, L. 2011. A unique cause of hemoperitoneum: spontaneous rupture of a splenic hemangiopericytoma. Int. J. Emerg. Med. 4, 13. Takamatsu, S., Nagano, H., Ohtsukasa, S., Kawachi, Y. and Maruyama, H. 2013. A case of spontaneous ruptured solid pseudopapillary tumor of pancreas resected by laparoscopic surgery. Case Rep. Med. 2013, 953240; doi: 10.1155/2013/953240 Tirumani, S.H., Ojili, V., Gunabushanam, G., Chintapalli, K.N., Ryan, J.G., Reinhold, C., Antonio, S., Haven, N., Hospital, T.O., Campus, C., Hospital, M.G., Centre, H. and Antonio, S. 2013. MDCT of abdominopelvic oncologic emergencies. Int. Cancer Imaging Soc. 13(2), 238–252; doi: 10.1102/1470-7330.2013.0025 Withrow, S.J. 2013. Cancer of the gastrointestinal tract. 5th ed. In Withrow & MacEwen’s small animal clinical oncology. Eds., Withrow, S.J., Vail, D.M. and Page, R.M. St. Louis, MO: Elsevier, pp: 401–402. | ||
| How to Cite this Article |
| Pubmed Style Shin D, Park K. First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management. Open Vet. J.. 2025; 15(8): 3899-3903. doi:10.5455/OVJ.2025.v15.i8.56 Web Style Shin D, Park K. First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management. https://www.openveterinaryjournal.com/?mno=263355 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.56 AMA (American Medical Association) Style Shin D, Park K. First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management. Open Vet. J.. 2025; 15(8): 3899-3903. doi:10.5455/OVJ.2025.v15.i8.56 Vancouver/ICMJE Style Shin D, Park K. First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3899-3903. doi:10.5455/OVJ.2025.v15.i8.56 Harvard Style Shin, D. & Park, . K. (2025) First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management. Open Vet. J., 15 (8), 3899-3903. doi:10.5455/OVJ.2025.v15.i8.56 Turabian Style Shin, Donghwi, and Kang-hyo Park. 2025. First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management. Open Veterinary Journal, 15 (8), 3899-3903. doi:10.5455/OVJ.2025.v15.i8.56 Chicago Style Shin, Donghwi, and Kang-hyo Park. "First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management." Open Veterinary Journal 15 (2025), 3899-3903. doi:10.5455/OVJ.2025.v15.i8.56 MLA (The Modern Language Association) Style Shin, Donghwi, and Kang-hyo Park. "First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management." Open Veterinary Journal 15.8 (2025), 3899-3903. Print. doi:10.5455/OVJ.2025.v15.i8.56 APA (American Psychological Association) Style Shin, D. & Park, . K. (2025) First canine case of nontraumatic hemoperitoneum secondary to pancreatic acinar cell carcinoma: Successful surgical management. Open Veterinary Journal, 15 (8), 3899-3903. doi:10.5455/OVJ.2025.v15.i8.56 |