| Research Article | ||
Open Vet. J.. 2025; 15(7): 2938-2947 Open Veterinary Journal, (2025), Vol. 15(7): 2938-2947 Research Article Sedative effects of detomidine and midazolam combination in horsesÉrika Ribeiro Gomes1, Larissa Alexsandra Felix1, Lucas Wamser Fonseca Gonzaga1, Naira Fernanda Dias da Silva1, Bruna Christina Fernandes Soares1, João Vitor Fernandes Cotrim de Almeida1, Gabriela Pereira Souza1, Rodrigo Norberto Pereira1, Marilda Onghero Taffarel2 and Marcos Ferrante1*1Department of Veterinary Medicine, Federal University of Lavras, Lavras, Brazil 2Department of Veterinary Medicine, State University of Maringá, Maringá, Brazil *Corresponding Author: Marcos Ferrante,. Department of Veterinary Medicine, Federal University of Lavras, Lavras, Brazil. Email: marcos.ferrante [at] ufla.br Submitted: 02/12/2024 Revised: 11/02/2025 Accepted: 10/06/2024 Published: 31/07/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: The combination of detomidine and midazolam may provide safer sedation with muscle-relaxant effects, which are particularly necessary during standing procedures. Aim: Thus, the objective of this study was to evaluate the sedative and muscle-relaxant effects of the detomidine/midazolam combination in horses through a pharmacodynamic study. Methods: Five horses were used and subjected to four experimental protocols: 15 µg/kg of detomidine (D15); a combination of detomidine (15 µg/kg) with 25 µg/kg (DM25), 50 µg/kg (DM50), or 75 µg/kg (DM75) of midazolam administered intravenously. The sedative and muscle-relaxant effects, as well as physiological parameters, were evaluated and statistically analyzed. Results: All protocols resulted in a decrease in head height, although no significant difference was observed among them. The acoustic stimulus response and tongue tone relaxation showed significant effects in the global analysis. Conclusion: This study contributes to our understanding of the application of this combination in equine sedation protocols. Keywords: Sedation, α-2 agonists, Benzodiazepines, Chemical restraint. IntroductionSedation techniques are widely used in horses to perform less invasive procedures, such as dental procedures, in which a sedative is administered along with a local block (Van Gils et al., 2017). A good sedation protocol is also essential for more invasive procedures performed with animal standing, such as castration and wound management (Dugdale and Taylor, 2016; Gozalo-Marcilla et al., 2017). The combination of drugs with different actions allows for a reduction in individual doses and adverse effects. However, more significant scientific evidence is still needed regarding the association between α2 agonists and benzodiazepines in horses for sedative procedures, as evidence supports the use of these drugs in combination with ketamine in a general anesthesia protocol (Grimsrud et al., 2009). At clinical doses, α2 agonists are the most recommended sedatives in horses because they provide sedation and somatic and visceral analgesia (Vigani and Garcia-Pereira, 2014). However, these agents only induce the necessary muscle relaxation at high doses, which can lead to deeper sedation and adverse effects, such as atrioventricular block and bradycardia (Grimm et al., 2015). On the other hand, benzodiazepines can promote skeletal muscle relaxation with minimal cardiorespiratory effects (Shini, 2000). Therefore, this study aimed to evaluate, through a pharmacodynamic study, the efficacy of detomidine, a primary drug used as an α-2 agonist, in combination with midazolam in different sedative protocols in horses. Materials and MethodsAnimalsSeven clinically healthy mixed-breed horses were selected from a herd belonging to the Federal University of Lavras. Among them, three were castrated males and four were females, with an average age of 11 ± 4 years and an average body weight of 382 ± 50 kg. The study was conducted in a randomized, double-blind, crossover design with a 7-day washout period. After completing all experimental evaluations, the horses were transferred to paddocks and monitored for approximately 2 hours to ensure their well-being and safety. Experimental designBefore the start of the experiment, the horses underwent a 6-hour fasting period. Subsequently, an initial clinical evaluation, animal weighing, and venous catheterization were performed using a 16G catheter (DESCARPACK®, Vila Hamburguesa, São Paulo, Brazil). In the crossover design, each horse was subjected to the following experimental protocols:
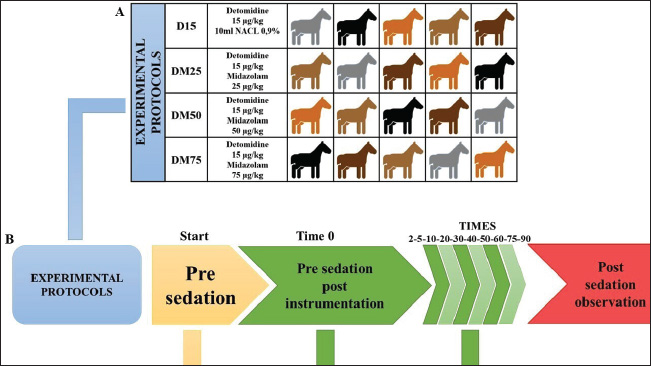
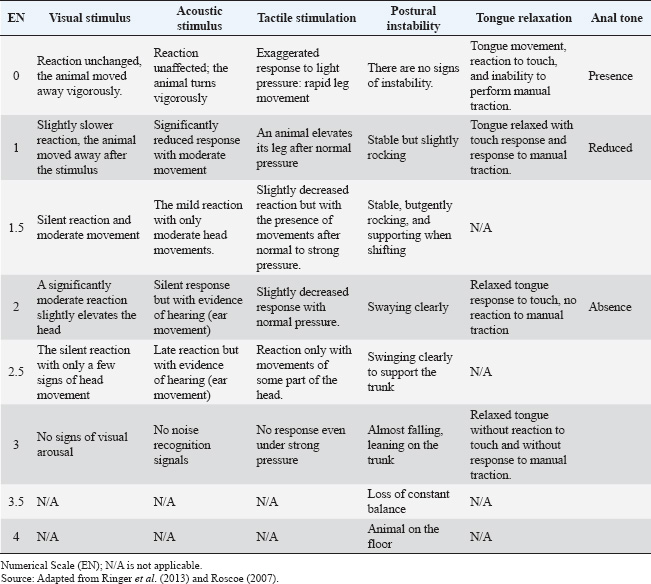
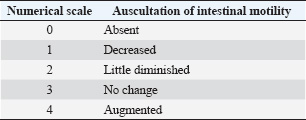
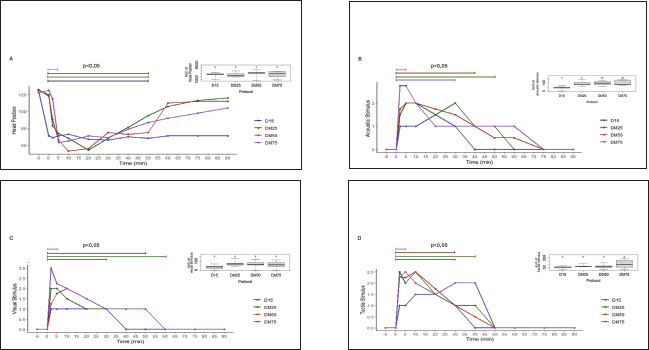
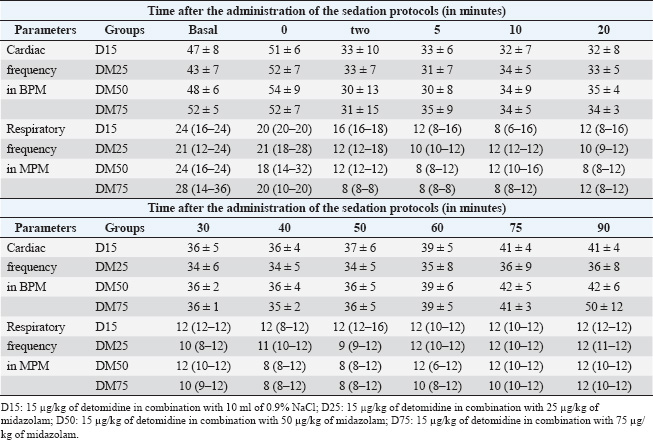
All drugs were administered intravenously. To ensure the double-blind nature of the study, in the D15 protocol, a 0.9% NaCl solution (10 ml) was administered using a separate syringe. In the protocols including midazolam, the volume of the solution containing the drug was adjusted to 10 ml with 0.9% NaCl solution to mask the treatment identity and ensure evaluator blinding. Sedative and muscle relaxant effects, as well as physiological variables, were assessed at multiple time points, starting before drug administration (baseline) and continuing at nine intervals after sedation: −5, 0, 5, 10, 20, 30, 45, 60, and 90 minutes. These evaluation points were selected to monitor the detailed progression of pharmacological effects over time. The experimental design, including the protocols and evaluation time points, is presented in Figure 1. Assessment of sedative effectsThe sedative effects were evaluated according to two main criteria: head height (HH) and response to stimuli. The HH was measured using a measuring tape fixed to the side of the animal’s body, recording the distance between the lower lip and the ground. Responses to acoustic stimuli (AS), tactile stimuli (TS), and visual stimuli (VS) were assessed using an adapted version of the numerical sedation scale described by Ringer et al. (2013). This scale, as detailed in Table 1, was used to assign scores that reflect the intensity of the horses’ responses to each stimulus type, enabling an objective and standardized evaluation of the sedation level. Assessment of myorelaxationMuscle relaxation was assessed using three main parameters: postural instability (PI), tongue relaxation (LR), and anal tone (AT). PI was measured using an adapted version of the numerical sedation scale described by Ringer et al. (2013), whereas LR was evaluated using a numerical scale adapted from Roscoe (2007). Both assessment methods are detailed in Table 1, which presents the criteria used to score the levels of muscle relaxation observed in the horses. Finally, AT was clinically evaluated as a complementary component of the muscle relaxation analysis. The physiological parameter evaluationPhysiological parameters were monitored by measuring heart rate and respiratory rate (RR) and by evaluating intestinal motility. Intestinal motility was analyzed using an adapted version of the numerical scale described by Gomes and Ribeiro Filho (2012) (Table 2). These parameters were selected as essential indicators of the horses’ physiological state, allowing for a comprehensive evaluation of the effects of the experimental protocols on the cardiovascular, respiratory, and gastrointestinal systems. Data analysisStatistical tests were conducted to evaluate normality, differences between protocols, and variations over time. The Shapiro–Wilk test was initially applied to assess data normality. For parametric data, a two-way repeated measures analysis of variance was used to compare the experimental protocols, followed by Tukey’s post hoc test to identify specific differences between groups. For the comparison of areas under the curve (AUC) across protocols for all parameters, the Friedman test was employed, which is appropriate for data in a complete block design without replications. However, the DM75 protocol was excluded from this analysis because of the early recumbency of one of the animals. The Nemenyi test was used as a post hoc method for pairwise comparisons among the remaining protocols. Additionally, to evaluate changes over time relative to baseline values, different approaches were adopted depending on the nature of the data. For nonparametric data, the Wilcoxon test was used, whereas paired t-tests were applied to parametric data. In all analyses, p 0.05 was considered statistically significant. Ethical animal researchThe Animal Use Ethics Committee of the Federal University of Lavras approved the present study (protocol 053/21). ResultsSedative effectsThe parameters of HH and responses to VS, AS, and TS were analyzed to assess sedation quality (Figure 2). All protocols induced a reduction in HH; however, this difference was not statistically significant. Similarly, responses to VS and TS were not significantly different between the protocols. However, a significant difference was observed in the frequency of responses to AS (p < 0.05) at 10 minutes, specifically between the D25 and D50 protocols. Additionally, midazolam administration demonstrated a more prolonged effect on auditory and VS responses compared with protocols that did not include this drug, as detailed in Figure 3.
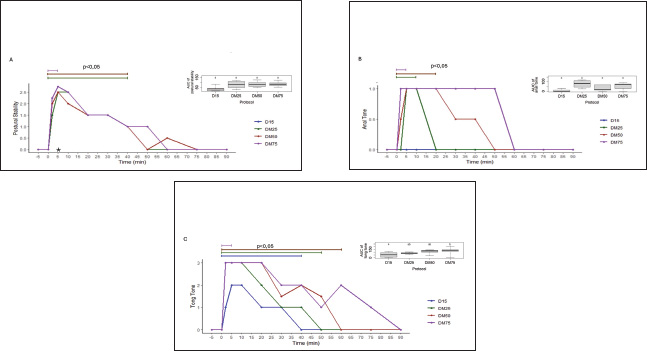
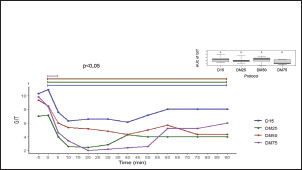
Fig. 1. Experimental design: (A) Experimental protocols and crossover design. (D15) 15 µg/kg of detomidine; (DM25) 15 µg/kg of detomidine combined with 25 µg/kg of midazolam; (DM50) 15 µg/kg of detomidine combined with 50 µg/kg of midazolam; (DM75) 15 µg/kg of detomidine combined with 75 µg/kg of midazolam. (B) Evaluation execution time. (HP) Head height. Effects of myorelaxationPI, AT, and lingual relaxation were assessed to evaluate the quality of myorelaxation (Fig. 3). PI was evident in protocols in which detomidine was combined with midazolam. However, only the DM75 protocol at time 10 significantly differed (p < 0.05) from the D15 protocol. The same trend occurred with lingual relaxation, which significantly differed (p < 0.05) from that in the DM50 protocol at 20 minutes. The AT did not differ significantly between the protocols. Midazolam administration resulted in a longer duration of PI and reduced lingual tone. Meanwhile, regarding AT, only a decrease relative to baseline was observed in the midazolam group. Effects of physiological parametersHR and RRs did not differ from baseline values, did not change over 90 minutes after drug administration, and remained within the reference range established for the species (Table 3). Intestinal motility decreased from baseline after administration of the drugs. However, there was no statistically significant difference (p < 0.05) between the protocols (Fig. 4). DiscussionTo the best of our knowledge, this study is the first to evaluate the harmacodynamics effects of detomidine combined with different doses of midazolam, in which an enhancement of sedative and myorelaxant effects was observed. Surgical procedures for equine species pose a major challenge for veterinarians. Although modern anesthetic, sedative, and monitoring techniques are available, few studies have detailed the spectrum of pharmacological effects in the frequently used dose ranges that allow the selection of the exact amount of anesthetic according to the need for this type of procedure. There are several references to the isolated use of detomidine alone or in combination with other drugs, both as an IV bolus and as a continuous intravenous infusion in horses (England et al., 1992; Roscoe, 2007; Grimsrud et al., 2009; Mama et al., 2009; Gozalo-Marcilla et al., 2017; Castro et al., 2023). However, only two studies have evaluated the effects of protocols using α-2 agonists combined with benzodiazepines (Marques et al., 2009; Castro et al., 2023). In a study by Marques et al. (2009), the use of 20 µg/kg detomidine intravenously administered with midazolam at a dose of 200 µg/kg intramuscularly was evaluated as an anesthetic premedication protocol for subsequent induction with ketamine. However, this study did not assess the sedative or myorelaxant effects of these agents. In a study by Castro et al. (2023), a dose of 20 µg/kg of detomidine combined with diazepam at 20 µg/kg was evaluated in horses undergoing dental procedures. The results demonstrated that the combination of the two drugs at those doses did not cause an increase in sedation or a reduction in tongue tone. However, in that study, only one dose of diazepam was administered. Therefore, the lack of potentiation of the effects of detomidine should not be considered as an inability of diazepam to potentiate the outcome, as could be explained by the use of an insufficient dose. According to a study by Müller and Stillbauer (1983), after oral administration, midazolam is 2.1 times more potent than diazepam; therefore, a dose of 20 µg/kg diazepam is equivalent to approximately 10 µg/kg midazolam. Therefore, to evaluate the potentiating effect of midazolam, a pilot study was carried out in which the dose range of midazolam (25–75 µg/kg) was estimated to be associated with the lowest indicated dose of detomidine (15 µg/kg), where synergistic effects could be demonstrated. Table 1. Numerical sedation scale scores for postural instability and behavioral responses, visual, acoustic, and tactile stimuli; tongue tone; myorelaxation; and anal tone in horses.
Table 2. Numerical classification scale for intestinal motility in horses, adapted from Gomes and Ribeiro Filho (2012).
HH is a clinical indicator frequently used to assess sedation (Mama et al., 2009; Santonastaso et al., 2014; Gaddini et al., 2022). All sedative protocols resulted in a decrease in HH, although the difference was not statistically significant. Mama et al. 2009 and Ringer et al. 2012 determined that a 50% reduction in HH compared with the baseline value indicates sedation. A decrease in the average HH compatible with this parameter was observed in the DM25 protocol at 10 minutes and in the DM50 protocol at 10 and 20 minutes, demonstrating a 50% difference from the baseline value. At DM75, a reduction of more than 50% was expected. However, this did not occur, which can be explained by the fact that the animals supported themselves against the containment trunk in search of the balance point to remain in the station. Therefore, it would be interesting to experiment with animals outside the containment trunk, as there would be no place to lean.
Fig. 2. (A) Average HH and AUC values for comparison of the protocols; (B) median AS values and AUCs for comparison of the protocols; (C) median VS values and AUCs for comparison of the protocols; (D) median TS values and AUCs for comparison of the protocols. The values were recorded before and after administration of the protocols of 15 µg/kg detomidine (D15) or 15 µg/kg detomidine combined with 25 µg/kg (DM25), 50 µg/kg (DM50) or 75 µg/kg midazolam (DM75). Significant differences (p < 0.05) are indicated by an asterisk (*).
Fig. 3. Scatter plots representing the values used in the assessment of myorelaxation effects: (A) Median PI values and AUC values for comparison of the protocols; (B) Median AT values and AUCs for comparison of the protocols; (C) Median lingual relaxation values and AUCs for comparison of the protocols. The values were recorded before and after administration of the protocols of 15 µg/kg detomidine (D15) or 15 µg/kg detomidine combined with 25 µg/kg (DM25), 50 µg/kg (DM50) or 75 µg/kg midazolam (DM75). Significant differences (p < 0.05) are indicated by an asteri Table 3. Mean and SD for heart rate and median and quartile (1–3); respiratory rate values recorded before and after administration of 15 µg/kg detomidine and 15 µg/kg detomidine associated with 25, 50, and 75 µg/kg midazolam (n=5).
Fig. 4. Scatter plot representing the median sum of the intestinal borborygmus scale values of the four quadrants. The assessments were performed before and after the administration of 15 µg/kg detomidine (D15) or 15 µg/kg detomidine in combination with 25 µg/kg (DM25), 50 µg/kg (DM50) or 75 µg/kg of midazolam (DM75). The effects on AC can be attributed to both detomidine and midazolam. Mama et al. 2009 evaluated the intravenous administration of 20 µg/kg detomidine in horses and reported a 50% reduction in the mean CA from 5 to 60 minutes of evaluation. Similarly, Hubbell et al. 2013, 50 and 100 µg/kg doses of midazolam intravenously in horses. A decrease in AC was observed; thus, the effect of reducing HH can be associated with both. In the present study, a shorter period was observed with an average AC lower than 50% of the observed baseline value, which can be attributed to the location of the experiments, since the study was carried out with the animals in a containment trunk. Responses to VS, AS, and TS are used to predict how a horse would respond to a noninvasive clinical or surgical procedure while under sedation (Gozalo-Marcilla et al., 2017). Several authors have evaluated auditory and visual stimuli as a single response, while others have separated them, as in this study (England et al., 1992; Mama et al., 2009; Love et al., 2011; Ringer et al., 2013). The stimuli applied to assess these responses also differ, as in the case of tactile stimulation, which can be described as touching the coronary band of the hoof or touching the inside of the ears with a blunt-tipped object (Bryant et al., 1991; Clarke et al., 1991; England et al., 1992). In the present study, there was a significant difference in the auditory stimulus in 10 minutes of evaluation between the midazolam and detomidine-only protocols. Similarly, Hubbell et al. (2013) described the pharmacokinetics of midazolam and reported a greater intensity of reduction in response to the stimulus applied during the blood collection procedure. The most significant decreases in response were approximately 5 and 15 minutes after the IV administration of midazolam at doses of 50g and 100 µg/kg, respectively, in healthy horses (Hubbell et al., 2013). In addition to this increase in intensity, the combination of midazolam also prolonged the duration of the reduction in response to stimuli compared with that of detomidine administered alone. This effect can be explained by the action of midazolam on Gamma-aminobutyric acid, which inhibits the central nervous system and promotes tranquilizing, hypnotic, anxiolytic, and myorelaxant activity (Marques et al., 2009). Although the evaluation of VS, AS, and TS is used to predict responsiveness, these results should not be interpreted directly as suitable for the performance of noninvasive clinical or surgical procedures; however, from these results, clinical studies evaluating protocols for specific procedures should be designed. Midazolam is known to produce intense muscle relaxation; consequently, the parameters of PI, AT, and TR were used to evaluate myorelaxation. The myorelaxant effect of benzodiazepines is one of the main factors underlying the frequent use of these agents in combination with ketamine or tiletamine (Allison et al., 2018; McIver et al., 2023). In the present study, there was a significant difference in PI (p < 0.05) at P10 between the DM75 and D15 protocols. Animals that received only detomidine showed mild ataxia, which returned to values close to baseline after 60 minutes. In contrast, midazolam was administered to animals with moderate ataxia for up to 75 minutes. The relationship between PI and midazolam administration may also be associated with the findings of other studies on donkeys and horses. Odette et al. (2022) reported that four of seven donkeys were recumbent after 100 µg/kg of midazolam IV administration. The same was reported in a study by Hubell et al. (2013) involving horses; one of the six animals that received a dose of 100 µg/kg of midazolam also became recumbent. These data differ from those of another study in which midazolam was administered at doses of 50, 100, or 150 µg/kg in 10 thoroughbred mares that were not recumbent at any of the other doses (Zamur and Neto, 2002). However, considering that midazolam is highly metabolized by Cytochrome P450 and that purebred horses have a high metabolic capacity and more significant muscle development, this tolerance to recumbency in could be due to greater midazolam metabolization or greater basal muscle tone (Gold et al., 2008; Schwilden and Schüttler, 2008). In brief, these findings indicate that midazolam doses above 75 µg/kg increase the risk of recumbency in horses. Furthermore, previous studies have demonstrated that racial issues are an essential variable to consider. TR was evaluated as a myorelaxation parameter for possible dental procedures. Greater relaxation was observed in the midazolam protocol from a 50 µg/kg dose at 20 minutes after the D15 protocol. These results are similar to those found in the evaluation of 20 µg/kg of detomidine combined with 20 µg/kg of diazepam in horses, where a lack of statistically significant differences was observed when evaluating this parameter (Castro et al., 2023). This finding can be explained by the fact that this dose of diazepam (20 µg/kg) was similar to the lowest quantity of midazolam (25 µg/kg) used in our study. Midazolam is more potent than diazepam, as demonstrated in studies on humans and ponies (Nuotto et al., 1992; Vries et al., 2016). The assessment of AT is not frequently performed in sedation protocols; however, in this study, a decrease in anal sphincter contraction was observed in protocols that included midazolam. Marques et al. (2009) also reported a reduction in AT in foals after the intramuscular administration of midazolam at a dose of 20 µg/kg and intravenous administration of detomidine at a dose of 20 µg/kg after 15 minutes. This reduction in AT can be explained by the mechanism of action of benzodiazepines, which can occur through the connection present in the GABAergic complex, increasing the influx of chloride ions and promoting muscle relaxation in most species (Hubbell et al., 2013). Regardless of the protocol administered, there were no changes in cardiorespiratory parameters, which remained within the values considered normal for this species (Feitosa, 2020). This difference likely occurred because of the use of a low-dose detomidine, which has dose-dependent cardiorespiratory effects (Gozalo-Marcilla et al., 2017). The incidence of cardiorespiratory changes in horses has not been reported for midazolam, which was confirmed in our study (Muir et al., 1982; Hubbell et al., 2013; Odette et al., 2022). Therefore, although the results suggest that protocols combining detomidine and midazolam are safe at the doses evaluated, additional studies are needed to assess the cardiorespiratory effects in greater detail using parameters such as invasive blood pressure, oscillometric blood pressure, and electrocardiography. Although there was a reduction in noise recorded through abdominal auscultation after drug administration, which indicates a decrease in Gastrointestinal tract (GIT) movements in all protocols, there was no statistically significant difference between the protocols. However, there was a tendency toward an increase in the reduction in GIT movements related to the increase in midazolam doses. Several studies using α2-agonists have reported decreased motility in horses (Singh et al., 1997; Cruz et al., 2011; Morton et al., 2011;). In the digestive system, α-2 adrenergic receptor agonist drugs induce analgesia by acting on central and peripheral pathways of the enteric nervous system and reducing GIT motility (Valverde, 2010; Van Gils et al., 2017). On the other hand, benzodiazepines delay transit through the gastrointestinal tract after midazolam administration in mice (Inada et al., 2004) and birds (Martel et al., 2018). In addition to cardiorespiratory effects, the reduction in motility may be dose-related. Furthermore, considering that the use of these drugs may decrease GIT motility, it is recommended that these parameters be monitored when these protocols are used. On the other hand, population studies with a greater number of animals are needed to determine the gastrointestinal safety of using these drugs in sedative protocols, as well as sedation-reversing drug (flumazenil and atipamezole) protocols that allow assistance when needed. Furthermore, in the future, the pharmacodynamic data obtained in this study can be integrated with data from pharmacokinetic studies of detomidine and midazolam to construct pharmacokinetic/pharmacodynamic models that predict the sedative and myorelaxant effects of different protocols with combinations of these drugs. ConclusionGiven the above findings, midazolam is believed to enhance the myorelaxant and sedative effects of detomidine. Therefore, this study contributes to the understanding and application of this combination treatment in sedative protocols in horses. Informed consentThe horses used in this study were obtained from the Faculty of Animal Science and Veterinary Medicine herd at the Federal University of Lavras. AcknowledgmentsThe authors appreciate all the collaborators and participants in this experiment, the residents of the large animal surgical clinic at the Federal University of Lavras for assisting during the investigation, and Professors Thiago Bernardes and Raquel Silva for making some animals available for this study. Conflict of interestsThe authors declare that they have no conflicts of interest. Author contributionsErika Ribeiro Gomes: Methodology, Investigation, Data Curation, Writing - Original Draft, Project administration Larissa Alexsandra Felix: Data Curation, Writing - Original Draft Lucas Wamser Fonseca Gonzaga: Formal analysis, Data Curation, Writing - Original Draft Naira Fernanda Dias: Data Curation, Writing - Original Draft Bruna Christina Fernandes Soares: Data Curation, Writing - Original Draft João Vitor Fernandes Cotrim de Almeida: Data Curation Gabriela Pereira Souza: Writing - Original Draft Rodrigo Norberto Pereira: Methodology Marilda Onghero Taffarel: Methodology, Writing - Review & Editing Marcos Ferrante: Methodology, Conceptualization, Formal analysis, Writing - Review & Editing, Supervision, Project administration. FundingThis research received no specific grant. Data availabilityThe data supporting the findings of this study are not openly available due to sensitivity reasons. However, they are available from the corresponding author upon reasonable request. ReferencesAllison, A., Robinson, R., Jolliffe, C. and Taylor, P.M. 2018. Evaluation of the use of midazolam as a co-induction agent with ketamine for anaesthesia in sedated ponies undergoing field castration. Equine Vet. J. 50, 321–326. Bryant, C.E., England, G.C.W. and Clarke, K.W. 1991. A comparison of the sedative effects of medetomidine and xylazine in the horse. J. Vet. Anaesth. 18, 55–57. Castro, M.L., da Silva, R.V., da Silva, A.K., Wenceslau, R.R., Beier, S.L., Fagundes, N., Pimenta, E.L.M., de Lima, J.T.B., Winter, I.C. and Palhares, M.S. 2023. Sedative and cardiorespiratory effects of detomidine combined or not with diazepam in horses subjected to dental examination. Res. Soc. Dev. 12, e12912338283. Clarke, K.W., England, G.C.W. and Goossens, L. 1991. Sedative and cardiovascular effects of romifidine, alone and in combination with butorphanol, in the horse. J. Vet. Anaesth. 18, 25–29. Cruz, F.S.F., Carregaro, A.B., Machado, M. and Antonow, R.R. 2011. Sedative and cardiopulmonary effects of buprenorphine and xylazine in horses. Can. J. Vet. Res. 1, 35–41. Dugdale, A.H.A. and Taylor, P.M. 2016. Equine anaesthesia-associated mortality: where are we now? Vet. Anaesth. Analg. 43, 242–255. England, G.C.W., Clarke, K.W. and Goossens, L. 1992. A comparison of the sedative effects of three α2-adrenoceptor agonists (romifidine, detomidine and xylazine) in the horse. J. Vet. Pharmacol. Ther. 15, 194–201. Feitosa, F.L.F. 2020. Semiologia veterinária - a arte do diagnóstico, 4th ed. São Paulo, Brazi: Roca. Gaddini, L.V., de Alencar, C.R.K., Azevedo, T.L.P., de Lima, G.G., Tomacheuski, R.M., Ferrante, M. and Taffarel, M.O. 2022. Sedative effect and physiological changes in horses treated with intramuscular injection of detomidine and morphine. Ciênc. Rural 52, e20200828. Gold, J.R., Warren, A.L., French, T.W. and Stokol, T. 2008. What is your diagnosis? Biopsy impression smear of a hepatic mass in a yearling Thoroughbred filly. Vet. Clin. Pathol. 37, 339–343. Gomes, C.L.N. and Ribeiro Filho, J.D. 2012. Efeitos laxativos do polietilenoglicol 3350 e de soluções eletrolíticas em equinos. Arq. Bras. Med. Vet. Zootec. 64, 833–840. Gozalo-Marcilla, M., Luna, S.P., Crosignani, N., Filho, J.N.P., Possebon, F.S., Pelligand, L. and Taylor, P.M. 2017. Sedative and antinociceptive effects of different combinations of detomidine and methadone in standing horses. Vet. Anaesth. Analg. 44, 1116–1127. Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A. and Robertson, S.A. 2015. Lumb & Jones Anestesiologia e Analgesia em Veterinária, 5th ed. Rio de Janeiro, Brazil: Roca. Grimsrud, K.N., Mama, K.R., Thomasy, S.M. and Stanley, S.D. 2009. Pharmacokinetics of detomidine and its metabolites following intravenous and intramuscular administration in horses. Equine Vet. J. 41, 361–365. Hubbell, J.A.E., Kelly, E.M., Aarnes, T.K., Bednarski, R.M., Lerche, P., Liu, Z. and Lakritz, J. 2013. Pharmacokinetics of midazolam after intravenous administration to horses. Equine Vet. J. 45, 721–725. Inada, T., Asai, T., Yamada, M. and Shingu, K. 2004. Propofol and midazolam inhibit gastric emptying and gastrointestinal transit in mice. Anesth. Analg. 99, 1102–1106. Love, E.J., Taylor, P.M., Murrell, J., Whay, H.R. and Waterman-Pearson, A.E. 2011. Assessment of the sedative effects of buprenorphine administered with 10 μg/kg detomidine in horses. Vet. Rec. 168, 379–379. Mama, K.R., Grimsrud, K., Snell, T. and Stanley, S. 2009. Plasma concentrations, behavioural and physiological effects following intravenous and intramuscular detomidine in horses. Equine Vet. J. 41, 772–777. Marques, J.A., Pereira, D.A. and Marques, I.C.S. 2009. Associação entre midazolam e detomidina na medicação pré-anestésica para indução da anestesia geral com cetamina em potros. Arq. Bras. Med. Vet. Zootec. 61, 1290–1296. Martel, A., Mans, C., Doss, G.A. and Williams, J.M. 2018. Effects of midazolam and midazolam-butorphanol on gastrointestinal transit time and motility in cockatiels (Nymphicus hollandicus). J. Avian. Med. Surg. 32, 286–293. McIver, K., Hofmeister, E., Boveland, S. and Clark-Price, S. 2023. Effects of tiletamine-zolazepam versus propofol on peri-induction intraocular pressure (IOP) in dogs: a prospective, randomized, blinded crossover study. Vet. Anaesth. Analg. 50, e113. Morton, A.J., Varney, C.R., Ekiri, A.B. and Grosche, A. 2011. Cardiovascular effects of N-butylscopolammonium bromide and xylazine in horses. Equine Vet. J. 43, 117–122. Muir, W.W., Sams, R.A., Huffman, R.H. and Noonan, J.S. 1982. Pharmacodynamic and pharmacokinetic properties of diazepam in horses. Am. J. Vet. Res. 43, 1756–1762. Müller, W.E. and Stillbauer, A.E. 1983. Benzodiazepine hypnotics: time course and potency of benzodiazepine receptor occupation after oral application. Pharmacol. Biochem. Behav.18, 545–549. Nuotto, E.J., Korttila, K.T., Lichtor, J.L., Ostman, P.L. and Rupani, G. 1992. Sedation and recovery of psychomotor function after intravenous administration of various doses of midazolam and diazepam. Anesth. Analg. 74, 265–271. Odette, O., Simon, B.T., Ebner, L.S., Lizarraga, I., Sun, X. and Cox, S.K. 2022. The pharmacokinetics and pharmacodynamics of midazolam after intravenous administration to donkeys (Equus africanus asinus). Can. J. Vet. Res. 86, 125. Ringer, S.K., Portier, K., Torgerson, P.R., Castagno, R. and Bettschart-Wolfensberger, R. 2013. The effects of a loading dose followed by constant rate infusion of xylazine compared with romifidine on sedation, ataxia and response to stimuli in horses. Vet. Anaesth. Analg. 40, 157–165. Ringer, S.K., Portier, K.G., Fourel, I. and Bettschart-Wolfensberger, R. 2012. Development of a xylazine constant rate infusion with or without butorphanol for standing sedation of horses. Vet. Anaesth. Analg. 39, 1–11. Roscoe, M.P. 2007. Avaliação de seis protocolos de sedação para procedimentos odontológicos em equinos (Dissertação). Universidade Federal de Minas Gerais - UFMG, Belo Horizonte. Santonastaso, A., Hardy, J., Cohen, N. and Fajt, V. 2014. Pharmacokinetics and pharmacodynamics of xylazine administered by the intravenous or intra-osseous route in adult horses. J. Vet. Pharmacol. Ther. 37, 565–570. Schwilden, H. and Schüttler, J. 2008. Modern Anesthetics. Berlin, Heidelberg: Springer. Shini, S. 2000. A review of diazepam and its use in the horse. J. Equine Vet. Sci. 20, 443–449. Singh, S., Young, S.S., McDonell, W.N. and O’Grady, M. 1997. Modification of cardiopulmonary and intestinal motility effects of xylazine with glycopyrrolate in horses. Can. J. Vet. Res. 61, 99. Valverde, A. 2010. Alpha-2 Agonists as pain therapy in horses. Vet. Clin. Equine Pract. 26, 515–532. Van Gils, M., Korhonen, I. and Yli-Hankala, A. 2017. Procedures for evaluating the adequacy of anesthesia. Crit. Rev. Biomed. Eng. 45, 187–218. Vigani, A. and Garcia-Pereira, F.L. 2014. Anesthesia and analgesia for standing equine surgery. Vet. Clin. North Am. Equine Pract. 30, 1–17. Vries, A., Pakkanen, S.A., Raekallio, M.R., Ekiri, A., Scheinin, M., Taylor, P.M. and Vainio, O.M. 2016. Clinical effects and pharmacokinetic variables of romifidine and the peripheral α2-adrenoceptor antagonist MK-467 in horses. Vet. Anaesth. Analg. 43, 599–610. Zamur, G. and Neto, A.Q. 2002. Comparison of the sedative and antinociceptive effects of midazolam and diazepam in horses. ARS Vet. 210–217. | ||
| How to Cite this Article |
| Pubmed Style Gomes �R, Felix LA, Gonzaga LWF, Silva NFDD, Soares BCF, , Souza GP, Pereira RN, Taffarel MO, Ferrante M. Sedative effects of detomidine and midazolam combination in horses. Open Vet. J.. 2025; 15(7): 2938-2947. doi:10.5455/OVJ.2025.v15.i7.5 Web Style Gomes �R, Felix LA, Gonzaga LWF, Silva NFDD, Soares BCF, , Souza GP, Pereira RN, Taffarel MO, Ferrante M. Sedative effects of detomidine and midazolam combination in horses. https://www.openveterinaryjournal.com/?mno=231218 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.5 AMA (American Medical Association) Style Gomes �R, Felix LA, Gonzaga LWF, Silva NFDD, Soares BCF, , Souza GP, Pereira RN, Taffarel MO, Ferrante M. Sedative effects of detomidine and midazolam combination in horses. Open Vet. J.. 2025; 15(7): 2938-2947. doi:10.5455/OVJ.2025.v15.i7.5 Vancouver/ICMJE Style Gomes �R, Felix LA, Gonzaga LWF, Silva NFDD, Soares BCF, , Souza GP, Pereira RN, Taffarel MO, Ferrante M. Sedative effects of detomidine and midazolam combination in horses. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 2938-2947. doi:10.5455/OVJ.2025.v15.i7.5 Harvard Style Gomes, �. R., Felix, . L. A., Gonzaga, . L. W. F., Silva, . N. F. D. D., Soares, . B. C. F., , Souza, . G. P., Pereira, . R. N., Taffarel, . M. O. & Ferrante, . M. (2025) Sedative effects of detomidine and midazolam combination in horses. Open Vet. J., 15 (7), 2938-2947. doi:10.5455/OVJ.2025.v15.i7.5 Turabian Style Gomes, Érika Ribeiro, Larissa Alexsandra Felix, Lucas Wamser Fonseca Gonzaga, Naira Fernanda Dias Da Silva, Bruna Christina Fernandes Soares, João Vitor Fernandes Cotrim De Almeida, Gabriela Pereira Souza, Rodrigo Norberto Pereira, Marilda Onghero Taffarel, and Marcos Ferrante. 2025. Sedative effects of detomidine and midazolam combination in horses. Open Veterinary Journal, 15 (7), 2938-2947. doi:10.5455/OVJ.2025.v15.i7.5 Chicago Style Gomes, Érika Ribeiro, Larissa Alexsandra Felix, Lucas Wamser Fonseca Gonzaga, Naira Fernanda Dias Da Silva, Bruna Christina Fernandes Soares, João Vitor Fernandes Cotrim De Almeida, Gabriela Pereira Souza, Rodrigo Norberto Pereira, Marilda Onghero Taffarel, and Marcos Ferrante. "Sedative effects of detomidine and midazolam combination in horses." Open Veterinary Journal 15 (2025), 2938-2947. doi:10.5455/OVJ.2025.v15.i7.5 MLA (The Modern Language Association) Style Gomes, Érika Ribeiro, Larissa Alexsandra Felix, Lucas Wamser Fonseca Gonzaga, Naira Fernanda Dias Da Silva, Bruna Christina Fernandes Soares, João Vitor Fernandes Cotrim De Almeida, Gabriela Pereira Souza, Rodrigo Norberto Pereira, Marilda Onghero Taffarel, and Marcos Ferrante. "Sedative effects of detomidine and midazolam combination in horses." Open Veterinary Journal 15.7 (2025), 2938-2947. Print. doi:10.5455/OVJ.2025.v15.i7.5 APA (American Psychological Association) Style Gomes, �. R., Felix, . L. A., Gonzaga, . L. W. F., Silva, . N. F. D. D., Soares, . B. C. F., , Souza, . G. P., Pereira, . R. N., Taffarel, . M. O. & Ferrante, . M. (2025) Sedative effects of detomidine and midazolam combination in horses. Open Veterinary Journal, 15 (7), 2938-2947. doi:10.5455/OVJ.2025.v15.i7.5 |