| Research Article | ||
Open Vet. J.. 2025; 15(7): 2982-2992 Open Veterinary Journal, (2025), Vol. 15(7): 2982-2992 Research Article Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cowsSuzanita Utama1, Fachruddin Aziz2, Syaiful Rizal3, Sri Mulyati1, Imam Mustofa1*, Aswin Rafif Khairullah4, Tita Damayanti Lestari1, Riza Zainuddin Ahmad4, Latifah Latifah5, Adeyinka Oye Akintunde6, Lili Anggraini5, Ertika Fitri Lisnanti7, Chairdin Dwi Nugraha5 and Arif Nur Muhammad Ansori8,9,101Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Master Program of Reproductive Biology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Research Center for Applied Zoology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 5Research Center for Animal Husbandry, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 7Program of Animal Husbandry, Faculty of Agriculture, Universitas Islam Kadiri, Kediri, Indonesia 8Postgraduate School, Universitas Airlangga, Surabaya, Indonesia 9Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, India 10Medical Biotechnology Research Group, Virtual Research Center for Bioinformatics and Biotechnology, Surabaya, Indonesia *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 07/02/2025 Revised: 15/06/2025 Accepted: 19/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
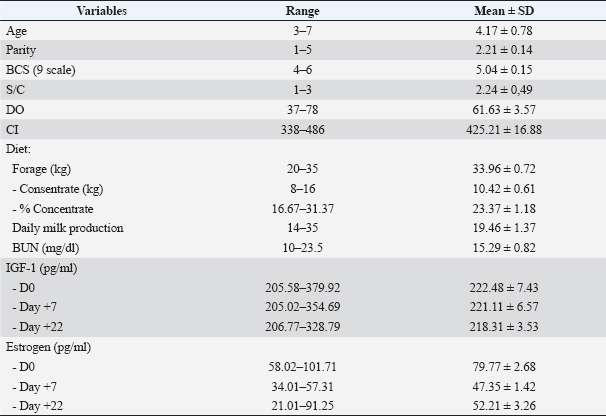
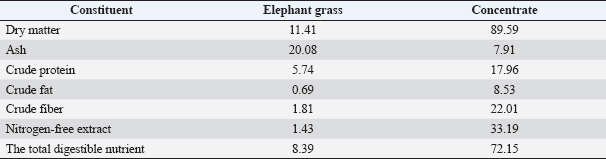
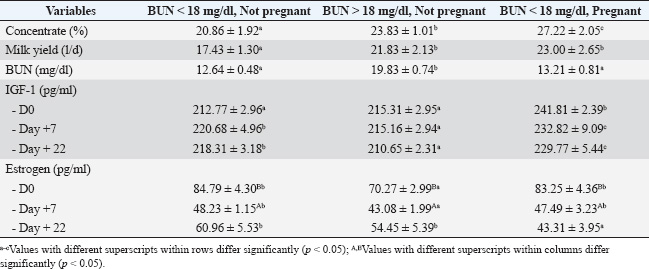
ABSTRACTBackground: Smallholder dairy farming is the largest fresh milk producer in Indonesia. The success of dairy cattle production depends on feed protein, increased blood or milk urea, increased crude feed protein, and increased degradation of feed protein. Aim: This study aimed to determine the effect of concentrate intake given to Friesian Holstein dairy cows on community farms on blood urea nitrogen (BUN), estrogen, and insulin-like growth factor-1 (IGF-1) levels. Methods: Gradually selecting the sample from 100, 50, and 21 dairy cows, we used the equality of variables as the control variable. Blood sampling was performed simultaneously on the day of artificial insemination (D0), the seventh (D+7), and D+22 (D+22) after artificial insemination. Pregnancy examination was performed using the rectal palpation technique three months after insemination. Results: Milk production and BUN levels increased with increasing concentrate intake up to a certain limit. Estrogen levels based on BUN and pregnancy on D0 and D+7 were not significantly different (p > 0.05) between the groups. In contrast to the group of nonpregnant cows with low BUN, estrogen concentrations in pregnant cows with low BUN and nonpregnant cows with high BUN were lower on D+22. IGF-1 levels in pregnant cows were higher for D0, D+7, and D+22 at unbalanced IGF-1 levels in cows with BUN >18 or <18 mg/dl. Conclusion: This research is an effort to increase milk production by increasing concentrate intake and must pay attention to BUN level, which affects up level of IGF-1, services per conception, and calving interval levels. Keywords: BUN, Estrogen, IGF-1, Milk yield, Pregnancy. IntroductionThe small holders dairy farming is the largest fresh milk producer in Indonesia. It is recommended that dairy cows get pregnant 2–3 months after calving in order to sustain high milk output (Mense et al., 2018). A key element of the extremely effective reproduction strategies of mammalian species is lactation. The role of feed is critical in dairy cows because it is a supporting source for the increased appearance of milk production and reproduction (Leduc et al., 2021). The addition of feed quality with a balanced ratio of forage and concentrate will provide good production and milk fat content. In this case, the formulation of dairy rations with a number of available feed ingredients is a very vital aspect, especially in order to balance the energy content and protein of the feed, of course without understating the role of other nutrients (Pesti and Choct, 2023). Pregnancy rates increased with higher intakes of soluble and fermentable neutral detergent fiber, whereas this measure decreased with higher milk output. Surprisingly, the percentage of pregnant women was positively correlated with the anticipated energy costs of urea generation (Rodney et al., 2015). Dairy cow fertility has decreased over the past 50 years as milk production per cow has increased in tandem with crude protein intake (Walsh et al., 2011). Cows that received more concentrate overall had greater energy and total dry matter intakes, which led to better milk output, lower body condition score loss, and negative energy balance (NEBAL) (Lawrence et al., 2015). A decrease in lactating cow fertility has been linked to increased milk production capacity (Butler, 2000). Reproduction depends on dietary proteins, and raised blood or milk urea, increased crude dietary proteins, and increased dietary protein degradability. Numerous studies have linked it to lower rates of conception and pregnancy. Blood urea nitrogen (BUN) or milk urea nitrogen (MUN) is a helpful metric for assessing the environmental effect and appropriateness of diets from dairy farms (Jonker and Kohn, 2001). Dairy cows that consume large amounts of rumen-degradable protein on a daily basis produce more milk, and they also have higher BUN concentrations (Rukkwamsuk, 2011). MUN is a useful and trustworthy signal for protein metabolism in dairy cattle, and it might be used in place of BUN as a urea status indicator in the field. Protein supply and fertility variations across herds have been predicted using urea nitrogen concentrations in bulk tank milk (Zhao et al., 2025). Reduced reproductive effectiveness has been linked to high BUN concentrations, which may be caused by changes in the intrauterine environment, including estrogen and insulin-like growth factor-1 (IGF-1) (Desta, 2024). Based on the physiology of cows, failed pregnancy could be caused by failure of oocyte maturation during folliculogenesis, fertilization, or implantation. High blood or milk urea levels were linked to conception or pregnancy (Raboisson et al., 2017). According to Utama et al. (2018), MUN, milk production, and reproductive performance were all higher in animals with a concentrate to forage ratio of greater than 30% than those with a lesser. In contrast, Putri et al. (2018) found that high concentration induced height BUN, in which BUN levels of more than 18 mg/dl failed cow to be pregnant. Hormone and other constituents are transported in blood plasma through the secretion of Mammary glands detected in milk and, of course, in the liquor of follicles (Mourupoju and Sundaresan, 2018). Several hormones, including IGF-1 and estrogen, are involved in follicle formation in the ovaries. The aim of this study was to determine the effect of concentrate intake given to Friesian Holstein dairy cows on community farms on BUN, estrogen, and IGF-1 levels. Materials and MethodsSpecifications of the cowsSmallholder dairy farms in Jambuwer Village, Malang District, East Java, Indonesia, have a total population of ±1,000 lactating dairy cows. Out of them, 100 cows were selected randomly based on criteria: not pregnant, lactation, parity more than one, milk yield, age, parity, health condition, and measured Body Condition Score (BCS) (1–9 scale). Parity is determined by the number of times a cow has given birth based on the inseminator’s recording, while milk yield is determined based on the farmer’s production records, which are measured during morning and evening milking. Fifty cows were further selected based on similarity in range X ± 0.25 SD of services per conception (S/C), calving interval (CI), number of days open (DO), and type of feed (forage and concentrate). These 50 cows were then grouped into three groups based on BUN levels and pregnancy status. Meanwhile, samples of forage and concentrate were collected for proximate analysis. Data and sample collectionBlood sampling was done through the coccygeal vein (5 ml per cow). Blood sampling was performed three times: on the day of artificial insemination (AI) (D0), 7 days after AI (D+7), and 22 days after AI (D+22). Two hours after collection, the blood samples were centrifuged at 2,000 rpm for 10 minutes, and the serum was separated and stored at −20ºC. Pregnancy detection was done 3 months after AI through rectal palpation. Measurement of BUN, estrogen, and IGF-1 levelsProximate analysis for forage and concentrate composition was conducted in the Animal Feed Laboratory, Department of Animal Husbandry, while the Elisa technique was used for measuring estrogen (DRG instrument GmbH, Germany) and IGF-1 (Fine Test® kit) conducted at the Laboratory of Endocrinology, Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga Surabaya. BUN was measured using the Berthelot method with a urea nitrogen diagnostic kit (Pointe Scientific, Inc) at the Center for Health Laboratory Surabaya. Statistical analysisStatistical analysis was performed to identify the data variable homogeneity of cows at each step of sampling by using Kolmogorov–Smirnov test. Statistical analyses were further performed with one-way analysis of variance and independent-samples t test for evaluate the probability of difference of the variables cows grouped based on BUN levels and pregnancy status of cows at a 95% significance level. Significance was also determined using a post hoc test. Ethical approvalThis study has obtained ethical approval from the Animal Care and Use Committee (ACUC), Faculty of Veterinary Medicine, Airlangga University. Number: 0290/HRECC.FODM/II/2024. ResultsData analysis was carried out by comparing variables in the first sampling group with the results of the second sampling to assess sample homogeneity and to compare whether the dairy cows from the second sampling represented the dairy cows from the first sampling (sampling in the population). The first stage of sample selection was carried out randomly to determine 100 dairy cows from the population to be selected based on the criteria of age 3–8 years, parity 2–5, BCS 3–8 (scale 9), and milk production 7–33 l/d. The second stage of sample selection involved randomly selecting 50 cows from 100 cows for reproductive efficiency and feed quantity. Samples were obtained with characteristics of age 3–7 years, parity 2–5, BCS 4–7, milk production 10–35 l/d, S/C 1–8, CI 339–697 days, DO 37–98 days, forage consumption 20–70, concentrate feed 5–15 kg/day, and percentage of concentrate in feed 7.89%–34.78%. Based on BUN levels and pregnancy status, out of a total of 50 cows, 7 cows had BUN levels <18 mg/dl and not pregnant (Group I), 24 cows with BUN levels >18 mg/dl and were pregnant (Group II), 19 cows had BUN levels <18 mg/dl and were not pregnant (Group III), and no pregnant cows had BUN levels <18 mg/dl. The third stage of random cattle sampling was to place 7 cows from Groups II and III so that the number of cows in each group was the same. There was no significant difference (p > 0.05) in the same variables among 100, 50, and 21 cows. Based on these results, we conclude that 21 cows represent the population at the study site. Homogeneity examination using the Kolmogorov–Smirnov test on the characteristics of 21 cows showed that the data were normally distributed (p > 0.05) (Table 1). Dairy farmers of Jambuwer Village in Malang District, East Java, Indonesia feed their dairy cows with elephant grass and concentrate. Proximate analysis of the feed is shown in Table 2. Concentrate, daily milk production, and BUN levelsThe increase in the percentage of concentrate was followed by an increase in milk production and BUN levels. In the non-pregnant cow group, the percentage of concentrate consumption was higher, followed by an increase in milk production and BUN levels, each at the limit of 23.83% ± 1.01% of concentrate. In the pregnant cow group, up to the limit of the percentage of concentrate consumption, there was no significant difference (p > 0.05) in milk production. In the pregnant cow group, a higher percentage of concentrate consumption was observed, but with lower BUN levels compared with the limit of 23.83% ± 1.01% concentrate. Insulin-like growth factor IThe serum IGF-1 profiles on day estrus (D0), day 7 (D+7), and day 22 (D+22) of estrus in all groups did not match the profile of the percentage of concentrate intake, milk production, and BUN levels, respectively. There were similarities in the IGF-1 level profiles of all groups on D+7 and D+22 of sampling. IGF-1 levels on all sampling days were higher in pregnant dairy cows than in non-pregnant dairy cows, both of which had lower BUN levels and higher BUN levels. In pregnant dairy cows with low BUN levels (Group III), IGF-1 D0 levels were higher in these cows than in nonpregnant dairy cows (Groups I and II). Meanwhile, in nonpregnant dairy cows with significantly higher BUN levels (p < 0.05), there was no difference in IGF-1 levels in D0. The IGF-1 hormone profile on D+7 and D+22 was relatively the same in nonpregnant dairy cows, both in dairy cows with high and low BUN levels. EstrogenSerum estrogen profiles on day estrus (D0), day 7 (D+7), and day 22 in all groups were not linear following the profile of the percentage of concentrate intake and milk production. However, the serum estrogen profiles on day estrus (D0) and day 7 of estrus (D+7) in all groups had profiles opposite to those of BUN levels. The average serum estrogen levels on D+7 of all groups were lower compared to D0. The group of nonpregnant cows with high BUN levels (Group II) had the lowest estrogen levels on D0 and D+7 compared with the other groups on the same day. On D+22, the estrogen levels of pregnant cows and low BUN levels (Group III) were lowest compared with the other groups. DiscussionThe addition of concentrate intake for dairy cows in order to gain an economic aspect of cost of nutrition versus reproductive efficiency, one calf a year, and milk production (Shortall et al., 2018). Milk production is an integrated part of the reproductive cycle and affects hormonal factors, particularly estrogen, placental lactogen, IGF-I, progesterone, growth hormone (GH), and prolactin (PRL). The milk-producing alveolus in the mammary gland is where milk is produced. The duct system connects the alveolus, a single layer of epithelial secretory cells encircling the lumen, which serves as the primary milk storage area. The quantity and effectiveness of functioning mammary epithelial cells, which are influenced by protein consumption, determine milk production capacity (Rezaei et al., 2016). Concentrate, daily milk yield, and BUNIn this study, an increase in the concentrate percentage (p < 0.05) was not linearly followed by an increase in milk yield and BUN level. Several studies have reported that higher milk production is influenced by a higher intake of concentrate, especially crude protein content (Butler, 2000). The forage to concentrate ratio of 50:50 was followed by a higher milk production (13.78 ± 0.45 kg/d) compared with the 70:30 ratio (12.28 ± 0.52 kg/d) (Beyero et al., 2015). In other hand, 70% good quality roughage and 30% concentrate affected the maximum milk yield 12–14 kg/d (Beyero et al., 2015). In the groups of not pregnant cows in this study, an increasing percentage of concentrate intake (20.86 ± 1.92%–23.83 ± 1.01%) followed by an increase in milk yield (17.43 ± 1.30–21.83 ± 2.13 l/d). In the same groups, there was an increase in BUN levels at the limit of 23.83% ± 1.01% concentrate. Up to the limit of percentage concentrate intake, milk yield remained high and cows were pregnant. In the group of pregnant cows, we found that a higher percentage of concentrate intake, specifically the level of BUN, was lower than the concentration limit. Table 1. The variables (mean ± SEM) of cows’ samples for the determination of percentage concentrate intake, daily milk yield, serum estrogen, and IGF-1 levels based on BUN and pregnancy status of smallholder dairy farms.
Table 2. Proximate analysis of elephant grass and concentrate used by dairy farmers in Jambuwer Village, Malang District, East Java, Indonesia.
In dairy cows that consume a higher percentage of concentrate intake, high BUN levels are observed, while others have low levels (Table 1). The movement of amino acids and peptides from the small intestine and the diffusion of ammonia over the rumen wall cause absorbed nitrogen in a dairy cow’s bloodstream (Zhao et al., 2025). The cow is poisoned by ammonia, which the liver quickly transforms into urea (Gimelli et al., 2023). Liver deamines the amino acids and peptides that are not needed for growth, maintenance, milk synthesis, or fetal development in order to produce urea (Torres et al., 2023). The BUN pool contains this urea. There are three possible outcomes for the BUN pool: recycling, milk secretion, and urine excretion. For microbial protein synthesis in ruminants, recycling urea through saliva, salivary mucins, and the rumen wall can be a significant source of nitrogen (Hailemariam et al., 2021). A certain amount of recycled urea permeates the hindgut, where it undergoes hydrolysis and may either be reabsorbed or integrated into the microbial cells that are expelled from the stool. The kidney removes urea from the blood, which is then expelled from the body as urine (Weiner et al., 2015). Animals have constant blood flow via their kidneys, which guarantees a constant blood filtration rate (milliliters of blood filtered per minute) independent of urine volume (Langenberg et al., 2005). Urea diffuses easily across cellular membranes due to its small size and neutrality. As the mammary gland produces milk, urea diffuses into and out of the gland, thereby balancing with blood urea. As a result, MUN is proportionate to and equilibrates with BUN (Aguilar et al., 2012). Total urine N excretion has been demonstrated to have a positive linear association with MUN since urea excretion is proportional to BUN content (Zhai et al., 2005; Salazar, 2014). MUN is a measure of lactating dairy cattle’s adequate diet and effective N use. MUN provides a straightforward and non-invasive method for dairy farmers to assess the protein content of the rations their calves are fed as a management tool. Farmers who regularly analyze MUN dairy products can modify the amount of protein in their diet to better suit the needs of their cows and possibly boost profitability by lowering feed expenses. In addition, MUN is a useful tool for estimating the amount of nitrogen excreted by nursing dairy calves. The effects of excessive N feeding on dairy cows in a watershed can be evaluated using MUN. Dairy farms’ shifting N impacts on watersheds can be identified by tracking their regular use of MUN (Jonker and Kohn, 2001). Farmers occasionally provide their cows with inadequate amounts of dietary energy and high urea levels in their diets. This could potentially reduce the cows’ fertility by raising the levels of urea nitrogen in their bodily fluids (Rukkwamsuk, 2011). BUN and creatinine levels were higher in area with higher thermal humidity index (Kekana et al., 2018). The results indicated that there was a disturbance of renal function according to the higher thermal humidity index. In our opinion, dairy cows with high BUN levels may have a low efficiency of urea clearance in the blood to be excreted into the urine. As milk supply increases after calving, nutritional needs rise quickly, leading to a NEBAL. NEBAL postpones the onset of ovulation by suppressing the frequency of the luteinizing hormone (LH) pulse and lowering blood glucose, insulin, and IGF-I levels, all of which limit the production of estrogen by dominant follicles. Ovulation is facilitated by the upregulation of peripheral IGF-I and LH pulses being up-regulated in conjunction with the NEBAL nadir. NEBAL lowers fertility and serum progesterone levels (Butler, 2000). A high milk output is supported by diets rich in crude proteins. The antecedent effects of NEBAL and the effects of high dietary protein are two nutritional interactions that lead to low fertility in high-producing dairy cows (Butler, 2000). A scenario known as physiological imbalance occurs when physiological variables diverge from normal, which puts cows at higher risk of disorders and lowers their ability to produce or reproduce (Bjerre-Harpøth et al., 2012). Increased BUN levels in cows are thought to indicate a less effective use of nitrogen in their diet (Bottini-Luzardo et al., 2016). Plasma urea nitrogen levels decreased in all cows during the restriction of nutrient density regardless of the presence of lactation (Orquera-Arguero et al., 2023). Low quantities of protein in the diet increase the likelihood of poor reproductive outcomes. In particular, excessive blood or milk urea can hinder conception. The correlation between low milk urea levels or variations in milk urea levels surrounding AI and conception. Cattle’s ability to reproduce may also be adversely affected by low or unstable urea levels. Reduced conception success was linked to a drop in urea levels from intermediate, 250, and 450 mg/kg (4.3 and 7.7 mM) before AI to low ≤150 mg/kg (2.6 mM) following AI, whereas cows appeared to tolerate an increase or a steady low level around AI (Albaaj et al., 2017). The reproductive effectiveness of dairy cows is affected by high BUN concentrations because they raise progesterone serum levels at D0 and decrease CR. Dairy cows with BUN levels same or more than 18 mg/dl on the day of insemination failed to become pregnant (Putri et al., 2018). Barley silage fed a higher protein level had significantly higher MUN levels than supplemental starch cows (Dyck et al., 2011). The concentration forage to ratio above 30% in dairy cattle can lead to higher MUN (15.7 ± 2.11, still less than 18 mg/dl), milk production, and reproductive performance than those with less than 30% (Utama et al., 2018). Significant relationships were found between milk production and certain blood metabolites (BUN/MUN) associated with physiological imbalance in dairy cows that returned to estrus following AI but did not conceive. The variables were not significantly correlated with animals that did not return to estrus after continuing their pregnancy (Fallahnezhad and Moghaddam, 2016). In dairy cows, reduced conception rates were linked to BUN values higher than 20 mg/dl. Overfeeding cows protein raises their BUN levels and lowers their uterine pH. The development of the embryo is negatively impacted by excess rumen-degradable protein in lactating cows, but not in nonlactating cows (Rukkwamsuk, 2011). IGF-1 and PregnancyThe profile of serum IGF-1 levels on day of estrus (D0), 7th day (D+7), and 22nd day of estrus in all groups was not in accordance with the profile of the percentage intake of concentrate, milk yield, and BUN level, respectively. There were similar profiles of IGF-1 levels in all groups, D+7 and D+22 of sampling. Levels of IGF-1 at all sampling days were higher in pregnant dairy cows (p < 0.05) compared with non-pregnant dairy cows, both of which had lower BUN levels and higher BUN levels. In pregnant dairy cows with low BUN levels (Group III), there was a higher IGF-1 D0 level (p < 0.05) compared to non-pregnant dairy cows (Groups I and II). In dairy cows that were not pregnant and had significantly higher BUN levels (p < 0.05) were not followed by differences in IGF-1 levels on D0. The IGF-1 hormone profiles on D+7 and D+22 were relatively the same in non-pregnant dairy cows, both in dairy cows with high BUN levels and those with low BUN levels. Once sampling of blood to measure IGF-1 concentrations in late pregnancy is completed, this can be used as a base selection tool for cows. The ability of IGF-binding protein-3 (IGFBP-3) to bind IGF-1 decreased before calving. GH receptor 1A and IGF-1 mRNA expression were lower at calving, and serum IGF-1 binding capabilities were likewise lower. Cows with low IGF-1 concentrations exhibited greater expression of IGF binding protein 4 mRNA; this binding protein limits IGF-1 activity at the tissue level and may lower IGF-1 bioavailability (Piechotta et al., 2013). In this study, IGF-1 levels D0 (when estrus), D+7, and day 22 in higher dairy cows compared with non-pregnant dairy cows, both with lower BUN levels and high BUN levels. The IGF-1 level profile on D0 (during estrus), D+7, and D+22 does not match the profile of BUN levels based on pregnancy status. In dairy cows that have low BUN levels, there is a higher IGF-1 level in D0, thatwhich is in pregnant dairy cows compared to non-pregnant dairy cows. In dairy cows that were not pregnant, BUN levels that were similar were not followed by differences in IGF-1 levels on D0. The IGF-1 hormone profile on the D+7 and D+22 is relatively the same in non-pregnant dairy cows, both in dairy cows with high BUN levels and those with low BUN levels (Table 2). IGF-1 is essential for developing embryos. These molecules work together to control important processes necessary for embryonic development to the blastocyst stage, such as proliferation (which is boosted by IGF-1) and the suppression of apoptosis and stress-mediated developmental arrest (which is facilitated by IGF-1 and CSF2) (Hansen et al., 2014). As a result, local synthesis and blood transudate are necessary for IGF-1 concentrations in the reproductive tract lumen. IGF-1 is locally expressed in the oviduct’s ampulla and isthmus endosalpinx, especially in the stroma beneath the luminal epithelium (Barton et al., 2020; Senn et al., 2024). On the other hand, the luminal epithelium is where proteins are most localized (Novoselsky et al., 2024). The expression of IGF-1 rises during estrus and then begins to diminish (Endo, 2022). Additionally, the uterine endometrium expresses IGF-1 (Waszkiewicz et al., 2020). On days 5–16 after estrus, there was minimal difference in the expression of lactose in cyclic or pregnant cows (Spencer et al., 2013). During the early stages of development, bovine embryos express IGF-1R. From the zygote stage until day three following fertilization, steady-state levels of IGF-1R mRNA decrease and then gradually increase until the blastocyst stage (Block et al., 2007). IGF-1 binding to the IGF-1R activates two key signal transduction pathways: the PI3K/AKT pathway, which prevents apoptosis, and the MAPK pathway, which promotes proliferation, growth, and differentiation (Werner, 2023). IGF-1’s effects on the preimplantation embryo show that the PI3K/AKT and MAPK pathways are both active. IGF-1 culture increases the proportion of embryos that reach the blastocyst stage (Bonilla et al., 2011). Because IGF-1 increased the number of cells in day 6 morulae, this effect most likely included enhanced proliferation (Afradiasbagharani et al., 2022). Additionally, IGF-1 prevents heat shock or the prooxidant menadione from triggering apoptosis (Jousan and Hansen, 2007). Since the administration of PI3K or AKT inhibitors prevented the reduction in apoptosis, the antiapoptotic effects of IGF-1 in heat-shocked embryos are mediated by PI3K/AKT (Barrera et al., 2023). IGF-1 also provides defense against cellular stress (Higashi et al., 2010). The embryo is resistant to IGF-1 in its early stages of development. The percentage of embryos that developed into blastocysts increased when IGF-1 was added on day 4 following fertilization, whereas IGF-1 had no effect from fertilization to day 4 (Lin et al., 2003). The ability of blastocysts produced with IGF-1 to achieve pregnancy is enhanced when they are transplanted to females (Block et al., 2007). IGF-1-treated blastocysts exhibited a higher cell count and a lower percentage of apoptotic cells, but variations in cell count and apoptosis (Jousan and Hansen, 2014). IGF-1 levels in the blood dropped from D0–16. Notably, compared with synchronized control cycles and nonpregnant cycles, maternal blood IGFBP4 concentrations were lower during pregnancy. It is hypothesized that an increase in free IGF-1 for local action could be caused by a decrease in IGFBP4 in maternal blood (Meyerholz et al., 2015). The somatotropic axis is involved in the intricate processes of metabolic adaptation throughout the early stages of pregnancy, as seen by systemic blood concentrations of the maternal GH/IGF system components (Meyerholz et al., 2015). IGF-1, IGF2, and GH: The levels of IGF-1 were similar in the pregnant cycle and various non-pregnant cycles. In contrast, the corresponding negative control cycle showed a notable decline from day 0 to 16 (Table 3), followed by a rise from day 16 to 18. There was no rise in IGF-1 levels between days 16 and 18 of pregnancy (Meyerholz et al., 2015). Table 3. Percentage of concentrate intake, daily milk yield, BUN, serum estrogen, and IGF-1 levels based on BUN and pregnancy status of smallholder dairy farms.
The physiological function of IGF-1 on development and glucose metabolism. IGF-1, like insulin, is essential for the metabolism of glucose, amino acid absorption, glycogen production, lipogenesis, and mitogenesis in cells (Kasprzak, 2021). IGF-1’s physiological effects are mediated through its binding to the type-1 IGF receptor, a heterotetramer consisting of two β-subunits and an α-subunit connected by disulfide bonds. The IGF-1 receptor belongs to the tyrosine kinase family, which autophosphorylates tyrosine residues in the carboxyl terminus of the intracellular domain after binding to agonist chemicals (IGF-1) and ultimately triggers a physiological response (Malekinejad and Rezabakhsh, 2015). Low protein intake is associated with decreased serum IGF-I concentrations (Meyer et al., 2017). The liver is the primary producer of IGF-1, a substance that affects practically every tissue in the body, including the mammary gland. The mitogenic factor most likely involved in the processes of apoptosis and proliferation is IGF. IGF receptor type 1 are the receptors via which IGFs interact with cells (Kappeler et al., 2008). Insulin affects the mammary gland’s ability to function and maintain its normal histological structure. This hormone controls the synthesis of lactose, milk lipids, and casein, as well as the consumption of glucose (for energy needs). Insulin promotes the synthesis of long-chain fatty acids, which increase the amount of fat in milk. Insulin increases milk production in ruminants. Insulin activates IGF-1 receptors; thus, its presence is necessary for GHs to interact with mammary gland cells (Błasiak and Molik, 2015). As the predominant growth factor, IGF-I is linked to cell cycle modification, which includes increased proliferation, prevention of apoptosis, and increased mitogenic activity. Colostrum and serum levels of IGF-I and IGFBP-3 are positively correlated (Hoeflich and Meyer, 2017). Because IGF-1 is a short single-chain polypeptide consisting of 70 amino acids and has a molecular weight of 7649 Da, it found its way into serum and may have leaked into ovarian follicles. Glycogen synthesis, mitogenesis, lipogenesis, amino acid absorption, and cellular glucose metabolism are all significantly impacted by IGF-1 (Kasprzak, 2021). Most IGFs in serum are part of a 150-kDa complex that consists of the acid-labile component IGFBP-3 and the IGF molecule (Yakar et al., 2002). Dairy cow reproduction traits can be identified using IGF-I concentrations as indications. IGF-I is one of the most significant hormones influencing reproduction and milk production. In dairy cows, postpartum ovarian activity and nutritional status are strongly correlated with IGF-I. Additionally, it promotes the production and secretion of milk (Kul and Erdem, 2018). Increased levels of IGF-1 and insulin have been favorably correlated with follicular development and ovulation rates in vivo, and they have been demonstrated to be essential for follicular cell proliferation and steroidogenesis. Insulin and IGF-1 plasma levels often decrease after calving (Dyck et al., 2011). One important signal transduction mechanism implicated in cell migration, proliferation, and death is the IGF pathway. The growth of the mammary gland in dairy cows is regulated by IGF family proteins and binding receptors, as well as their intracellular binding partners (Ha et al., 2016). IGF-1 and BUN had a substantial positive correlation, and IGF-1 and estrogen had a positive correlation. Early postpartum variations in serum IGF-I may be useful in predicting dairy cattle’s nutritional and reproductive health (Zulu et al., 2002). From days 0 to 18, IGF-1 levels dropped, and from days 18 to 100 of pregnancy, it then gradually increased (Mense et al., 2018). On days 7 and 11 of the cycle, the uterine IGF-1 concentration was more than five times that of the plasma, whereas on days 3 and 15, the values were comparable (Costello et al., 2013). The levels of insulin and IGF-I in preovulatory follicles were approximately 205 pg/ml and 146 ng/ml, respectively. Since IGF-I is crucial for the last stage of follicle growth, it is possible that aberrant amounts of these metabolic hormones could cause follicular malfunction, which could result in low-quality oocytes that ultimately fail to fertilize (Braw-Tal et al., 2009). Estrogen levels, daily milk yield, and pregnancyThe profiles of serum estrogen on day of estrus (D0), 7th day (D+7), and 22nd day of estrus in all groups were not linear, followed by the profiles of the percentage intake of concentrate and milk yield, respectively. However, the profile of serum estrogen on day of estrus (D0) and at 7th day of estrus (D+7) in all groups had the opposite profile as that of BUN level. The average serum estrogen levels at D+7 in all groups were lower (p < 0.05) than those at D0. The group of not pregnant cows with high BUN levels (Group II) had the lowest (p < 0.05) estrogen levels at D0 and D+7 compared with those of the other groups at the same day. At D+22 levels of estrogen in pregnant cows and lower BUN levels (Group III) were lowest compared with those of the other groups. In addition estrogen level in this study was not in accordance with daily milk yield profiles (Table 3), which may have caused the cows to be in the lactation phase, not in the mamogenesis process again. The biggest detrimental effect on fertility should be given priority in attempts to address the issue. First, reduce the NEBAL and treat any postpartum uterine infections. Second, estrus expression and detection was performed followed by high-quality semen insemination (D0). Third, a high-quality oocyte is fertilized during ovulation (day 1). Fourth, the corpus luteum’s early rise in progesterone secretion (days 3–7). Fifth, in order to promote embryo growth, the uterine endometrium must create an early and suitable environment (days 6–13). Sixth, a big embryo produces enough interferon tau (days 14–18) to change the release of uterine prostaglandins and alert the mother to the pregnancy (days 16–18) (Walsh et al., 2011). Even if a threshold of 6.5 mM or 18 mg/dl serum cannot be ruled out with confidence, this threshold is the most appropriate in terms of pregnancy or conception success (Putri et al., 2018). High nitrogen exposure before artificial insemination was more strongly associated with pregnancy or conception than high urea concentrations following artificial insemination (Raboisson et al., 2017). The serum estrogen level of the D+7 cows was lower than that of the 0th day (at the time of estrus), indicating that all dairy cows under study ovulated (Table 2). Estrogen serum levels will decline after ovulation (Sumiyoshi et al., 2014; Stevenson and Pulley, 2016; Sauls et al., 2017). Most physiological, behavioral, and morphological needs for female vertebrate reproduction depend on estrogens (Malekinejad and Rezabakhsh, 2015). Estrogens are necessary for the mammary gland’s long-term development. When an animal reaches sexual maturity, progesterone and estrogen are present during ductal mammogenesis, or mammogenesis of the duct system, occurs. Additionally, ductal and lobuloalveolar mammogenesis is positively impacted by estrogens. Estrogens directly affect the tissues of the mammary gland because the gland has estrogen receptors. The stimulation of PRL secretion from the pituitary gland and an increase in the quantity of PRL receptors in the mammary gland are indirect effects of estrogens. Additionally, estrogens boost glandular cell sensitivity and the release of growth factors (IGF-1 and TGF-α). Estrogens are essential for lactogenesis because they make the mammary glands more sensitive to lactogenic hormones. However, milk production declines when estrogen concentration is high. The right ratio of estrogen to progesterone is essential for the start of lactation (Błasiak and Molik, 2015). Differences in estrogen levels between groups only occurred on D0 and day 22. On D0, the lowest level of esrogen occurs in non-pregnant dairy cows with a BUN level >18 mg/dl, whereas on D+22, the lowest estrogen level occurs in pregnant cows with a BUN level <18 mg/dl (Table 2). Pregnancy establishment may be inhibited when progesterone or estradiol levels are lower during AI (Sauls et al., 2017). Pregnant heifers had higher levels of progesterone and lower levels of estrogen than nonpregnant heifers, as well as on days 16 and 18 of the control cycle (Meyerholz et al., 2015). Progesterone levels remain relatively high during pregnancy, which prevents estrogens from stimulating PRL action. Greater production of PRL and GH occurs when progesterone levels decrease during the perinatal period. Additionally, the mammary gland produces more milk because it is sensitive to lactogenic hormones due to estrogens (Błasiak and Molik, 2015). In pregnant cows, estrogen levels 22 days after estrus were lower compared to non-pregnant dairy cows, both with low and high BUN levels (Table 2). It caused negative feedback of progesterone on the hypothalamus–hypophiseal axil to suppress FSH secretion, follicle development did not occur, and finally, estrogen levels in circulation were low (Stevenson and Pulley, 2016). ConclusionThe results of this study indicate that the occurrence of pregnancy can be affected by BUN concentrations induced by suppressed IGF-1 and estrogen concentrations. Increased concentrations of BUN ≥18 mg/dl were associated with likely false-positive pregnancy based on estrogen concentration and lowered conception rates. Dairy cows with higher feed concentrate intakes are followed by increased milk production, but to some extent, they also produce high levels of BUN. AcknowledgmentsThe authors gratefully acknowledge Dikko Yudha Hidayat DVM, Nowo Siswo Yuworo DVM, M. Vet., who was pleased to provide survey access and sampling for this study. Conflict of interestThe authors declare no conflict of interest. FundingThis study was supported by the Skema Penelitian Dasar Unggulan (PDU) Universitas Airlangga, Year 2023 (grant number: 1286/UN3.1.6/PT/2023. Author’s contributionsSU, FA, SR, EFL, and IM: conceived the idea and manuscript drafting. CDN, SM, LL, TDL, and AOA: acquisition, analysis, and interpretation of data. ANMA, ARK, LA, and RZA: critically read and revised the manuscript for intellectual content. All authors have read and approved the final manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data are available in the manuscript. ReferencesAfradiasbagharani, P., Hosseini, E., Allahveisi, A. and Bazrafkan, M. 2022. The insulin-like growth factor and its players: their functions, significance, and consequences in all aspects of ovarian physiology. Middle East Fertil. Soc. J. 27(1), 27. Aguilar, M., Hanigan, M.D., Tucker, H.A., Jones, B.L., Garbade, S.K., McGilliard, M.L., Stallings, C.C., Knowlton, K.F. and James, R.E. 2012. Cow and herd variation in milk urea nitrogen concentrations in lactating dairy cattle. J. Dairy Sci. 95(12), 7261–7268. Albaaj, A., Foucras, G. and Raboisson, D. 2017. Changes in milk urea around insemination are negatively associated with conception success in dairy cows. J. Dairy Sci. 100(4), 3257–3265. Barrera, S.S., Naranjo-Gomez, J.S. and Rondón-Barragán, I.S. 2023. Thermoprotective molecules: effect of insulin-like growth factor type I (IGF-1) in cattle oocytes exposed to high temperatures. Heliyon 9(3), e14375. Barton, B.E., Herrera, G.G., Anamthathmakula, P., Rock, J.K., Willie, A., Harris, E.A., Takemaru, K.I. and Winuthayanon, W. 2020. Roles of steroid hormones in oviductal function. Reproduction 159(3), R125–R137. Beyero, N., Kapoor, V. and Tewatia, B.S. 2015. Effect of different roughage: concentrate ratio on milk yield and its fatty acid profile in dairy cows. J. Biol. Agric. Healthc. 5(13), 176–185. Bjerre-Harpøth, V., Friggens, N.C., Thorup, V.M., Larsen, T., Damgaard, M.B., Ingvartsen, K.L. and Moyes, K. 2012. Metabolic and production profiles of dairy cows in response to decreased nutrient density to increase physiological imbalance at different stages of lactation. J. Dairy Sci. 95(5), 2362–2380. Błasiak, M. and Molik, E. 2015. Role of hormones and growth factors in initiating and maintaining the lactation of seasonal animals. Med. Weter. 71(8), 467–471. Block, J., Fischer-Brown, A.E., Rodina, T.M., Ealy, A.D. and Hansen, P.J. 2007. The effect of in vitro treatment of bovine embryos with IGF-1 on subsequent development in utero to Day 14 of gestation. Theriogenology 68(2), 153–161. Bonilla, A.Q., Ozawa, M. and Hansen, P.J. 2011. Timing and dependence upon mitogen-activated protein kinase signaling for pro-developmental actions of insulin-like growth factor 1 on the preimplantation bovine embryo. Growth Horm. IGF Res. 21(2), 107–111. Bottini-Luzardo, M.B., Aguilar-Pérez, C.F., Centurión-Castro, F.G., Solorio-Sánchez, F.J. and Ku-Vera, J.C. 2016. Milk yield and blood urea nitrogen in crossbred cows grazing Leucaena leucocephala in a silvopastoral system in the Mexican tropics. Trop. Grassl.-Forrajes Trop. 4(3), 159–167. Braw-Tal, R., Pen, S. and Roth, Z. 2009. Ovarian cysts in high-yielding dairy cows. Theriogenology 72(5), 690–698. Butler, W.R. 2000. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 60–61(1), 449–457. Costello, L.M., O’Boyle, P., Diskin, M.G., Hynes, A.C. and Morris, D.G. 2014. Insulin-like growth factor and insulin-like growth factor-binding proteins in the bovine uterus throughout the oestrous cycle. Reprod. Fertil. Dev. 26(4), 599–608. Desta, A.G. 2024. The effect of crude protein and energy on conception of dairy cow: a review. Discov. Anim. 1(1), 29. Dyck, B.L., Colazo, M.G., Ambrose, D.J., Dyck, M.K. and Doepel, L. 2011. Starch source and content in postpartum dairy cow diets: Effects on plasma metabolites and reproductive processes. J. Dairy Sci. 94(9), 4636–4646. Endo, N. 2022. Possible causes and treatment strategies for the estrus and ovulation disorders in dairy cows. J. Reprod. Dev. 68(2), 85–89. Fallahnezhad, F.N. and Moghaddam, G.A. 2016. The relationships between milk production and some blood metabolites and their effects on returning to estrus in lactating Holstein dairy cows. Iran. J. Rum. Health Res. 1(2), 35–45. Gimelli, A., Pupin, R.C., Guizelini, C.C., Gomes, D.C., Franco, G.L., Vedovatto, M., Gaspar, A.O. and Lemos, R.A.A. 2023. Urea poisoning in cattle: a brief review and diagnostic approach. Pesq. Vet. Bras. 43(1), e07228. Ha, W.T., Jeong, H.Y., Lee, S.Y. and Song, H. 2016. Review effects of the insulin-like growth factor pathway on the regulation of mammary gland development. Dev. Reprod. 20(3), 179–185. Hailemariam, S., Zhao, S., He, Y. and Wang, J. 2021. Urea transport and hydrolysis in the rumen: A review. Anim. Nutr. 7(4), 989–996. Hansen, P.J., Denicol, A.C. and Dobbs, K.B. 2014. Maternal embryokines that regulate development of the bovine preimplantation embryo. Turk. J. Vet. Anim. Sci. 38(6), 589–598. Higashi, Y., Sukhanov, S., Anwar, A., Shai, S.Y. and Delafontaine, P. 2010. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol. Metab. 21(4), 245–254. Hoeflich, A. and Meyer, Z. 2017. Functional analysis of the IGF-system in milk. Best Pract. Res. Clin. Endocrinol. Metab. 31(4), 409–418. Jonker, J.S. and Kohn, R.A. 2001. Using milk urea nitrogen to evaluate diet formulation and environmental impact on dairy farms. Sci. World J. 1(Suppl 2), 852–859. Jousan, F.D. and Hansen, P.J. 2004. Insulin-like growth factor-I as a survival factor for the bovine preimplantation embryo exposed to heat shock. Biol. Reprod. 71(5), 1665–1670. Jousan, F.D. and Hansen, P.J. 2007. Insulin-like growth factor-I promotes resistance of bovine preimplantation embryos to heat shock through actions independent of its anti-apoptotic actions requiring PI3K signaling. Mol. Reprod. Dev. 74(2), 189–196. Kappeler, L., Filho, C.D.M., Dupont, J., Leneuve, P., Cervera, P., Périn, L., Loudes, C., Blaise, A., Klein, R., Epelbaum, J., Le Bouc, Y. and Holzenberger, M. 2008. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 6(10), e254. Kasprzak, A. 2021. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 22(12), 6434. Kekana, T.W., Nherera-Chokuda, F.V., Muya, M.C., Manyama, K.M. and Lehloenya, K.C. 2018. Milk production and blood metabolites of dairy cattle as influenced by thermal-humidity index. Trop. Anim. Health Prod. 50(4), 921–924. Kul, E. and Erdem, H. 2018. Relationships between milk insulin-like growth factor-I (IGF-I) concentration and body condition score with reproductive performance and milk yield. Large Anim. Rev. 24(2), 65–70. Langenberg, C., Bellomo, R., May, C., Wan, L., Egi, M. and Morgera, S. 2005. Renal blood flow in sepsis. Crit. Care. 9(4), R363–R374. Lawrence, D.C., O’Donovan, M., Boland, T.M., Lewis, E. and Kennedy, E. 2015. The effect of concentrate feeding amount and feeding strategy on milk production, dry matter intake, and energy partitioning of autumn-calving Holstein-Friesian cows. J. Dairy Sci. 98(1), 338–348. Leduc, A., Souchet, S., Gelé, M., Le Provost, F. and Boutinaud, M. 2021. Effect of feed restriction on dairy cow milk production: a review. J. Anim. Sci. 99(7), skab130. Lin, T.C., Yen, J.M., Gong, K.B., Hsu, T.T. and Chen, L.R. 2003. IGF-1/IGFBP-1 increases blastocyst formation and total blastocyst cell number in mouse embryo culture and facilitates the establishment of a stem-cell line. BMC Cell. Biol. 4(1), 14. Malekinejad, H. and Rezabakhsh, A. 2015. Hormones in dairy foods and their impact on public health - a narrative review article. Iran. J. Public Health 44(6), 742–758. Mense, K., Heidekorn-Dettmer, J., Wirthgen, E., Brockelmann, Y., Bortfeldt, R., Peter, S., Jung, M., Höflich, C., Hoeflich, A. and Schmicke, M. 2018. Increased concentrations of insulin-like growth factor binding protein (IGFBP)-2, IGFBP-3, and IGFBP-4 are associated with fetal mortality in pregnant cows. Front. Endocrinol. (Lausanne) 9(1), 310. Meyer, Z., Höflich, C., Wirthgen, E., Olm, S., Hammon, H.M. and Hoeflich, A. 2017. Analysis of the IGF-system in milk from farm animals - occurrence, regulation, and biomarker potential. Growth Horm. IGF Res. 35(1), 1–7. Meyerholz, M.M., Mense, K., Lietzau, M., Kassens, A., Linden, M., Knaack, H., Wirthgen, E., Hoeflich, A., Raliou, M., Richard, C., Sandra, O., Schuberth, H.J., Hoedemaker, M. and Schmicke, M. 2015. Serum IGFBP4 concentration decreased in dairy heifers towards day 18 of pregnancy. J. Vet. Sci. 16(4), 413–421. Mourupoju, A. and Sundaresan, V. 2018. Milk-hormones: narrative review. J. Adv. Dairy Res. 6(3), 1–5. Novoselsky, R., Harnik, Y., Yakubovsky, O., Katina, C., Levin, Y., Halpern, K.B., Pencovich, N., Nachmany, I. and Itzkovitz, S. 2024. Intracellular polarization of RNAs and proteins in the human small intestinal epithelium. PLoS Biol. 22(12), e3002942. Orquera-Arguero, K.G., Blanco, M., Bertolín, J.R., Ferrer, J. and Casasús, I. 2023. Performance and milk fatty acid profile of beef cows with a different energy status with short nutrient restriction and refeeding. J. Anim. Sci. 101(1), skad053. Pesti, G.M. and Choct, M. 2023. The future of feed formulation for poultry: toward more sustainable production of meat and eggs. Anim. Nutr. 15(1), 71–87. Piechotta, M., Kedves, K., Araujo, M.G., Hoeflich, A., Metzger, F., Heppelmann, M., Muscher-Banse, A., Wrenzycki, M.C. and Kaske, M. 2013. Hepatic mRNA expression of acid labile subunit and deiodinase 1 differs between cows selected for high versus low concentrations of insulin-like growth factor 1 in late pregnancy. J. Dairy Sci. 96(6), 3737–3749. Putri, K.Y., Srianto, P., Lestari, T.D., Utama, S., Wurlina, W. and Mustofa, I. 2018. Reproductive efficiency and serum progesterone concentration on dairy cattle based on blood urea nitrogen (BUN) concentrations. Iraqi J. Vet. Sci. 32(2), 143–148. Raboisson, D., Albaaj, A., Nonne, G. and Foucras, G. 2017. High urea and pregnancy or conception in dairy cows: a meta-analysis to define the appropriate urea threshold. J. Dairy Sci. 100(9), 7581–7587. Rezaei, R., Wu, Z., Hou, Y., Bazer, F.W. and Wu, G. 2016. Amino acids and mammary gland development: nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotech. 7(1), 20. Rodney, R.M., Celi, P., Scott, W., Breinhild, K. and Lean, I.J. 2015. Effects of dietary fat on fertility of dairy cattle: a meta-analysis and meta-regression. J. Dairy Sci. 98(8), 5601–5620. Rukkwamsuk, T. 2011. Effect of nutrition on reproductive performance of postparturient dairy cows in the tropics: a review. Thai J. Vet. Med. 41(1), 103–107. Salazar, J.H. 2014. Overview of urea and creatinine. Lab. Med. 45(1), e19–e20. Sauls, J.A., Voelz, B.E., Hill, S.L., Mendonça, L.G.D. and Stevenson, J.S. 2017. Increasing estrus expression in the lactating dairy cow. J. Dairy Sci. 100(1), 807–820. Senn, L.K., Peterson, K.D., Edwards, J.L., Payton, R.R. and Mathew, D.J. 2024. Oviduct and endometrial epithelium improve in vitro produced bovine embryo developmental kinetics. Reproduction 167(5), e240008. Shortall, J., Foley, C., Sleator, R.D. and O’Brien, B. 2018. The effect of concentrate supplementation on milk production and cow traffic in early and late lactation in a pasture-based automatic milking system. Animal 12(4), 853–863. Spencer, T.E., Forde, N., Dorniak, P., Hansen, T.R., Romero, J.J. and Lonergan, P. 2013. Conceptus-derived prostaglandins regulate gene expression in the endometrium prior to pregnancy recognition in ruminants. Reproduction 146(4), 377–387. Stevenson, J.S. and Pulley, S.L. 2016. Feedback effects of estradiol and progesterone on ovulation and fertility of dairy cows after gonadotropin-releasing hormone-induced release of luteinizing hormone. J. Dairy Sci. 99(4), 3003–3015. Sumiyoshi, T., Tanaka, T. and Kamomae, H. 2014. Relationships between the appearances and changes of estrous signs and the estradiol-17β peak, luteinizing hormone surge and ovulation during the periovulatory period in lactating dairy cows kept in tie-stalls. J. Reprod. Dev. 60(2), 106–114. Torres, N., Tobón-Cornejo, S., Velazquez-Villegas, L.A., Noriega, L.G., Alemán-Escondrillas, G. and Tovar, A.R. 2023. Amino acid catabolism: an overlooked area of metabolism. Nutrients 15(15), 3378. Utama, S., Mulyati, S., Wurlina, W. and Mustofa, I. 2018. Effect of concentrate to forage ratio on milk urea nitrogen, milk production and reproductive performance of dairy cows. Philipp. J. Vet. Med. 55(SI), 25–34. Walsh, S.W., Williams, E.J. and Evans, A.C. 2011. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 123(3–4), 127–138. Waszkiewicz, E.M., Kozlowska, W., Zmijewska, A. and Franczak, A. 2020. Expression of insulin-like growth factor 1 (IGF-1) and epidermal growth factor (EGF) receptors and the effect of IGF-1 and EGF on androgen and estrogen release in the myometrium of pigs-in vitro study. Animals (Basel) 10(5), 915. Weiner, I.D., Mitch, W.E. and Sands, J.M. 2015. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 10(8), 1444–1458. Werner, H. 2023. The IGF1 signaling pathway: from basic concepts to therapeutic opportunities. Int. J. Mol. Sci. 24(19), 14882. Yakar, S., Rosen, C.J., Beamer, W.G., Ackert-Bicknell, C.L., Wu, Y., Liu, J.L., Ooi, G.T., Setser, J., Frystyk, J., Boisclair, Y.R. and LeRoith, D. 2002. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 110(6), 771–781. Zhai, S.W., Liu, J.X. and Ma, Y. 2005. Relation between milk urea content and nitrogen excretion from lactating cows. Acta Agric. Scand. A Anim. Sci. 55(2–3), 113–115. Zhao, X., Zheng, N., Zhang, Y. and Wang, J. 2025. The role of milk urea nitrogen in nutritional assessment and its relationship with phenotype of dairy cows: a review. Anim. Nutr. 20(1), 33–41. Zulu, V.C., Sawamukai, Y., Nakada, K., Kida, K. and Moriyoshi, M. 2002. Relationship among insulin-like growth factor-I, blood metabolites and postpartum ovarian function in dairy cows. J. Vet. Med. Sci. 64(10), 879–885. | ||
| How to Cite this Article |
| Pubmed Style Utama S, Aziz F, Rizal S, Mulyati S, Mustofa I, Khairullah AR, Lestari TD, Ahmad RZ, Latifah L, Akintunde AO, Anggraini L, Lisnanti EF, Nugraha CD, Ansori ANM. Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows. Open Vet. J.. 2025; 15(7): 2982-2992. doi:10.5455/OVJ.2025.v15.i7.9 Web Style Utama S, Aziz F, Rizal S, Mulyati S, Mustofa I, Khairullah AR, Lestari TD, Ahmad RZ, Latifah L, Akintunde AO, Anggraini L, Lisnanti EF, Nugraha CD, Ansori ANM. Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows. https://www.openveterinaryjournal.com/?mno=241574 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.9 AMA (American Medical Association) Style Utama S, Aziz F, Rizal S, Mulyati S, Mustofa I, Khairullah AR, Lestari TD, Ahmad RZ, Latifah L, Akintunde AO, Anggraini L, Lisnanti EF, Nugraha CD, Ansori ANM. Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows. Open Vet. J.. 2025; 15(7): 2982-2992. doi:10.5455/OVJ.2025.v15.i7.9 Vancouver/ICMJE Style Utama S, Aziz F, Rizal S, Mulyati S, Mustofa I, Khairullah AR, Lestari TD, Ahmad RZ, Latifah L, Akintunde AO, Anggraini L, Lisnanti EF, Nugraha CD, Ansori ANM. Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 2982-2992. doi:10.5455/OVJ.2025.v15.i7.9 Harvard Style Utama, S., Aziz, . F., Rizal, . S., Mulyati, . S., Mustofa, . I., Khairullah, . A. R., Lestari, . T. D., Ahmad, . R. Z., Latifah, . L., Akintunde, . A. O., Anggraini, . L., Lisnanti, . E. F., Nugraha, . C. D. & Ansori, . A. N. M. (2025) Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows. Open Vet. J., 15 (7), 2982-2992. doi:10.5455/OVJ.2025.v15.i7.9 Turabian Style Utama, Suzanita, Fachruddin Aziz, Syaiful Rizal, Sri Mulyati, Imam Mustofa, Aswin Rafif Khairullah, Tita Damayanti Lestari, Riza Zainuddin Ahmad, Latifah Latifah, Adeyinka Oye Akintunde, Lili Anggraini, Ertika Fitri Lisnanti, Chairdin Dwi Nugraha, and Arif Nur Muhammad Ansori. 2025. Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows. Open Veterinary Journal, 15 (7), 2982-2992. doi:10.5455/OVJ.2025.v15.i7.9 Chicago Style Utama, Suzanita, Fachruddin Aziz, Syaiful Rizal, Sri Mulyati, Imam Mustofa, Aswin Rafif Khairullah, Tita Damayanti Lestari, Riza Zainuddin Ahmad, Latifah Latifah, Adeyinka Oye Akintunde, Lili Anggraini, Ertika Fitri Lisnanti, Chairdin Dwi Nugraha, and Arif Nur Muhammad Ansori. "Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows." Open Veterinary Journal 15 (2025), 2982-2992. doi:10.5455/OVJ.2025.v15.i7.9 MLA (The Modern Language Association) Style Utama, Suzanita, Fachruddin Aziz, Syaiful Rizal, Sri Mulyati, Imam Mustofa, Aswin Rafif Khairullah, Tita Damayanti Lestari, Riza Zainuddin Ahmad, Latifah Latifah, Adeyinka Oye Akintunde, Lili Anggraini, Ertika Fitri Lisnanti, Chairdin Dwi Nugraha, and Arif Nur Muhammad Ansori. "Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows." Open Veterinary Journal 15.7 (2025), 2982-2992. Print. doi:10.5455/OVJ.2025.v15.i7.9 APA (American Psychological Association) Style Utama, S., Aziz, . F., Rizal, . S., Mulyati, . S., Mustofa, . I., Khairullah, . A. R., Lestari, . T. D., Ahmad, . R. Z., Latifah, . L., Akintunde, . A. O., Anggraini, . L., Lisnanti, . E. F., Nugraha, . C. D. & Ansori, . A. N. M. (2025) Effects of concentrate intake on blood urea nitrogen, estrogen, and IGF-1 levels in relation to pregnancy status and milk yield in smallholder dairy cows. Open Veterinary Journal, 15 (7), 2982-2992. doi:10.5455/OVJ.2025.v15.i7.9 |