| Case Report | ||
Open Vet. J.. 2025; 15(8): 3904-3911 Open Veterinary Journal, (2025), Vol. 15(8): 3904-3911 Case Report Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insightsKosuke Kobayashi1,*, Yuga Kawabata2, Sho Kadekaru2, Osamu Sakai1, Ryohei Yoshitake1, Yohei Mochizuki1, Shin-ichi Nakamura2 and Kenji Kutara11Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan 2The Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan *Corresponding Author: Kosuke Kobayashi. Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan. Email: k-kobayashi [at] ous.ac.jp Submitted: 16/05/2025 Revised: 02/07/2025 Accepted: 15/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Encapsulating peritoneal sclerosis (EPS) is a rare clinical syndrome, either idiopathic or secondary to abdominal inflammation, characterized by fibrotic thickening of the peritoneum that encases the abdominal organs. Herein, we describe a case involving a relatively young cat initially suspected of having EPS based on clinical imaging findings, but ultimately diagnosed postmortem with peritoneal dissemination of exocrine pancreatic adenocarcinoma. Case Description: A 2-year-old castrated male domestic shorthair cat presented with abdominal distension and anorexia. The blood test results revealed no significant abnormalities. Imaging revealed severe ascites, digestive tract consolidation, pancreatic enlargement, and irregular peritoneal thickening with nodules. The ascitic fluid was negative for the feline coronavirus gene, and no increase in anti-feline coronavirus antibody titer was found in the blood. Given the patient’s age, neoplastic disease was considered unlikely, and EPS was suspected based on the imaging findings. Despite receiving medical treatment with prednisolone, the cat died 19 days later. Postmortem examination revealed an exocrine pancreatic adenocarcinoma with abdominal and lung metastases. The peritoneal tissue formed a mass within the tumor, encapsulated the upper small intestine, caused ileus, and exhibited severe fibrosis. The cat was diagnosed with cancerous peritonitis, mimicking EPS based on histopathological findings. Conclusion: In the present case, peritoneal dissemination of exocrine pancreatic adenocarcinoma resulted in encapsulation of the gastrointestinal tract by thickened peritoneal tissue, producing clinical features that closely resembled EPS. Although antemortem diagnosis was challenging in this case, malignancy should be considered in the differential diagnosis when EPS is suspected. In addition, the possibility of neoplasia should be carefully considered, even in relatively young cats. Keywords: Autopsy, Cat, Encapsulating peritoneal sclerosis, Pancreatic adenocarcinoma. IntroductionEncapsulating peritoneal sclerosis (EPS) is a rare condition characterized by fibrotic thickening with or without inflammation of the peritoneal tissue that encases the abdominal organs, such as the gastrointestinal tract. In humans, EPS is diagnosed as idiopathic or secondary to peritoneal dialysis, abdominal surgery, and foreign body (Danford et al., 2018). In addition to treating the underlying condition, medical therapies, such as corticosteroids and tamoxifen, and surgical interventions are usually employed to control disease progression and relieve symptoms. However, the response to treatment is typically poor, and the prognosis remains unfavorable. In veterinary medicine, five cases of EPS in cats have previously been reported: three classified as idiopathic (Hardie et al., 1994; Kiniger et al., 2023) and two as secondary to bacterial peritonitis (Gremillet et al., 2022). Among the idiopathic cases, two showed long-term survival with early surgical intervention, while the remaining case resulted in death or euthanasia shortly after diagnosis. There is no established treatment yet for this condition due to the limited information available, especially for the advanced stages. Hence, feline EPS is considered to have a poor prognosis. Additionally, peritoneal carcinomatosis mimicking EPS has been previously reported in cats with carcinomatosis of unknown origin (Nam et al., 2023) and with pancreatic adenocarcinoma (Kim et al., 2024). EPS was initially suspected in both cases, suggesting that differentiating EPS from malignant peritoneal dissemination based solely on imaging can be challenging. In this report, we describe the clinical course, diagnostic imaging findings, and postmortem histopathological results of a cat initially suspected of having EPS based on imaging, but ultimately diagnosed postmortem with peritoneal dissemination of exocrine pancreatic adenocarcinoma. Case DetailsA 2-year-old castrated male domestic shorthair cat, weighing 5.3 kg, presented with a 2-month history of abdominal distension and a 4-day history of anorexia. There was no history of illness or abdominal surgery. Physical examination revealed severe abdominal distension with a fluid wave and mild hypothermia (37.1°C). Blood tests revealed mild elevations in blood glucose (178 mg/dl; reference interval 71–148 mg/dl) and serum amyloid A (5.27 µg/ml; reference interval <3.75 µg/ml) levels, while lipase activity was within normal limits (23.9 U/l; reference interval <30 U/l). Abdominal radiography revealed increased radiopacity and digestive tract consolidation (Fig. 1). Abdominal ultrasonography revealed severe ascites, small intestine hypoperistalsis, pancreatic enlargement, and irregular thickening of the peritoneum with several nodules (Fig. 2). Echocardiography revealed no abnormality. In the ascitic fluid analysis, the total nucleated cell count was 1,000 cells/μl with no evidence of neoplastic cells or bacterial infection, and the specific gravity of the supernatant was 1.024, confirming the ascites as a modified transudate (Fig. 3). Feline infectious peritonitis was considered unlikely due to the absence of feline coronavirus genes in the ascitic fluid and no elevation in blood anti-coronavirus antibody titers. Based on the imaging findings, such as peritoneal thickening, gastrointestinal tract consolidation, and severe ascites, EPS was initially suspected. These findings are consistent with the typical presentation of EPS, characterized by fibrotic thickening of the peritoneum and encasement of abdominal organs (Gremillet et al., 2022). However, the possibility of malignancy was deemed unlikely due to the cat’s relatively young age. A trial treatment with prednisolone (10 mg/head, q24 h) and enrofloxacin (25 mg/head, q24 h) was initiated to manage the suspected inflammatory process and potential bacterial infection, respectively. However, no clinical improvement was observed, suggesting that the underlying condition might be more complex than initially suspected. A non-sedated computed tomography (CT) scan was performed to obtain more detailed diagnostic information on day 12 after presentation. A Siwtenn-slice multi-slice CT scanner (Aquilion™ Lightning, Canon Medical Systems, Japan) was used without anesthesia, with the patient restrained in an acrylic cage (CT capsule, Terucom, Japan). The following technical parameters were used: rotation time=0.75 s; slice thickness=1 mm; reconstruction interval=0.5 mm; table speed=16 mm/rotation; helical pitch=16.0; X-ray tube voltage=120 kV; and X-ray tube current=100 mA. The CT scan revealed a tumor-like enlargement of the pancreas, irregular scattered nodular formations aggregating dorsally in the peritoneum, and ground-glass opacity in the right lower lobe of the lung (Fig. 4). Because angiography was not performed, the vascular characteristics and more detailed differentiation of the soft tissue masses could not be assessed. A pancreatic tumor was suspected; however, diagnostic and therapeutic surgical interventions were deemed difficult due to the animal’s condition. In addition, the owner declined further invasive procedures, such as fine-needle aspiration or laparoscopy. Despite prednisolone treatment (5 mg/head, 24 h), ascitic fluid drainage, and fluid therapy, the cat died 19 days after the first presentation.
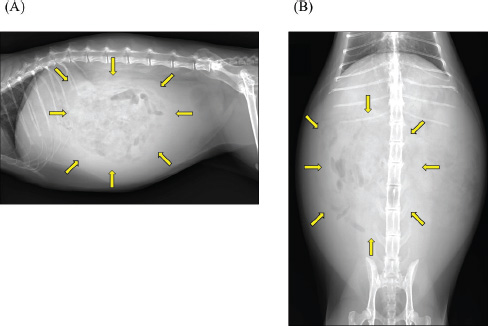
Fig. 1. Abdominal radiographs. (A) Right lateral and (B) ventrodorsal radiographs are shown. The images show severe abdominal distension and a generalized decrease in contrast, suggesting ascites. Mild gas retention and clustering of the intestines were observed (arrow).
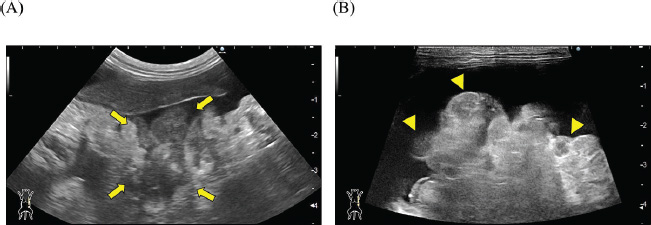
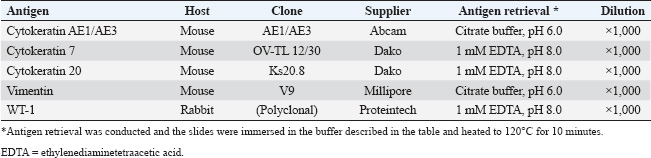
Fig. 2. Abdominal ultrasonography. Severe ascites were observed throughout the abdomen. (A) A hypoechoic irregular area (arrow) was observed in the left upper abdomen, which was considered to be an enlarged pancreas. (B) The peritoneum exhibited irregular thickening, with scattered hypoechoic nodular formations internally (arrowhead). Postmortem ExaminationsAfter obtaining the owner’s consent, a postmortem examination was performed on the day of death. The cat was submitted for autopsy, modified with cosmetic techniques. Sampling for histopathological examination was limited to the thoracic and peritoneal organs, including the thoracic trachea, lung, heart, spleen, esophagus, stomach, intestine, liver, pancreas, kidney, bladder, adrenal gland, coelomic lymph nodes, and coelomic serosa. The samples were fixed in 10% neutral-buffered formalin, routinely processed with paraffin, and finally embedded, sectioned, and stained with hematoxylin and eosin (HE) stain. Grimelius, Azan, periodic acid–Schiff, and immunohistochemical staining were performed to confirm tumor differentiation and fibrosis. The primary antibodies and antigen retrieval method are listed in Table 1. To prevent endogenous reactions, the deparaffinized sections were incubated with 0.5% hydrogen peroxide in methanol at 24°C for 20 minutes. After heat-induced antigen retrieval (citrate buffer, pH 6.0 or 1 mM EDTA, pH 8.0) by heating to 120℃ for 10 minutes, sections were incubated in Tris-buffered saline containing 5% skim milk at room temperature for 20 minutes to prevent non-specific reactions. Sections were incubated overnight at 4°C with primary antibodies, reacted with the secondary antibody Histofine MAX-PO MULTI (Nichirei, Tokyo, Japan) for 60 minutes at room temperature, and visualized using ImmPACT DAB (Vector Laboratories, Burlingame, CA, USA). The accuracy of each primary antibody reaction and Grimelius stain was evaluated by the positive reaction of the internal positive controls in the case.
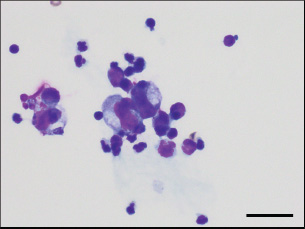
Fig. 3. Cytology of ascitic fluid sediment. The smear revealed low cellularity, with a few mildly degenerated neutrophils and macrophages. No evidence of neoplastic cells or bacterial infection was observed. Diff-Quik® stain, ×1,000. Scale bar=50 μm. As gross findings of the autopsy, severe ascites were seen, and a small amount of fibrin adhered to the serosal surface of the abdominal organs (Fig. 5A). The omentum, lesser omentum, and mesentery were thick and hard, with multiple nodule formations (Fig. 5B). The pancreas and upper small intestine were covered by these peritoneum tissues and formed a mass in the upper abdomen, resulting in loss of mobility. Numerous irregular nodules were observed on the abdominal wall and the ventral side of the diaphragm (Fig. 5C and D). Abdominal lymph nodes were not visible upon overall examination. Histopathologically, the pancreas exhibited infiltrative proliferation of tumor cells, forming ductal structures and sheets a proliferative pattern with necrosis in the center of the neoplastic tissue and partial mucus production, indicating a primary pancreatic tumor (Fig. 6A and B). In the omentum, lesser omentum, mesentery, and peritoneal wall, infiltrative proliferation of tumor cells with similar characteristics, mild presence of inflammatory cells, and remarkable fibrosis were observed (Fig. 6C and D). Metastatic lesions were observed in the abdominal wall, diaphragm, bladder serosa, and lungs. The neoplastic cells were positive for anti-cytokeratin AE1/AE3, anti-cytokeratin 7, and anti-cytokeratin 20, and negative for vimentin, suggesting that the neoplastic tissue differentiated into pancreatic exocrine cells, especially pancreatic ductal epithelial cells (Fig. 6E–G). Although these findings are consistent with the diagnostic criteria for exocrine pancreatic adenocarcinoma, the immunopositivity for cytokeratins 7 and 20 was approximately 20% or less (Cony et al., 2023). Neoplastic cells were negative for anti-WT1 antibody and Grimelius staining, ruling out mesothelioma and neuroendocrine tumors, respectively (Fig. 6H and I). The cat was ultimately diagnosed with cancerous peritonitis due to exocrine pancreatic adenocarcinoma, with suspected pancreatic ductal adenocarcinoma (T1N0M1). The pancreatic mass, along with the peritoneum exhibiting sclerosis and thickening resembling EPS, caused gastrointestinal ileus and severe ascites due to compression of the vascular system. Table 1. Details of primary antibodies and protocols for immunohistochemistry.
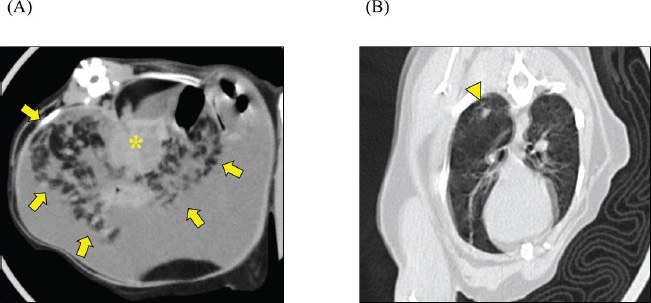
Fig. 4. CT scan. Transverse CT images of (A) upper abdomen and (B) chest are shown. The mesenteric fat exhibited scattered nodules with irregular contours (arrow) centered around the enlarged and tumorous pancreas (*). Ground-glass opacity was observed in the right lower lobe of the lung (arrowhead).
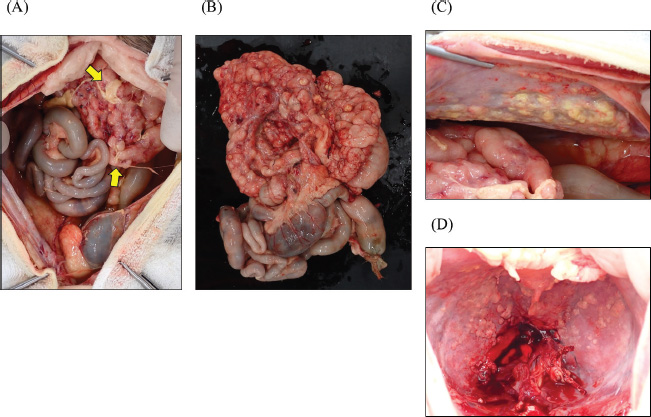
Fig. 5. Gross findings at autopsy. (A) The irregularly thickened peritoneum (omentum, lesser omentum, and mesentery) formed a mass in the upper abdomen. Small amounts of fibrin deposition were observed on the serosal surfaces of the abdominal organs (arrow). (B) The upper small intestine was covered by an irregularly thickened peritoneum, resulting in a loss of mobility. Multiple white to yellowish-white nodules, measuring 3−5 mm, were observed on the (C) left abdominal wall and (D) the ventral surface of the diaphragm. DiscussionExocrine pancreatic adenocarcinoma of ductular or acinar origin is very rare and more commonly reported in older cats aged ≥10 years (Linderman et al., 2013; Cony et al., 2023). The patient in the current case, at only 2 years and 7 months of age, was relatively young. Reports on undifferentiated carcinoma of the pancreas in cats younger than 1 year (Cony et al., 2023) and neuroendocrine carcinoma in cats aged 2 years and 11 months (Michishita et al., 2017) had been documented previously. Therefore, significant pancreatic enlargement warrants the consideration of malignant tumors as a differential diagnosis, even in younger cats. Feline pancreatic cancer typically demonstrates high metastatic potential, with a median survival time of approximately 3 months (Linderman et al., 2013; Rosario et al., 2023). The presence of ascites has been reported as a poor prognostic factor. In the present case, severe ascites were observed, and the survival time after presentation was short (19 days). While showing histological features of pancreatic ductal adenocarcinoma, the relatively low expression of cytokeratins 7 and 20, which are immunohistochemical markers of this type of cancer (Cony et al., 2023), suggested a lower degree of differentiation; the latter could contribute to its strong invasive and metastatic potential. Some cases of feline pancreatic cancer have been reported to show prolonged survival after surgical intervention, chemotherapy, and targeted molecular therapy (Linderman et al., 2013; Rosario et al., 2023). This case was advanced, making antemortem diagnosis and therapeutic intervention difficult. Further research is warranted to establish non-invasive diagnostic techniques for the early detection of pancreatic cancer in cats. In previous reports, cancerous peritonitis mimicking EPS was documented in a feline case of carcinoma of unknown origin and in both a cat and a dog with pancreatic adenocarcinoma (Tsukada et al., 2022; Nam et al., 2023; Kim et al., 2024). The clinical and histopathological features in the previously reported cases closely resembled those of the present case; however, as the cats were 9 and 13 years old, the current case represents the youngest reported case to date with similar characteristics. In human medicine, the most common etiology of EPS is long-term, repetitive peritoneal dialysis, with chronic inflammation recognized as the primary underlying mechanism (Danford et al., 2018). In contrast, rapidly progressive malignant neoplasms are not typically regarded as a causative factor for EPS. Therefore, while such cases may fall within the broader spectrum of EPS, it remains controversial whether peritoneal encapsulation secondary to peritoneal carcinomatosis should be classified as true EPS. This uncertainty persists even when imaging demonstrates encapsulation of abdominal organs by markedly thickened peritoneum, and pronounced peritoneal fibrosis is revealed by histopathology. The molecular mechanisms underlying fibrosis in these pathological conditions remain unclear. In human EPS, fibrotic progression is suggested to involve pathways such as transforming growth factor signaling and α-smooth muscle actin expression (Danford et al., 2018); however, these factors are not specific to EPS and are also implicated in desmoplasia associated with human pancreatic cancer (Merika et al., 2012). Therefore, it is challenging to determine whether the fibrosis observed arises from tumor-associated desmoplasia or from EPS-like mechanisms. A prior pathological review of feline pancreatic tumors noted frequent peritoneal dissemination (Cony et al., 2023), although EPS was not explicitly addressed, raising the possibility that EPS-like conditions may have gone unrecognized. These findings suggest that feline pancreatic adenocarcinoma may exhibit clinical features that closely mimic EPS. Conversely, a canine case of EPS associated with intraperitoneal bleeding caused by hepatocellular carcinoma, without evidence of peritoneal metastasis, has also been reported (Barnes, 2015). Collectively, these observations underscore the necessity of including malignant neoplasia in the differential diagnosis when EPS is suspected in companion animals.
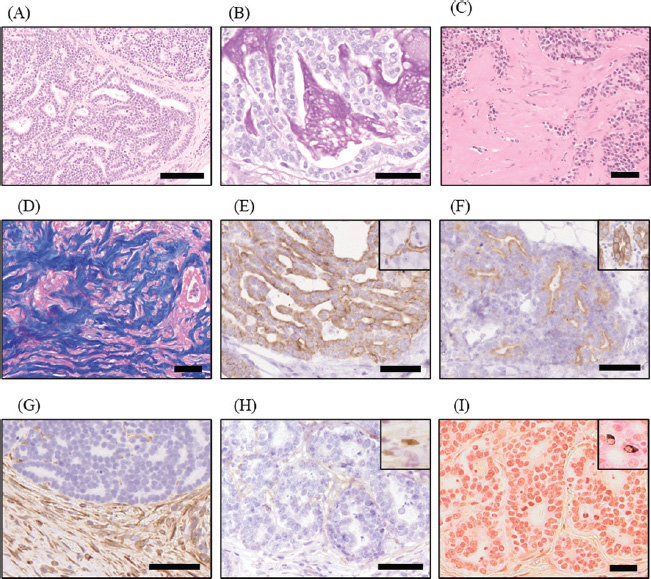
Fig. 6. Histopathological findings. (A) Neoplastic tissue proliferated, forming a ductal structure and a sheet pattern. HE stain; scale bar=100 µm. (B) Mucus in the inner cavity of the neoplastic ductal structure is positive for Periodic acid–Schiff (PAS) reaction. PAS stain; scale bar=50 µm. (C) Severe fibrosis was observed in the stroma of neoplastic tissue in the peritoneum. HE stain; scale bar=50 µm. (D) The fibrosis area in the stroma of neoplastic tissue was stained deep blue by Azan stain; scale bar=50 μm. (E) Neoplastic cells were positive for anti-cytokeratin 7. Ductal cells in pancreas (inset) in the case were positive for the same antibody; scale bar=50 µm. (F) Neoplastic cells were positive for anti-cytokeratin 20. Intestinal epithelial cell (inset) in the case was positive for the same antibody. Scale bar=50 µm. (G) The neoplastic cells were negative for anti-vimentin antibody, while the surrounding stromal cells, including fibroblast, were positive. Scale bar=50 μm. (H) The neoplastic cells were negative for anti-WT1 antibody. Mesothelial cells (inset) in the case were positive for the same antibody. Scale bar=50 μm. (I) The neoplastic cells were negative for Grimelius stain. Neuroendocrine cells (inset) distributed in intestinal epithelial cells are positive for Grimelius stain. Scale bar=25 μm. In humans, EPS is clinically diagnosed through a combination of clinical symptoms and course, imaging (including ultrasonography and CT scans), and histopathological examination. The imaging results typically show a thickened peritoneum, peritoneal calcification, dilated bowel loops, small bowel tethering, and loculated ascites. The histopathological features of peritoneal tissue in EPS include loss of the mesothelial cell layer, proliferation of fibroblasts, and deposition of fibrous collagen. A classification of the four different stages has been proposed (Nakayama et al., 2014; Danford et al., 2018). In the inflammatory stage (stage 2) of EPS, infiltration of inflammatory cells is observed, whereas in the pre-EPS (stage 1), encapsulation (stage 3), and chronic (stage 4) stages, inflammation is either absent or mild. In humans, EPS is often observed as a complication of peritoneal dialysis; the pathogenesis may differ in dogs and cats. Therefore, whether these findings and the clinical staging can be applied directly to the animals remains unclear. EPS is rarely diagnosed in veterinary medicine. A previous study summarizing the imaging findings in seven dogs and two cats with a presumptive diagnosis of EPS identified some common features, such as ascites, dilation, and stasis of the gastrointestinal tract, indicative of ileus. In addition, some cases showed peritoneal thickening in CT scans, raising the suspicion of calcification (Gremillet et al., 2022). The findings in this case were consistent with those previously reported in humans, dogs, and cats, confirming a clinical and pathological condition that mimicked EPS. The lack of significant inflammation in the peritoneum might be attributable to the use of prednisolone. Moreover, the absence of peritoneal calcification, which is otherwise present in advanced stages of EPS (Danford et al., 2018), could be attributed to the underlying aggressive pancreatic cancer, which was presumed to have progressed rapidly. In humans, treatment for EPS includes management of the underlying condition, along with treatment options tailored for the clinical stage (Danford et al., 2018). Corticosteroids are often used in the early stages (stages 1 and 2), characterized by inflammatory signs but minimal fibrosis. In the later stages (stages 2 and 3), when fibrosis becomes severe, tamoxifen may be used to inhibit fibrosis. In the chronic stage (stage 4), in which there is no response to medical treatment, surgical intervention is appropriate. Although treatment responsiveness varies depending on the stage, the mortality rate is approximately 30%, and the median survival time is <2 years, indicating a poor prognosis. The treatment of small animals with EPS is challenging due to the limited availability of information. A case of improvement using tamoxifen combined with methylprednisolone was reported in a dog that developed EPS after surgical treatment for a gastrointestinal foreign body (Etchepareborde et al., 2010). Tamoxifen was also administered in a feline case of pancreatic carcinoma-associated carcinomatosis mimicking EPS; however, due to the impact of the underlying malignancy, it showed no apparent effect in suppressing the progression of the EPS-like pathology (Kim et al., 2024). Therefore, the efficacy of tamoxifen in the treatment of EPS and EPS-like conditions in veterinary medicine remains unclear. Published cases of feline EPS induced by septic peritonitis had to be euthanized soon after diagnosis due to deterioration of the condition (Gremillet et al., 2022; Kiniger et al., 2023). In contrast, a cat with idiopathic EPS survived for over 11 months after surgical intervention and corticosteroid administration (Hardie et al., 1994). Additionally, another cat with EPS suspected to be caused by prior abdominal surgery showed over 30 months of survival following surgical treatment (Kiniger et al., 2023). Therefore, long-term prognosis might be possible in cases of feline EPS without underlying diseases that are detected early and are amenable to surgical intervention. These findings suggest that although the prognosis of EPS and EPS-like conditions associated with malignancy remains poor, early identification of underlying disease and aggressive management, including surgical intervention, may improve outcomes in idiopathic or non-neoplastic cases. Further accumulation of case reports and clinical experience is essential to establish effective management strategies and improve prognostic accuracy in veterinary patients with EPS and conditions that mimic it. ConclusionThis case report describes the clinical and histopathological findings of a relatively young cat initially suspected of having EPS, but ultimately diagnosed postmortem with peritoneal dissemination of exocrine pancreatic adenocarcinoma. This case highlights the importance of including malignant neoplasia in the differential diagnosis when EPS is suspected. While similar cases have been previously reported (Nam et al., 2023; Kim et al., 2024), it remains a subject of ongoing debate whether EPS-like conditions secondary to malignancy should be classified as true EPS. Both exocrine pancreatic adenocarcinoma and EPS are rare in cats and are associated with a poor prognosis. Further accumulation of case reports and further research are essential for improving diagnostic accuracy and developing effective treatment strategies for these challenging conditions. AcknowledgmentsThe authors thank the owners of the cat for their cooperation and are grateful to the members of the Veterinary Medical Teaching Hospital of Okayama University of Science for their contributions. Conflict of interestThe authors declare no potential conflicts of interest with respect to this article’s research, authorship, and/or publication. FundingThis research received no specific grant. Authors’ contributionsK.Kobayashi contributed to the writing of the manuscript, literature review, and clinical evaluation of the case. O.S. and Y.M. also performed the clinical evaluation of the patient. R.Y. and K. Kutara contributed to the interpretation and description of the imaging findings. Y.K., S.K., and S.N. examined the histopathology. All authors have read and approved the final version of the manuscript. Ethical approvalEthical approval was not required for this case report, as it involved a single clinical case with no experimental intervention. All procedures were conducted in accordance with the institutional ethical guidelines of Okayama University of Science. Written informed consent for the diagnostic procedures and publication of this case report was obtained from the owner. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesBarnes, K. 2015. What is your diagnosis? Sclerosing encapsulating peritonitis. J. Am. Vet. Med. Assoc. 247, 43–45. Cony, F.G., Slaviero, M., Santos, I.R., Cecco, B.S., Bandinelli, M.B., Panziera, W., Sonne, L., Pavarini, S.P. and Driemeier, D. 2023. Pathological and immunohistochemical characterization of pancreatic carcinoma in cats. J. Comp. Pathol. 201, 123–129. Danford, C.J., Lin, S.C., Smith, M.P. and Wolf, J.L. 2018. Encapsulating peritoneal sclerosis. World J. Gastroenterol. 24, 3101–3111. Etchepareborde, S., Heimann, M., Cohen-Solal, A. and Hamaide, A. 2010. Use of tamoxifen in a German shepherd dog with sclerosing encapsulating peritonitis. J. Small Anim. Pract. 51, 649–653. Gremillet, B.C.H., Porsmoguer, C., Bolen, G., Billen, F., Noël, S., Brutinel, F. and Busoni, V. 2022. Imaging findings in dogs and cats with presumptive sclerosing encapsulating peritonitis. Front. Vet. Sci. 9, 891492. Hardie, E.M., Rottman, J.B. and Levy, J.K. 1994. Sclerosing encapsulating peritonitis in four dogs and a cat. Vet. Surg. 23, 107–114. Kim, C., Sanggu, K., Park, J., Lee, D., Chae, Y., Yun, T., Chang, D., Kang, B.-T., Lee, S., Kim, S. and Kim, H. 2024. Case report: sclerosing encapsulating peritonitis in a cat with disseminated pancreatic adenocarcinoma of presumed ductal origin. Front. Vet. Sci. 11, 1406223. Kiniger, C., Janssen, J.N., Lederer, K.A., Lipnik, K. and Doulidis, P.G. 2023. Sclerosing encapsulating peritonitis in cats: a two-case report and literature review. JFMS Open. Rep. 9, 20551169231178447. Linderman, M.J., Brodsky, E.M., de Lorimier, L.-P., Clifford, C.A. and Post, G.S. 2013. Feline exocrine pancreatic carcinoma: a retrospective study of 34 cases. Vet. Comp. Oncol. 11, 208–218. Merika, E.E., Syrigos, K.N. and Saif, M.W. 2012. Desmoplasia in pancreatic cancer. Can we fight it?. Gastroenterol. Res. Pract. 2012, 781765. Michishita, M., Takagi, M., Kishimoto, T.E., Nakahira, R., Nogami, T., Yoshimura, H., Hatakeyama, H., Azakami, D., Ochiai, K. and Takahashi, K. 2017. Pancreatic neuroendocrine carcinoma with exocrine differentiation in a young cat. J. Vet. Diagn. Invest. 29, 325–330. Nakayama, M., Miyazaki, M., Honda, K., Kasai, K., Tomo, T., Nakamoto, H. and Kawanishi, H. 2014. Encapsulating peritoneal sclerosis in the era of a multi-disciplinary approach based on biocompatible solutions: the NEXT-PD study. Perit. Dial. Int. 34, 766–774. Nam, S.J., Song, S.H., Lee, S.H., Jeung, S.Y., Ah, J.G., Lee, S.H. and Ryu, M.O. 2023. Peritoneal carcinomatosis with desmoplasia and osseous metaplasia mimicking encapsulating peritoneal sclerosis in a cat: case report. Front. Vet. Sci. 10, 1298736. Rosario, C.O., Musser, M.L., Yuan, L., Mochel, J.P., Talbott, J., Johannes, C.M. and Berger, E.P. 2023. Retrospective evaluation of toceranib phosphate (Palladia) use in the treatment of feline pancreatic carcinoma. Can. Vet. J. 64, 1143–1148. Tsukada, Y., Park, Y.T., Mitsui, I., Murakami, M. and Tsukamoto, A. 2022. Sclerosing encapsulating peritonitis in a dog with pancreatic ductal adenocarcinoma. BMC Vet. Res. 18, 383. | ||
| How to Cite this Article |
| Pubmed Style Kobayashi K, Kawabata Y, Kadekaru S, Sakai O, Yoshitake R, Mochizuki Y, Nakamura S, Kutara K. Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights. Open Vet. J.. 2025; 15(8): 3904-3911. doi:10.5455/OVJ.2025.v15.i8.57 Web Style Kobayashi K, Kawabata Y, Kadekaru S, Sakai O, Yoshitake R, Mochizuki Y, Nakamura S, Kutara K. Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights. https://www.openveterinaryjournal.com/?mno=258596 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.57 AMA (American Medical Association) Style Kobayashi K, Kawabata Y, Kadekaru S, Sakai O, Yoshitake R, Mochizuki Y, Nakamura S, Kutara K. Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights. Open Vet. J.. 2025; 15(8): 3904-3911. doi:10.5455/OVJ.2025.v15.i8.57 Vancouver/ICMJE Style Kobayashi K, Kawabata Y, Kadekaru S, Sakai O, Yoshitake R, Mochizuki Y, Nakamura S, Kutara K. Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3904-3911. doi:10.5455/OVJ.2025.v15.i8.57 Harvard Style Kobayashi, K., Kawabata, . Y., Kadekaru, . S., Sakai, . O., Yoshitake, . R., Mochizuki, . Y., Nakamura, . S. & Kutara, . K. (2025) Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights. Open Vet. J., 15 (8), 3904-3911. doi:10.5455/OVJ.2025.v15.i8.57 Turabian Style Kobayashi, Kosuke, Yuga Kawabata, Sho Kadekaru, Osamu Sakai, Ryohei Yoshitake, Yohei Mochizuki, Shin-ichi Nakamura, and Kenji Kutara. 2025. Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights. Open Veterinary Journal, 15 (8), 3904-3911. doi:10.5455/OVJ.2025.v15.i8.57 Chicago Style Kobayashi, Kosuke, Yuga Kawabata, Sho Kadekaru, Osamu Sakai, Ryohei Yoshitake, Yohei Mochizuki, Shin-ichi Nakamura, and Kenji Kutara. "Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights." Open Veterinary Journal 15 (2025), 3904-3911. doi:10.5455/OVJ.2025.v15.i8.57 MLA (The Modern Language Association) Style Kobayashi, Kosuke, Yuga Kawabata, Sho Kadekaru, Osamu Sakai, Ryohei Yoshitake, Yohei Mochizuki, Shin-ichi Nakamura, and Kenji Kutara. "Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights." Open Veterinary Journal 15.8 (2025), 3904-3911. Print. doi:10.5455/OVJ.2025.v15.i8.57 APA (American Psychological Association) Style Kobayashi, K., Kawabata, . Y., Kadekaru, . S., Sakai, . O., Yoshitake, . R., Mochizuki, . Y., Nakamura, . S. & Kutara, . K. (2025) Postmortem diagnosis of exocrine pancreatic adenocarcinoma mimicking encapsulating peritoneal sclerosis in a cat: Clinical and histopathological insights. Open Veterinary Journal, 15 (8), 3904-3911. doi:10.5455/OVJ.2025.v15.i8.57 |