| Review Article | ||
Open Vet. J.. 2025; 15(7): 2925-2937 Open Veterinary Journal, (2025), Vol. 15(7): 2925-2937 Review Article The biology of the avian influenza virus: A comprehensive review with insights into novel therapeutic strategiesKhaled M. Mekkawy*, Fatma Abdalla and Ahmed A. H. AliDepartment of Virology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Khaled M. Mekkawy. Department of Virology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: khaledatia60 [at] gmail.com Submitted: 02/06/2025 Revised: 12/06/2025 Accepted: 15/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
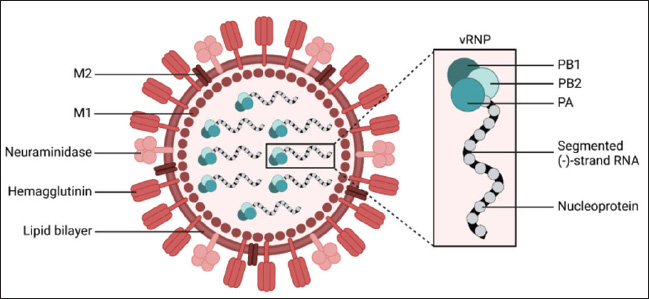
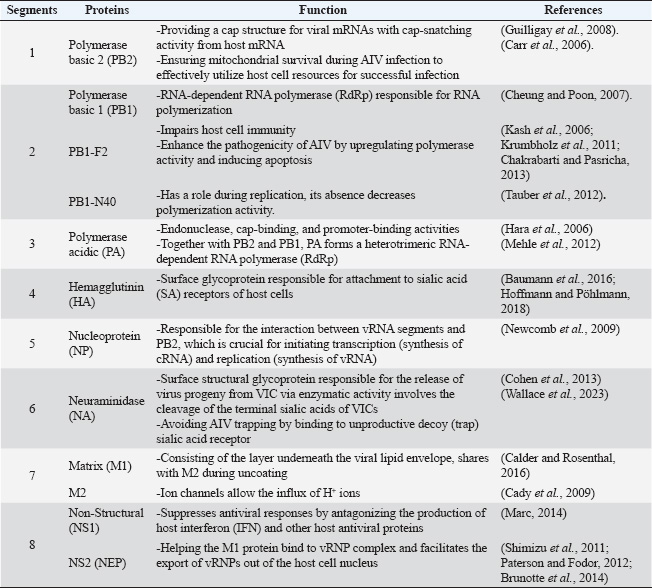
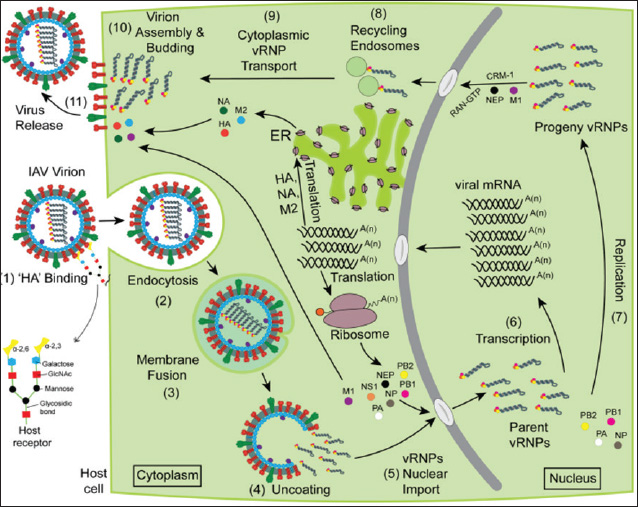
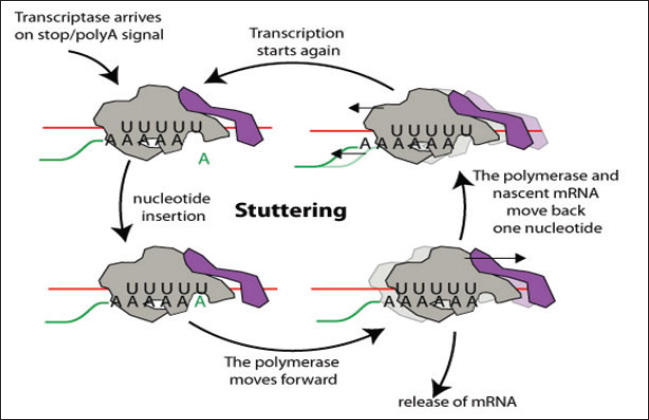
ABSTRACTAvian influenza, commonly referred to as bird flu, is a severe infectious respiratory disease affecting millions of birds each year that causes extensive losses in the poultry industry and poses a risk to human health. Avian influenza causes substantial economic losses to the poultry industry because outbreaks of highly pathogenic avian influenza can severely impact poultry populations, necessitate the culling of infected birds, and impose significant financial burdens. Zoonotic infections can lead to significant morbidity and mortality, highlighting the economic and public health consequences of influenza. The pathogenicity of influenza viruses is a consequence of two factors: the virus’s contagiousness via direct contact and aerosol transmission and its existence as a quasispecies. Annual vaccination is a fundamental preventive strategy against influenza. Due to the rapid evolution of influenza viruses through antigenic drift and antigenic shift, it is necessary to update vaccines annually to correlate with the most prevalent field strains. To overcome the limitations of this influenza vaccine program, there is a critical need for effective anti-influenza therapies. The majority of presently approved drugs exhibit limited efficacy and are liable to rapid development of resistance. Consequently, it is imperative to investigate novel treatment alternatives. As a result, the key characteristics of avian influenza are discussed in this review, along with novel approaches that exhibit promising activities against the virus. Keywords: Avian influenza, Anti-influenza drugs, Influenza therapeutics, Antivirals, Vaccines. IntroductionInfluenza A viruses, belonging to the Orthomyxoviridae family in the viral taxonomy, are characterized by their negative-sense, single-stranded Ribonucleic acid (RNA) genomes (Mohamed et al., 2019) and are the primary causes of avian influenza. Hemagglutinin (HA) subtypes 1–18 and neuraminidase (NA) subtypes 1–11 are used to classify these viruses according to the antigenicity differences in their main surface glycoproteins. Avian influenza comprises two main groups: low-pathogenic avian influenza viruses and highly pathogenic avian influenza viruses. The most dangerous subtypes of birds are H5, H7, and H9. Specifically, some strains of H5 and H7 can result in high incidences and deaths in birds (Tong et al., 2013). Concerns about the potential spread of the virus have been raised by the introduction of novel strains of HPAI viruses, which occurred in Egypt throughout 2021 and 2022. Wild migratory birds are considered key carriers of the transmission of AIV, which can be transferred to domestic birds (Kandeil et al., 2022). In order to reduce the transmission of AIV, it is necessary to assess how well existing vaccinations work and to strengthen biosecurity measures (Salaheldin et al., 2022). Because vaccine production is time-consuming and there is no vaccine readily available at the beginning of a pandemic, the emergence of a novel or unexpected influenza strain raises a significant challenge, as demonstrated during the 2009 influenza (H1N1) pandemic (Girard et al., 2010). The latest developments in antiviral therapy against influenza A have produced various approved drugs with distinctive mechanisms of action. These drugs target the vital phases of the influenza virus life cycle to suppress viral replication and transmission (Dobrovolny and Beauchemin, 2017). Three groups of drugs have been approved by the FDA based on how they work. The M2 inhibitors amantadine and rimantadine are included in one group. Another group includes the RdRp inhibitor baloxavir marboxil (marketed as Xofluza) was also included. Lastly, the NA inhibitors such as zanamivir, oseltamivir, and peramivir are also available (Li et al., 2024). Unfortunately, the majority of the approved treatments are not very effective because of the emergence of resistant strains. Natural products, such as peptides produced by animals and bacteria, and herbal metabolites, are vital potential new treatment possibilities (Shahrajabian et al., 2020; Eichberg et al., 2022). When it comes to creating treatments and ways to prevent influenza, nanotechnology is becoming increasingly crucial. Investigating antivirals based on nanotechnology and studying how they affect the viral life cycle and host cells is helping us understand viruses better and is resulting in new nanomedicines (Wieczorek et al., 2020). Structure and chemical composition of Avian Influenza VirusThe influenza virus (Fig. 1) contains an envelope composed of a lipid membrane obtained from the cell membrane of the virus-infected cell (VIC) during the viral budding process. Three viral proteins are integrated into the lipid membrane: HA, NA, and the M2 proton channel. Several proteins that are responsible for critical functions are located inside the virion (Table 1) (Cheung and Poon, 2007). Replication of AIVNovel and effective targets for antiviral drug development can be identified through an in-depth study of the viral life cycle. The replication cycle, as shown in Figure 2, starts when the HA1 subunit of the HA spike interacts with the α2,3 sialic acid receptor of the target cell, triggering clathrin-mediated endocytosis (Rust et al., 2004). When AIV-containing late endosomes are acidified, the HA2 subunit undergoes a structural change that facilitates virion membrane fusion with the endosomal membrane. The subsequent opening of the viral membrane’s M2 ion channels allows H+ to enter the viral core. Virus ribonucleoproteins (vRNPs) are released when the M1 protein breaks down and enters the cytoplasm of host cells (Cady et al., 2009). After entering the host cell nucleus, the polymerase complex PA/PB1/PB2 starts the transcription process, turning viral vRNA into viral mRNA. In the cytoplasm of host cells, mRNAs are translated into viral proteins. An RdRp complex mediates a cap-snatching mechanism essential for vRNA transcription (Plotch et al., 1981; Rialdi et al., 2017). PB1 uses its polymerase activity and the stolen cap to generate viral mRNAs with a poly A tail at the 3’ end. The tail is formed by a reiterative stuttering action, as shown in Figure 3 (Polymerase stuttering ~ ViralZone). Then, transcription is terminated (Jorba et al., 2009). Ribosomes in the cytoplasm translate viral mRNAs for polymerase subunits (PB2, PB1, and PA), nucleoprotein (NP), NS1, NEP, and M1. Subsequently, all the substances are transported to the nucleus. At the same time, ribosomes attached to the endoplasmic reticulum translate the surface glycoproteins (HA, NA) and ion channels (M2), which then make their way to the plasma membrane (Dou et al., 2018). The replication process, which is cap-independent, results in the generation of cRNA, which is not capped and is not polyadenylate. The newly synthesized cRNAs are used to amplify more copies of vRNA (Vreede et al., 2004). In the cytoplasm, vRNPs are transported to the cytoplasm for assembly with other viral proteins (Neumann et al., 2000). The hydrolysis of glycosidic bonds between carbohydrate molecules and SA residues is facilitated by the catalytic activity of the NA glycoprotein. Consequently, it facilitates the release of newly synthesized virions via budding from the plasma membrane (Palese et al., 1974; McAuley et al., 2019). Pathogenesis and transmission of AIVThe susceptibility of birds to AIV, as well as the severity of the resulting infection, depends on a complex interplay of factors related to both the host and the virus. According to Franɫa and Brown, there are several factors that contribute to the spread of avian influenza, such as the species of bird infected, the bird’s age when infected, the availability of receptors on the target cells, the availability of enzymes in host cells needed for viral entry and its replication, the bird’s immune competency, any other infections the bird may be experiencing at the time of infection, and the strain of AIV (Wright et al., 2013; Franҫa and Brown, 2014). Predominantly, AIV is transmitted from bird to bird by fecal–oral, fecal–fecal, and fecal–respiratory routes of direct contact (Krammer et al., 2018).
Fig. 1. Schematic representation of AIV virion (Eichberg et al., 2022). Table 1. Proteins encoded by the eight segments of the influenza A virus genome with their functions.
Multiple factors contribute to the development of severe influenza illness, including the virus’s cytopathic effects, an increase in the body’s inflammatory response, and ischemia due to thrombosis. As a result, interventions should aim to decrease viral load as well as alleviate the detrimental inflammatory reaction (De Jong et al., 2006). Mechanism of AIV evolutionThere are two main processes that accelerate influenza virus evolution: antigenic drift and antigenic shift. The term “antigenic drift” describes the slow but steady accumulation of point mutations in the virus genome, particularly in the segments encoding HA and NA proteins that enable the virus to attach to host cells. This occurs when mistakes arise during replication because the viral RNA polymerase does not have proofreading activity. As a consequence, the virus is able to escape detection by the immune system because there are changes in the epitopes of HA and NA that decrease the recognition of antibodies produced by earlier infections or vaccines (Carrat and Flahault, 2007; Peter et al., 2013).
Fig. 2. Schematic of the avian influenza life cycle (Chauhan and Gordon, 2022). This explains the prevalence of seasonal influenza, which requires periodic influenza vaccine updates to maintain adequate antigenic closeness between the vaccine and circulating field virus strains (Dey et al., 2023). When multiple influenza viruses infect the same cell, they give a great chance for a mechanism known as an antigenic shift occurring. This factor is an important player in the occurrence of influenza pandemics. In this process, viral RNA segments reassort, giving rise to new strains with significantly different antigenicity. These novel strains pose a serious health risk because they can spread rapidly in people who do not yet have immunity. Because of reassortment, human influenza strains can acquire HA and, in rare cases, NA from other animal influenza viruses. This occurred during the pandemics of 1957, 1968, and 2009 (Lowen, 2018). Control of AIV prophylaxisBiosecurity measures should be prioritized to combat AIV in poultry. These include education, monitoring, quick detection, and depopulation of contaminated flocks to prevent the virus from spreading to healthy flocks. In addition to strong biosecurity, effective vaccination decreases morbidity and mortality in exposed birds while avoiding financial burdens (Swayne et al., 2011; Swayne and Spackman, 2013). AIV vaccinationWhen it comes to pandemics or drifting strains of influenza, seasonal vaccinations are not effective. As a result, improving vaccinations to produce wider immunity is still an important goal for public health. Future vaccine developments will focus on more universal and less mutable viral targets, such as the hemagglutinin stem (Varghese et al., 2022). Live attenuated influenza vaccineLive attenuated influenza vaccines (LAIVs) are live viruses that have been either selected for low virulence in nature or developed by reducing the infectiousness of a wild strain of influenza by passing it through fertile chicken eggs or virus-susceptible cell lines (Blanco-Lobo et al., 2019).
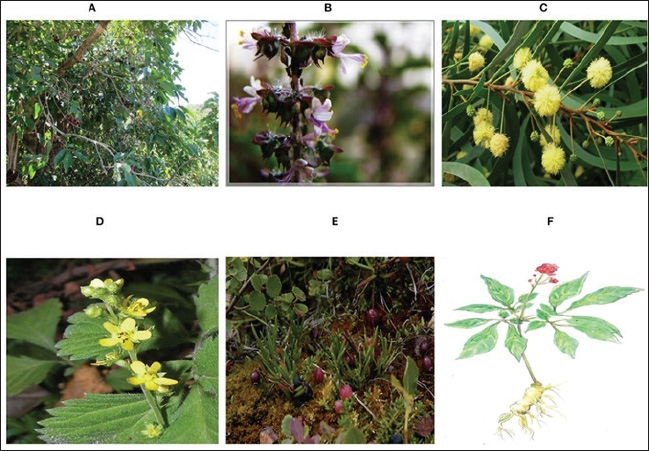
Fig. 3. Negative-stranded RNA virus polymerase stuttering (“Polymerase stuttering ~ ViralZone”). There are security problems related to the use of LAIV because LAIV administration provides a chance for reassortment in cases in which the recipient catches a circulating seasonal field strain of AIV at the same time of vaccination. In addition, certain groups, such as those with immunocompromised status, should not receive LAIV (Sridhar et al., 2015). Inactivated influenza vaccinesThese vaccines have an excellent safety record and can be classified into three types: whole-virion inactivated vaccine, split-virion influenza vaccine, and subunit inactivated vaccine (Wong and Webby, 2013). Whole-virus-inactivated vaccinesTo make these vaccines, the virus is first grown in chicken embryos and then heated or exposed to chemicals such as formalin to make it less infectious and less capable of multiplying. Adjuvants are added to the final vaccine preparation to further enhance its immunogenicity (Sabbaghi et al., 2019). Whole-virus inactivated vaccines can promote humoral immunity and produce significant levels of specific IgG antibodies because they contain a complete attenuated virus in addition to its surface antigens (Ainai et al., 2017). Nevertheless, when tested against influenza variations involving distinct genotypes or subtypes, these vaccines failed to offer any protection. Another disadvantage of this approach is that it only elicits a modest response of cell-mediated immunity (Chen et al., 2001). Split-virion influenza vaccineDeoxycholate (Fluarix®) and Triton X-100 (Fluzone®) are examples of splitting agents that are used to prepare these from entire viruses (Kon et al., 2016). By cleaving the viral envelope, these drugs liberate the nucleic acids inside the cell. Viral proteins with large molecular weights, including HA, NA, and some matrix (M) and NP, are eliminated, but the components that are active and antigenic. This particular vaccine formulation causes a substantial increase in antibody production by efficiently concentrating the antigenic proteins (Hu et al., 2020). Compared with inactivated whole-virus vaccinations, split-virion influenza vaccinations are safer because of the absence of nonantigenic viral components, which helps limit adverse effects (Li et al., 2014). Subunit influenza vaccinesThe initial approach for making these vaccines involved obtaining a pure and concentrated form of the virus’s HA and NA proteins and then adding adjuvants. Currently, the vaccine is made by expressing the recombinant antigenic proteins utilizing plasmids that code for HA and NA. The final subunit vaccines are prepared by combining these recombinant proteins with adjuvants. The safety, immunogenicity, and tolerance of subunit influenza vaccination have all been well-documented. One drawback of this approach is that vaccines are expensive to produce because of the large dosages needed (Chen et al., 2021). Viral-vectored influenza vaccinesThe production of these vaccines involves the use of viral vectors such as adenovirus, baculovirus, and Newcastle disease virus to produce antigenic IAV proteins. Using this approach, vaccinated people can express IAV antigenic proteins at a high level. Viral-vectored influenza vaccines, such as LAIVs, are able to elicit robust cellular and humoral immune responses (Sayedahmed et al., 2020). The continuous proliferation of the vaccine vector also causes non-specific immunological effects in vaccinated hosts against the vector (Suarez and Pantin-Jackwood, 2017; Krammer and Palese, 2019; Wong et al., 2019). The ability to rapidly develop polyvalent antibodies with enhanced cross-protection across various genotypes is another benefit of this type of influenza vaccine (Lingel et al., 2017). mRNA influenza vaccinesmRNA vaccines work by mimicking the behavior of DIPs present. Instead of viral genes that encode structural proteins, cells obtain parts of mRNA encoding virus antigens (Vasou et al., 2017) and take advantage of mRNA replication without production of infectious viral particles (Demento et al., 2009). Several challenges to the efficiency of vaccinationBecause vaccines may not always have all the possible combinations of antigenic properties necessary to match circulating virus strains, the co-circulation of different AIV subtypes such as H5, H7, and H9, makes vaccination attempts more difficult. Consequently, it is essential to vaccinate against several subtypes of HA (Suarez and Schultz-Cherry, 2000). In addition, AIV-vaccinated birds may have a compromised immune response if they were previously infected with mycotoxins or were concurrently infected with an immunosuppressive disease (Hegazy et al., 2011). In vaccinated birds, the risk of infection is still present, even if the vaccine might alleviate the severity of clinical illness. Because of this, the virus can remain in a state of persistent “silent” circulation, increasing the possibility that it will be transferred from chickens to the human population (Gilbert et al., 2017). The frequency of antigenic drift increases when the virus evolves to mitigate the host immune response, which can be accelerated by the immunological pressure from vaccination (Cattoli et al., 2011). The length of protection is uncertain; however, vaccine-induced immunity usually peaks 3-4 weeks after immunization (Swayne and Kapczynski, 2008). It is also possible that immunization of young birds will be hindered by maternally inherited immunity in vaccinated breeding flocks (Abdelwhab et al., 2012). Eventually, vaccine manufacturing problems such as vaccine strain quality and purity, as well as inappropriate handling and delivery, can all have a significant impact on the success rate of AIV vaccination (Flint et al., 2020). Anti-influenza therapiesAntivirals are a class of antimicrobials that can be obtained from a wide variety of sources, including both natural and synthetic ones. Their main function is to inhibit viral multiplication, and they do this in two ways: by engaging the viruses directly or by influencing components within the host cell required for the virus life cycle (Kausar et al., 2021). Antiviral treatments work by hindering the growth of viruses rather than eradicating them, as is the case with most antibiotics (He, 2013). Major challenging limitations of anti-influenza therapiesBecause viral multiplication is extremely dependent on the host cell metabolism machinery, most drugs that target virus replication affect host cells by hampering these metabolic processes. Antiviral development requires a high degree of safety with the selective inhibition of influenza (Flint et al., 2020). Developing new anti-influenza drugs to combat potential future outbreaks is heavily influenced by the rise of influenza virus mutations and the emergence of resistant viral mutants to current antivirals. An essential factor for eradicating resistance is potency, even low replication rates when an inhibitor is present provide resistant mutants a chance for survival (Eshaghi et al., 2014; Dobrovolny and Beauchemin, 2017). Focusing on highly common conserved targets (less mutable) among influenza subtypes potentially remains effective regardless of AIV antigenic drift and shift, delays resistance, and achieves broad-spectrum activity (Holthausen et al., 2017). Promising antivirals and their putative mechanism of actionToxins and defensive peptides from animalsAnimal poisons and venoms often have effects on the circulatory system or cause neurological manifestations, but many of these toxins also have substantial antibacterial and antiviral properties, which might make them useful in therapeutics (Kachel et al., 2018). Two extracts from scorpion venom, micropores and its improved form, mucroporin-M1, have shown antiviral activity against several influenza strains, effectively inhibiting infection at nanomolar and micromolar concentrations (Dai et al., 2008; Li et al., 2011). With an EC50 value of 0.40 μM, the peptide melittin, which is obtained from bee venom, has the ability to lower the infectivity of influenza (Uddin et al., 2016). Another example is urumin, a host defense peptide obtained from the Indian frog Hydrophlax bahuvistara’s skin, which showed micromolar IC50 concentration in MDCK cells against HA1 (Holthausen et al., 2017). Peptide BF-30, which was derived from the venom of the banded krait (Bungarus fasciatus), is another potential candidate that has been studied in vitro and in vivo. This peptide causes the virus to fuse its membranes during the attachment and penetration phases. It has no effect on HA and NA, which are viral glycoproteins that can develop resistance to drugs through adaptive changes. Therefore, BF-30 is a promising candidate that should be investigated further for therapeutic applications (Xu et al., 2019). Fungal extracts with anti-influenza propertiesThe efficacy of diverse fungal extracts in suppressing various strains of influenza in MDCK cells was demonstrated, with IC50 values < 1 µM. Among these, cyclosporin A from Tolypocladium inflatum and a tetrahydrofuran derivative from Chaetomium have been highlighted for their antiviral properties (Sacramento et al., 2015), and L435-3, an ophiobolin derivative from the phytopathogenic fungus Bipolaris oryzae, has also been identified as effective (Wang et al., 2016). Bacterial extracts with anti-influenza propertiesBacteria are an excellent source of new antimicrobial therapeutics. Cyanobacteria and Pseudomonas lectins exhibit antiviral activity against influenza by binding to viral spikes. By blocking viral-host cell attachment hinders viral multiplication with an IC50 of approximately 1 nM (Smee et al., 2008; Morimoto and Sato, 2016). Streptomyces microflavus is the source of two promising substances with anti-influenza activity: fattiviracin A1 and fattiviracin 8. Their antiviral activity was demonstrated against the H1N1 influenza virus was demonstrated, with an EC50 of 1.34 µM (Habib et al., 2001). More naturally produced nucleoside and nucleotide analogs that are effective against influenza include Streptomyces candidus-derived formycin A and B, which have a nanomolar IC50 value against H1N1 (Kunimoto et al., 1968; Buchanan et al., 1980) . One potential option, DAS181 (Fludase®), has demonstrated the ability to break down α-(2,3)- and α-(2,6)-linked sialic acid, which in turn blocks the virus from accessing the host cell. To validate tolerance, tolerance was evaluated in human airway epithelial cultures as well as in many cell lines (MDCK, A549, and Caco2). The EC50 values were reported using nanomolars in viral inhibition experiments (Malakhov et al., 2006). Herbal antivirals against AIVTraditional Chinese Medicine (TCM) is a great source of natural herbal antivirals against AIV. Several plants (Fig. 4) have several ways in which TCM therapies work together to combat viruses and boost the immune system of the host. Antiviral activity against AIV has been discovered in several plant-derived phytochemicals with low molecular weights, such as polyphenols, flavonoids, terpenes, glucosides, and alkaloids (Gangehei et al., 2010; Grienke et al., 2016). Virucidal activity is another potential way in which herbal extracts, such as Ocimum sanctum terpenoids, Acacia arabica crude extract polyphenols, and Agrimonia pilosa extract, work against viruses by affecting the structural characteristics of the virus (Shin et al., 2010; Ghoke et al., 2018). The actions of Ginseng saponins derived from the plant stems and leaves point to a host-acting mechanism that relies on the regulation of the host immune system response, specifically the humoral one that was significantly enhanced after these saponins were added to drinking water (Wang et al., 2018). In addition to Ocimum sanctum, Tinospora cordifolia, Glycyrrhiza glabra, and Allium sativum, other medicinal herbs that have comparable immunomodulatory properties are Zingiber officinale, Cocos nucifera, and Ocimum tenuiflorum (Abbas et al., 2022). Nanotechnology potentials as antiviral therapyWhen it comes to the worldwide poultry sector, nanotechnology is one of the breakthrough technologies with an endless number of potential applications from both economic and social perspectives (Abd El-Ghany et al., 2021). Gene silencing is a nanotechnology strategy. This strategy aims to deactivate gene expression in specific genomic or transcriptome parts using silencers such as antisense oligonucleotides (ASO), DNAzymes (Kesy et al., 2019; Michalak et al., 2019; Piasecka et al., 2020). The ASOs, 12–30 nucleotides of single-stranded Deoxyribonucleic acid (DNA) or modified (RNA) engage with the target RNA inducing several mechanisms, including activation of RNase H cleavage of messenger RNA in DNA/RNA duplexes or attachment to the ribosome, providing a steric barrier at the translational start site causing the nascent transcripts to be degraded or destabilized (Mescalchin and Restle, 2011). Levina et al. (2015, 2016, 2018) showed promising results with ASOs nanocomposites consisting of positive-sense RNA or DNA sequences that target the –RNA-conserved sequence of segment 5 of AIV H5N1 and also designed –RNA to bind with +RNA transcripts of the virus of segment 5. AIV replication in the MDCK cell line was selectively and successfully suppressed by nanocomposites that contained all of the specified sequences coupled to NPs such as TO2 or silicon-organic through a polylysine linker. Simultaneously, these nanocomposites inhibiting H5N1 were evaluated in MDCK and demonstrated strong antiviral efficacy with moderate cytotoxicity (Levina et al., 2015, 2016, 2018). DNAzymes can break complementary RNA because they are pieces of DNA with catalytic properties. There are several synthetic DNAzymes, but no naturally occurring ones have been found. Immobilized DNA enzymes on TiO2 NPs coated with polylysine linker showed anti-influenza properties by targeting influenza virus segment 5’s coding region. The virus replication was significantly reduced by the cleaving action of TiO2 DNAzymes (Repkova et al., 2017).
Fig. 4. Herbal therapeutics controlling avian influenza virus. (A) Eugenia jambolana, (B) Ocimum sanctum, (C) Acacia arabica, (D) Agrimonia pilosa. (E) cranberry and (F) ginseng (Abbas et al., 2022). ConclusionInfluenza continues to threaten world health despite extensive research. Due to its wide animal reservoir and genetic plasticity, influenza poses a pandemic risk in addition to periodic epidemics with fluctuating fatality rates and substantial economic costs. However, therapy choices are limited. In most countries, only M2 channel blockers and neuraminidase inhibitors are authorized. Both kinds of inhibitors are ineffective and prone to resistance. Thus, innovative influenza antiviral therapies are urgently needed. In recent decades, innovative influenza virus treatments have been developed. Natural products are a great starting point for discovering new influenza virus-fighting chemicals due to their variety and structural complexity. In this review, we discuss current research on natural products such as toxins, anti-influenza extracts from fungi and bacteria, and herbal extracts with insights into employing nanotechnology in this field. Most of these substances are in early antiviral development. Comparing compounds’ antiviral medication potential is challenging for many reasons. First, the compound evaluation methods differ significantly. Many research studies use different cell lines. In addition, viral replication inhibition assays vary. Many researchers use plaque assays to measure viral titers, whereas others use hemagglutination or virus-induced cytopathic effect to measure antiviral activity. Therefore, standardizing antiviral effectiveness assessment methodologies would clarify the comparisons. Second, many chemicals have unknown mechanisms of action. However, therapeutic development requires understanding how these compounds work. Third, most compounds lack resistance development data. In addition, few candidates have in vivo data. Consider a combination treatment in addition to monotherapy with one drug. Targeting different viral targets has shown synergistic effects and reduced resistance. AcknowledgmentsThe authors thank the Faculty of Veterinary Medicine, Zagazig University. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Author’s contributionsKMM drafted the manuscript. AAHA and FA revised and edited the manuscript. All authors have read and approved the final version of the manuscript. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAbbas, G., Yu, J. and Li, G. 2022. Novel and alternative therapeutic strategies for controlling avian viral infectious diseases: focus on infectious bronchitis and avian influenza. Front. Vet. Sci. 9, 933274; doi: 10.3389/fvets.2022.933274 Abd El-Ghany, W.A., Shaalan, M. and Salem, H.M. 2021. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 77, 1001–1025; doi: 10.1080/00439339.2021.1960235 Abdelwhab, E.M., Grund, C., Aly, M.M., Beer, M., Harder, T.C. and Hafez, H.M. 2012. Influence of maternal immunity on vaccine efficacy and susceptibility of one day old chicks against Egyptian highly pathogenic avian influenza H5N1. Vet. Microbiol. 155, 13–20; doi: 10.1016/j.vetmic.2011.08.004 Ainai, A., Suzuki, T., Tamura, S. and Hasegawa, H. 2017. Intranasal administration of whole inactivated influenza virus vaccine as a promising influenza vaccine candidate. Viral Immunol. 30, 451–462; doi: 10.1089/vim.2017.0022 Baumann, J., Kouassi, N.M., Foni, E., Klenk, H.D. and Matrosovich, M. 2016. H1N1 Swine influenza viruses differ from avian precursors by a Higher pH Optimum of Membrane Fusion. J. Virol. 90, 1569–1577; doi: 10.1128/JVI.02332-15 Blanco-Lobo, P., Nogales, A., Rodríguez, L. and Martínez-Sobrido, L. 2019. Novel approaches for the development of live attenuated influenza vaccines. Viruses 11, 190; doi: 10.3390/v11020190 Brunotte, L., Flies, J., Bolte, H., Reuther, P., Vreede, F. and Schwemmle, M. 2014. The nuclear export protein of H5N1 influenza A viruses recruits matrix 1 (M1) protein to the viral ribonucleoprotein to mediate nuclear export. J. Biol. Chem. 289, 20067–20077; doi: 10.1074/jbc.M114.569178 Buchanan, J.G., Stobie, A. and Wightman, R.H. 1980. A new synthesis of pyrazofurin. J. Chem. Soc. Chem. Commun. 916–917; doi: 10.1039/C39800000916 Cady, S.D., Luo, W., Hu, F. and Hong, M. 2009. Structure and function of the influenza A M2 proton channel. Biochemistry 48, 7356–7364. Calder, L.J. and Rosenthal, P.B. 2016. Cryomicroscopy provides structural snapshots of influenza virus membrane fusion. Nat. Struct. Mol. Biol. 23, 853–858. Carr, S.M., Carnero, E., García-Sastre, A., Brownlee, G.G. and Fodor, E. 2006. Characterization of a mitochondrial-targeting signal in the PB2 protein of influenza viruses. Virology 344, 492–508; doi: 10.1016/J.VIROL.2005.08.041 Carrat, F. and Flahault, A. 2007. Influenza vaccine: the challenge of antigenic drift. Vaccine 25, 6852–6862; doi: 10.1016/j.vaccine.2007.07.027 Cattoli, G., Fusaro, A., Monne, I., Coven, F., Joannis, T., Abd El-Hamid, H.S., Hussein, A.A., Cornelius, C., Amarin, N.M. and Mancin, M. 2011. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine 29, 9368–9375. Chakrabarti, A.K. and Pasricha, G. 2013. An insight into the PB1F2 protein and its multifunctional role in enhancing the pathogenicity of the influenza A viruses. Virology 440, 97–104. Chauhan, R.P. and Gordon, M.L. 2022. An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. Virus Genes 58, 255–269; doi: 10.1007/s11262-022-01904-w Chen, J., Wang, J., Zhang, J. and Ly, H. 2021. Advances in development and application of influenza vaccines. Front. Immunol. 12, 711997. Chen, W., Calvo, P.A., Malide, D., Gibbs, J., Schubert, U., Bacik, I., Basta, S., O’Neill, R., Schickli, J., Palese, P., Henklein, P., Bennink, Y. and JR, J.W. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7, 1306–1312. Cheung, T.K.W. and Poon, L.L.M. 2007. Biology of influenza a virus. Ann N Y Acad Sci 1102, 1–25; doi: 10.1196/annals.1408.001 Cohen, M., Zhang, X.Q., Senaati, H.P., Chen, H.-W., Varki, N.M., Schooley, R.T. and Gagneux, P. 2013. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol. J. 10, 321; doi: 10.1186/1743-422X-10-321 Dai, C., Ma, Y., Zhao, Z., Zhao, R., Wang, Q., Wu, Y., Cao, Z. and Li, W. 2008. Mucroporin, the first cationic host defense peptide from the venom of Lychas mucronatus. Antimicrob. Agents Chemother. 52, 3967–3972. De Jong, M.D., Simmons, C.P., Thanh, T.T., Hien, V.M., Smith, G.J., Chau, T.N.B., Hoang, D.M., Van Vinh Chau, N., Khanh, T.H. and Dong, V.C. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207. Demento, S.L., Eisenbarth, S.C., Foellmer, H.G., Platt, C., Caplan, M.J., Saltzman, W.M., Mellman, I., Ledizet, M., Fikrig, E. and Flavell, R.A. 2009. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine 27, 3013–3021. Dey, P., Ahuja, A., Panwar, J., Choudhary, P., Rani, S., Kaur, M., Sharma, A., Kaur, J., Yadav, A.K., Sood, V., Suresh Babu, A.R., Bhadada, S.K., Singh, G. and Barnwal, R.P. 2023. Immune control of avian influenza virus infection and its vaccine development. Vaccines 11, 593; doi: 10.3390/vaccines11030593 Dobrovolny, H.M. and Beauchemin, C.A.A. 2017. Modelling the emergence of influenza drug resistance: the roles of surface proteins, the immune response and antiviral mechanisms. PLOS one 12, 1–26; doi: 10.1371/journal.pone.0180582 Dou, D., Revol, R., Östbye, H., Wang, H. and Daniels, R. 2018. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 9, 1581; doi: 10.3389/fimmu.2018.01581 Eichberg, J., Maiworm, E., Oberpaul, M., Czudai-Matwich, V., Lüddecke, T., Vilcinskas, A. and Hardes, K. 2022. Antiviral potential of natural resources against influenza virus infections. Viruses 14, 2452; doi: 10.3390/v14112452 Eshaghi, A., Shalhoub, S., Rosenfeld, P., Li, A., Higgins, R.R., Stogios, P.J., Savchenko, A., Bastien, N., Li, Y., Rotstein, C. and Gubbay, J.B. 2014. Multiple influenza A (H3N2) mutations conferring resistance to neuraminidase inhibitors in a bone marrow transplant recipient. Antimicrob. Agents 58, 7188–7197. Flint, S.J., Racaniello, V.R., Rall, G.F., Hatziioannou, T. and Skalka, A.M. 2020. Vaccines. In Principles of virology, volume 2: pathogenesis and control. John Wiley & Sons, pp: 231–243. Franҫa, M.S. and Brown, J.D. 2014. Influenza pathobiology and pathogenesis in avian species. In Influenza pathogenesis and control, vol. I. Eds., Compans, R.W. and Oldstone, M.B.A. Cham : Springer International Publishing, pp: 221–242; doi: 10.1007/82_2014_385 Gangehei, L., Ali, M., Zhang, W., Chen, Z., Wakame, K. and Haidari, M. 2010. Oligonol a low molecular weight polyphenol of lychee fruit extract inhibits proliferation of influenza virus by blocking reactive oxygen species-dependent ERK phosphorylation. Phytomedicine 17, 1047–1056; doi: 10.1016/j.phymed.2010.03.016 Ghoke, S.S., Sood, R., Kumar, N., Pateriya, A.K., Bhatia, S., Mishra, A., Dixit, R., Singh, V.K., Desai, D.N., Kulkarni, D.D., Dimri, U. and Singh, V.P. 2018. Evaluation of antiviral activity of Ocimum sanctum and Acacia arabica leaves extracts against H9N2 virus using embryonated chicken egg model. BMC Complement. Altern. Med. 18, 174; doi: 10.1186/s12906-018-2238-1. Gilbert, M., Xiao, X. and Robinson, T.P. 2017. Intensifying poultry production systems and the emergence of avian influenza in China: a “One Health/Ecohealth” epitome. Arch. Public Health 75, 48; doi: 10.1186/s13690-017-0218-4 Girard, M.P., Tam, J.S., Assossou, O.M. and Kieny, M.P. 2010. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 28, 4895–4902; doi: 10.1016/j.vaccine.2010.05.031 Grienke, U., Richter, M., Walther, E., Hoffmann, A., Kirchmair, J., Makarov, V., Nietzsche, S., Schmidtke, M. and Rollinger, J.M. 2016. Discovery of prenylated flavonoids with dual activity against influenza virus and Streptococcus pneumoniae. Sci. Rep. 6, 27156; doi: 10.1038/srep27156 Guilligay, D., Tarendeau, F., Resa-Infante, P., Coloma, R., Crepin, T., Sehr, P., Lewis, J., Ruigrok, R.W.H., Ortin, J., Hart, D.J. and Cusack, S. 2008. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat. Struct. Mol. Biol. 155(15), 500–506; doi: 10.1038/nsmb.1421 Habib, E.S.E., Yokomizo, K., Nagao, K., Harada, S. and Uyeda, M. 2001. Antiviral activity of fattiviracin FV-8 against human immunodeficiency virus type 1 (HIV-1). Biosci. Biotechnol. Biochem. 65, 683–685; doi: 10.1271/bbb.65.683 Hara, K., Schmidt, F.I., Crow, M. and Brownlee, G.G. 2006. Amino acid residues in the N-terminal region of the PA subunit of influenza A virus RNA polymerase play a critical role in protein stability, endonuclease activity, cap binding, and virion RNA promoter binding. J Virol. 80, 7789–7798. He, H. 2013. Vaccines and antiviral agents. In Current issues in molecular virology - viral genetics and biotechnological applications. Ed. Romanowski, V. Rijeka, Croatia: IntechOpen, pp: 239–248; doi: 10.5772/56866 Hegazy, A., Abdallah, F., El Abd-Samie, L. and Nazim, A. 2011. The relation between some immunosuppressive agents and widespread nature of highly pathogenic avian influenza (HPAI) post vaccination. J. Am. Sci. 7, 66–72. Hoffmann, M. and Pöhlmann, S. 2018. Cell entry of influenza A viruses: sweet talk between HA and CaV1.2. Cell Host Microbe 23, 697–699; doi: 10.1016/j.chom.2018.05.019 Holthausen, D.J., Lee, S.H., Kumar, V.T., Bouvier, N.M., Krammer, F., Ellebedy, A.H., Wrammert, J., Lowen, A.C., George, S. and Pillai, M.R. 2017. An amphibian host defense peptide is virucidal for human H1 hemagglutinin-bearing influenza viruses. Immunity 46, 587–595. Hu, Y., Shao, M., Hu, Y., Liang, Q., Jia, N., Chu, K., Xu, L., Li, J., Li, C. and Zhu, F. 2020. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine: a randomized, double-blind, controlled phase III clinical trial in children aged 6–35 months in China. Hum. Vaccines Immunother. 16, 1691–1698. Jorba, N., Coloma, R. and Ortín, J. 2009. Genetic trans-complementation establishes a new model for influenza virus RNA transcription and replication. PLOS Pathog. 5, e1000462. Kachel, H.S., Buckingham, S.D. and Sattelle, D.B. 2018. Insect toxins–selective pharmacological tools and drug/chemical leads. Curr. Opin. Insect Sci. 30, 93–98. Kandeil, A., Moatasim, Y., El Taweel, A., El Sayes, M., Rubrum, A., Jeevan, T., McKenzie, P.P., Webby, R.J., Ali, M.A., Kayali, G. and El-shesheny, R. 2022. Genetic and antigenic characteristics of highly pathogenic avian influenza A(H5N8) viruses circulating in domestic poultry in Egypt, 2017–2021. Microorganisms 10, 595; doi: 10.3390/microorganisms10030595 Kash, J.C., Tumpey, T.M., Proll, S.C., Carter, V., Perwitasari, O., Thomas, M.J., Basler, C.F., Palese, P., Taubenberger, J.K., García-Sastre, A., Swayne, D.E. and Katze, M.G. 2006. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443, 578–581. Kausar, S., Said Khan, F., Ishaq Mujeeb Ur Rehman, M., Akram, M., Riaz, M., Rasool, G., Hamid Khan, A., Saleem, I., Shamim, S. and Malik, A. 2021. A review: mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 35, 20587384211002621; doi: 10.1177/20587384211002621 Kesy, J., Patil, K.M., Kumar, S.R., Shu, Z., Yong, H.Y., Zimmermann, L., Ong, A.A.L., Toh, D.F.K., Krishna, M.S., Yang, L., Decout, J.L., Luo, D., Prabakaran, M., Chen, G. and Kierzek, E., 2019. A short chemically modified dsRNA-binding PNA (dbPNA) inhibits influenza viral replication by targeting viral RNA panhandle structure. Bioconjug. Chem. 30, 931–943; doi: 10.1021/acs.bioconjchem.9b00039 Krammer, F. and Palese, P. 2019. Universal influenza virus vaccines that target the conserved hemagglutinin stalk and conserved sites in the head domain. J. Infect. Dis. 219, S62–S67. Krammer, F., Smith, G.J.D., Fouchier, R.A.M., Peiris, M., Kedzierska, K., Doherty, P.C., Palese, P., Shaw, M.L., Treanor, J., Webster, R.G. and García-Sastre, A. 2018. Influenza. Nat. Rev. Dis. Primer. 4, 1–21; doi: 10.1038/s41572-018-0002-y Krumbholz, A., Philipps, A., Oehring, H., Schwarzer, K., Eitner, A., Wutzler, P. and Zell, R. 2011. Current knowledge on PB1-F2 of influenza A viruses. Med. Microbiol. Immunol. 200, 69–75. Kunimoto, T., Wakashiro, T., Okamura, I., Asajima, T. and Hori, M. 1968. Structural requirements for formygin activity. J. Antibiot. (Tokyo) 21, 468–470. Levina, A.S., Repkova, M.N., Bessudnova, E.V., Filippova, E.I., Mazurkova, N.A. and Zarytova, V.F. 2016. High antiviral effect of TiO2· PL–DNA nanocomposites targeted to conservative regions of (−) RNA and (+) RNA of influenza A virus in cell culture. Beilstein J. Nanotechnol. 7, 1166–1173. Levina, A.S., Repkova, M.N., Mazurkova, N.A., Makarevich, E.V., Ismagilov, Z.R. and Zarytova, V.F. 2015. Knockdown of different influenza A virus subtypes in cell culture by a single antisense oligodeoxyribonucleotide. Int. J. Antimicrob. Agents 46, 125–128. Levina, A.S., Repkova, M.N., Shikina, N.V., Ismagilov, Z.R., Yashnik, S.A., Semenov, D.V., Savinovskaya, Y.I., Mazurkova, N.A., Pyshnaya, I.A. and Zarytova, V.F. 2018. Non-agglomerated silicon–organic nanoparticles and their nanocomplexes with oligonucleotides: synthesis and properties. Beilstein J. Nanotechnol. 9, 2516–2525. Li, Q., Zhao, Z., Zhou, D., Chen, Y., Hong, W., Cao, L., Yang, J., Zhang, Y., Shi, W., Cao, Z., Wu, Y., Yan, H. and Li, W. 2011. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides 32, 1518–1525; doi: 10.1016/j.peptides.2011.05.015 Li, S., Li, L., Ai, X., Yang, L., Bai, Y., Wang, Z., Han, H., Lu, Q., Luo, F. and Zhang, Z. 2014. A randomized, controlled, blinded study of the safety, immunogenicity and batch consistency of Aleph inactivated split influenza vaccine made in China in Chinese people. Hum. Vaccines Immunother. 10, 557–565. Li, Y., Huo, S., Yin, Z., Tian, Z., Huang, F., Liu, P., Liu, Y. and Yu, F. 2024. Retracted and republished from: “The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs.” mBio 15, e00175-24; doi: 10.1128/mbio.00175-24 Lingel, A., Bullard, B.L. and Weaver, E.A. 2017. Efficacy of an adenoviral vectored multivalent centralized influenza vaccine. Sci. Rep. 7, 14912; doi: 10.1038/s41598-017-14891-y Lowen, A.C. 2018. It’s in the mix: reassortment of segmented viral genomes. PLOS Pathog. 14, e1007200; doi: 10.1371/journal.ppat.1007200 Malakhov, M.P., Aschenbrenner, L.M., Smee, D.F., Wandersee, M.K., Sidwell, R.W., Gubareva, L.V., Mishin, V.P., Hayden, F.G., Kim, D.H. and Ing, A. 2006. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob. Agents Chemother. 50, 1470–1479. Marc, D. 2014. Influenza virus non-structural protein NS1: interferon antagonism and beyond. J. Gen. Virol. 95, 2594–2611; doi: 10.1099/vir.0.069542-0 McAuley, J.L., Gilbertson, B.P., Trifkovic, S., Brown, L.E. and McKimm-Breschkin, J.L. 2019. Influenza virus neuraminidase structure and functions. Front. Microbiol. 10, 39; doi: 10.3389/fmicb.2019.00039 Mehle, A., Dugan, V.G., Taubenberger, J.K. and Doudna, J.A. 2012. Reassortment and mutation of the avian influenza virus polymerase PA subunit overcome species barriers. J. Virol. 86, 1750–1757; doi: 10.1128/JVI.06203-11 Mescalchin, A. and Restle, T. 2011. Oligomeric nucleic acids as antivirals. Molecules 16, 1271–1296. Michalak, P., Soszynska-Jozwiak, M., Biala, E., Moss, W.N., Kesy, J., Szutkowska, B., Lenartowicz, E., Kierzek, R. and Kierzek, E. 2019. Secondary structure of the segment 5 genomic RNA of influenza A virus and its application for designing antisense oligonucleotides. Sci. Rep. 9, 3801; doi: 10.1038/s41598-019-40443-7 Mohamed, M.E.M., Ahmed, H.A., Erfan, A.M., Abdelkarim, L. and Awadallah, M.A.I. 2019. Endemic status and zoonotic potential of avian influenza viruses in Egypt, 2006–2019. Adv. Anim. Vet. Sci. 7, 154–162; doi: 10.17582/journal.aavs/2019/7.s2.154.162 Morimoto, K. and Sato, Y. 2016. Anti-influenza virus activity of high-mannose binding lectins derived from genus Pseudomonas. Virus Res. 223, 64–72. Neumann, G., Hughes, M.T. and Kawaoka, Y. 2000. Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1. EMBO J. 19, 6751–6758. Newcomb, L.L., Kuo, R.-L., Ye, Q., Jiang, Y., Tao, Y.J. and Krug, R.M. 2009. Interaction of the influenza A virus nucleocapsid protein with the viral RNA polymerase potentiates unprimed viral RNA replication. J. Virol. 83, 29–36; doi: 10.1128/JVI.02293-07 Palese, P., Tobita, K., Ueda, M. and Compans, R.W. 1974. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology 61, 397–410. Paterson, D. and Fodor, E. 2012. Emerging roles for the influenza A virus nuclear export protein (NEP). PLoS Pathog. 8(12), e1003019. Piasecka, J., Lenartowicz, E., Soszynska-Jozwiak, M., Szutkowska, B., Kierzek, R. and Kierzek, E. 2020. RNA secondary structure motifs of the influenza A virus as targets for siRNA-mediated RNA interference. Mol. Ther. Nucleic Acids 19, 627–642; doi: 10.1016/j.omtn.2019.12.018 Plotch, S.J., Bouloy, M., Ulmanen, I. and Krug, R.M. 1981. A unique cap(m7G pppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 23, 847–858. Polymerase stuttering ~ ViralZone [WWW Document]. n.d. Negat.-stranded RNA virus polym. Stuttering. Available via https://viralzone.expasy.org/1916 (Accessed 7 July 2024). Repkova, M., Levina, A., Chelobanov, B., Ismagilov, Z., Shatskaya, N., Baiborodin, S., Filippova, E., Mazurkova, N. and Zarytova, V. 2017. Efficient inhibition of influenza A viral replication in cells by deoxyribozymes delivered by nanocomposites. Int. J. Antimicrob. Agents 49, 703–708; doi: 10.1016/j.ijantimicag.2017.01.026 Rialdi, A., Hultquist, J., Jimenez-Morales, D., Peralta, Z., Campisi, L., Fenouil, R., Moshkina, N., Wang, Z.Z., Laffleur, B., Kaake, R.M., McGregor, M.J., Haas, K., Pefanis, E., Albrecht, R.A., Pache, L., Chanda, S., Jen, J., Ochando, J., Byun, M., Basu, U., García-Sastre, A., Krogan, N., Bakel, H. and Marazzi, I. 2017. The RNA exosome syncs IAVRNAPII transcription to promote viral ribogenesis and infectiv ity. Cell 169, 679–692. Rust, M.J., Lakadamyali, M., Zhang, F. and Zhuang, X. 2004. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 11, 567–573. Sabbaghi, A., Miri, S.M., Keshavarz, M., Zargar, M. and Ghaemi, A. 2019. Inactivation methods for whole influenza vaccine production. Rev. Med. Virol. 29, e2074. Sacramento, C.Q., Marttorelli, A., Fintelman-Rodrigues, N., de Freitas, C.S., de Melo, G.R., Rocha, M.E., Kaiser, C.R., Rodrigues, K.F., da Costa, G.L. and Alves, C.M. 2015. Aureonitol, a fungi-derived tetrahydrofuran, inhibits influenza replication by targeting its surface glycoprotein hemagglutinin. PLoS One 10, e0139236. Salaheldin, A.H., Elbestawy, A.R., Abdelkader, A.M., Sultan, H.A., Ibrahim, A.A., Abd El-Hamid, H.S. and Abdelwhab, E.M. 2022. Isolation of genetically diverse H5N8 avian influenza viruses in Poultry in Egypt, 2019–2021. Viruses 14, 1431; doi: 10.3390/v14071431 Sayedahmed, E.E., Elkashif, A., Alhashimi, M., Sambhara, S. and Mittal, S.K. 2020. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines 8, 574. Shahrajabian, M.H., Sun, W. and Cheng, Q. 2020. Traditional herbal medicine for the prevention and treatment of cold and flu in the autumn of 2020, overlapped with COVID-19. Nat. Prod. Commun. 15, 1934578X2095143; doi: 10.1177/1934578X20951431 Shimizu, T., Takizawa, N., Watanabe, K., Nagata, K. and Kobayashi, N. 2011. 124Crucial role of the influenza virus NS2 (NEP) C-terminal domain in M1 binding and nuclear export of vRNP. FEBS Lett. 585, 41–46. Shin, W.J., Lee, K.H., Park, M.H. and Seong, B.L. 2010. Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol. Immunol. 54, 11–19; doi: 10.1111/j.1348-0421.2009.00173.x Smee, D.F., Bailey, K.W., Wong, M.-H., O’Keefe, B.R., Gustafson, K.R., Mishin, V.P. and Gubareva, L.V. 2008. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antiviral Res. 80, 266–271. Sridhar, S., Brokstad, K.A. and Cox, R.J. 2015. Influenza vaccination strategies: comparing inactivated and live attenuated influenza vaccines. Vaccines 3, 373–389. Suarez, D.L. and Pantin-Jackwood, M.J. 2017. Recombinant viral-vectored vaccines for the control of avian influenza in poultry. Vet. Microbiol. 206, 144–151; doi: 10.1016/j.vetmic.2016.11.025 Suarez, D.L. and Schultz-Cherry, S. 2000. Immunology of avian influenza virus: a review. Dev. Comp. Immunol. 24, 269–283; doi: 10.1016/S0145-305X(99)00078-6 Swayne, D.E. and Kapczynski, D. 2008. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 225, 314–331; doi: 10.1111/j.1600-065x.2008.00668.x Swayne, D.E., Pavade, G., Hamilton, K., Vallat, B. and Miyagishima, K. 2011. Assessment of national strategies for control of high-pathogenicity avian influenza and lowpathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Rev. Sci. Tech. OIE 30, 839–870; doi: 10.20506/rst.30.3.2081 Swayne, D.E., Spackman, E., 2013. Current status and future needs In Diagnostics and vaccines for high pathogenicity avian influenza. In Vaccines and diagnostics for transboundary animal diseases: International Symposium, Ames, Iowa, September 2012: Proceedings. Eds., Roth, J.A., Richt, J.A. and Morozov, I.A. Basel, Switzerland: Karger Medical and Scientific Publishers, pp: 79–94; doi: 10.1159/000325276 Tauber, S., Ligertwood, Y., Quigg-Nicol, M., Dutia, B.M. and Elliott, R.M. 2012. Behaviour of influenza A viruses differentially expressing segment 2 gene products in vitro and in vivo. J. Gen. Virol. 93, 840–849; doi: 10.1099/vir.0.039966-0 Tong, S., Zhu, X., Li, Y., Shi, M., Zhang, J., Bourgeois, M., Yang, H., Chen, X., Recuenco, S. and Gomez, J. 2013. New world bats harbor diverse influenza A viruses. PLoS Pathog. 9, e1003657. Uddin, M.B., Lee, B.H., Nikapitiya, C., Kim, J.H., Kim, T.H., Lee, H.C., Kim, C.G., Lee, J.S., and Kim, C.J. 2016. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 54, 853–866. Varghese, P.M., Kishore, U. and Rajkumari, R. 2022. Innate and adaptive immune responses against influenza A virus: immune evasion and vaccination strategies. Immunobiology 227, 152279; doi: 10.1016/j.imbio.2022.152279 Vasou, A., Sultanoglu, N., Goodbourn, S., Randall, R.E. and Kostrikis, L.G. 2017. Targeting pattern recognition receptors (PRR) for vaccine adjuvantation: from synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses 9, 186. Vreede, F.T., Jung, T.E. and Brownlee, G.G. 2004. Model suggesting that replication of influenza virus is regulated by stabilization of replicative intermediates. J. Virol. 78, 9568–9572; doi: 10.1128/jvi.78.17.9568-9572.2004 Wallace, L.E., de Vries, E., van Kuppeveld, F.J.M. and de Haan, C.A.M. 2023. Neuraminidase-dependent entry of influenza A virus is determined by hemagglutinin receptor-binding specificity. J. Virol. 97, e0060223; doi: 10.1128/jvi.00602-23 Wang, S., Luo, X., Yan, R., Wang, Q., Qi, Q., Chi, X., Zhang, L., Yu, Z., Cai, B. and Chen, J.L. 2016. 3-Anhydro-6-hydroxy-ophiobolin A displays high in vitro and in vivo efficacy against influenza A virus infection. Protein Cell 7, 839–843. Wang, Y., Jung, Y.J., Kim, K.H., Kwon, Y., Kim, Y.J., Zhang, Z., Kang, H.S., Wang, B.Z., Quan, F.S. and Kang, S.M. 2018. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses 10, 471; doi: 10.3390/v10090471 Wieczorek, K., Szutkowska, B. and Kierzek, E. 2020. Anti-influenza strategies based on nanoparticle applications. Pathogens 9, 1020. https://doi.org/10.3390/pathogens9121020 Wong, S.S. and Webby, R.J. 2013. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 26, 476–492. Wong, Y.C., Croft, S., Smith, S.A., Lin, L.C.W., Cukalac, T., La Gruta, N.L., Drexler, I. and Tscharke, D.C. 2019. Modified vaccinia virus ankara can induce optimal CD8 + T cell responses to directly primed antigens depending on vaccine design. J. Virol. 93, e01154-19; doi: 10.1128/JVI.01154-19 Wright, F.P., Neumann, G. and Kawaoka, Y. 2013. Orthomyxoviruses. In Fields virology. Eds., David, M.K. and Peter, M.H. Philadelphia, PA: Lippincott Williams and Wilkins, pp: 1186–1243. Xu, J., Chen, S., Jin, J., Ma, L., Guo, M., Zhou, C. and Dou, J. 2019. Inhibition of peptide BF-30 on influenza A virus infection in vitro/vivo by causing virion membrane fusion. Peptides 112, 14–22. | ||
| How to Cite this Article |
| Pubmed Style Mekkawy KM, Abdalla F, Ali AAH. The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies. Open Vet. J.. 2025; 15(7): 2925-2937. doi:10.5455/OVJ.2025.v15.i7.4 Web Style Mekkawy KM, Abdalla F, Ali AAH. The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies. https://www.openveterinaryjournal.com/?mno=262422 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.4 AMA (American Medical Association) Style Mekkawy KM, Abdalla F, Ali AAH. The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies. Open Vet. J.. 2025; 15(7): 2925-2937. doi:10.5455/OVJ.2025.v15.i7.4 Vancouver/ICMJE Style Mekkawy KM, Abdalla F, Ali AAH. The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 2925-2937. doi:10.5455/OVJ.2025.v15.i7.4 Harvard Style Mekkawy, K. M., Abdalla, . F. & Ali, . A. A. H. (2025) The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies. Open Vet. J., 15 (7), 2925-2937. doi:10.5455/OVJ.2025.v15.i7.4 Turabian Style Mekkawy, Khaled M., Fatma Abdalla, and Ahmed A. H. Ali. 2025. The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies. Open Veterinary Journal, 15 (7), 2925-2937. doi:10.5455/OVJ.2025.v15.i7.4 Chicago Style Mekkawy, Khaled M., Fatma Abdalla, and Ahmed A. H. Ali. "The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies." Open Veterinary Journal 15 (2025), 2925-2937. doi:10.5455/OVJ.2025.v15.i7.4 MLA (The Modern Language Association) Style Mekkawy, Khaled M., Fatma Abdalla, and Ahmed A. H. Ali. "The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies." Open Veterinary Journal 15.7 (2025), 2925-2937. Print. doi:10.5455/OVJ.2025.v15.i7.4 APA (American Psychological Association) Style Mekkawy, K. M., Abdalla, . F. & Ali, . A. A. H. (2025) The biology of avian influenza virus: a comprehensive review with insights into novel therapeutic strategies. Open Veterinary Journal, 15 (7), 2925-2937. doi:10.5455/OVJ.2025.v15.i7.4 |