| Review Article | ||
Open Vet. J.. 2025; 15(1): 18-34 Open Veterinary Journal, (2024), Vol. 15(1): 18-34 Research Article Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overviewMohamed Tharwat1*, Abdulrahman A. Alkheraif2 and Shin Oikawa31Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 3Veterinary Herd Health, Department of Veterinary Medicine, School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Hokkaido, Japan *Corresponding Author: Mohamed Tharwat. Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia. Email: atieh [at] qu.edu.sa Submitted: 05/11/2024 Accepted: 31/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
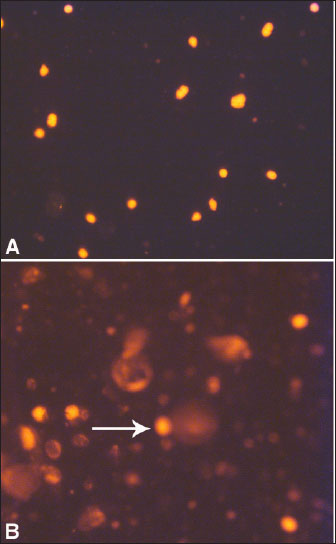
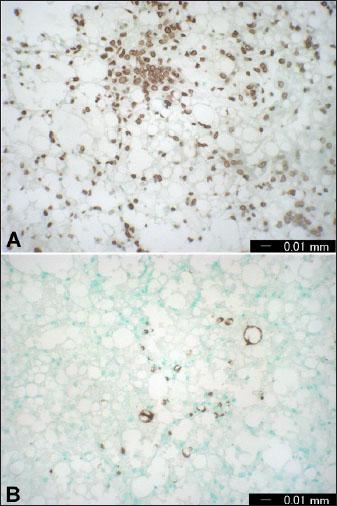
AbstractThe rising demand of the rapidly growing global population for animal-derived foods and other products requires intense animal farming. However, the husbandry and breeding of livestock are associated with a conflict between the economic requirements of producers and the biological needs of the animals. This conflict is rapidly gaining recognition not only by veterinarians, animal scientists, and producers but also by the general public. Any defect in animal feeding, housing, or breeding strategy may trigger the development of production diseases (PDs), leading to decreased producer income, reduced consumer product quality, and impaired animal welfare. Because of the need for high production during the past decades, several animals have been subjected to intense genetic selection, thus increasing animal productivity to a standard where the requirement for nutrients from the ration and internal tissue stores has greatly increased. Therefore, the inability to address the high metabolic needs of increased production is also elevated. In farm animals, high production is greatly challenged by various risk factors, such as improper nutrition, infection, stress, and housing systems. Thus, new data concerning understanding the physiology, detection, treatment, and prevention of PD are urgently required. This review highlights the most important PDs that may influence different livestock species, including cows, sheep, camels, and goats. Recently, PD has been widened to include other conditions, such as fatty infiltration of the liver, sub-acute ruminal acidosis, hepatic abscess, caudal vena cava thrombosis, endocarditis, abomasal ulcerations, displacement of the abomasum, pregnancy toxemia, and mastitis. Because many PDs emerge during the transition period, the first section of this review focuses on a series of physiological events that occur during this period. Following, different partial discharges including will be discussed. Keywords: Animals, Diagnosis, Diseases, Pathology, Production. IntroductionProduction diseases (PDs) occur when the level of production is inconsistent with nutrient intake, inadequate diet, unsuitable environment, improper breeding practices, or a combination of these factors. Although the transition period (TP) or the periparturient period (PP) of 21 days before until 21 days after parturition is greatly associated with a peak of PD, the influence of these diseases on animal productivity and health extends more into the coming lactation cycles (Mulligan and Doherty, 2008). PP is therefore considered the most critical stage for the health of dairy animals (Tharwat et al., 2012a,b,c; Tharwat and Al-Sobayil, 2015; Tharwat et al., 2015a,b; Tharwat, 2020). Traditionally, PD includes hypocalcemia, downer syndrome, hypophosphatemia, hypomagnesemia, and ketosis (Constable et al., 2017). More recently, the term PD has been widened to include other conditions, such as fatty infiltration of the liver, sub-acute ruminal acidosis, liver abscess, caudal vena cava thrombosis, endocarditis, abomasal ulcerations, displacement of the abomasum, pregnancy toxemia (PT), and mastitis (Tharwat et al., 2024a,b). In PD, output is higher than input because of the overproduction level; therefore, no diet can keep the animal in nutritional equilibrium, or due to an unbalanced diet or inadequate nutrient density. For instance, a diet may contain adequate protein required for the production of milk but does not contain sufficient glucose precursors to substitute for the energy secreted in the milk. This review is designed to emphasize the most important partial discharge that may affect different livestock, including cattle, sheep camels, and goats. Because several PDs arise during the TP, the initial section of this review focuses on the key physiological changes and events characteristic of this critical phase. This stage will especially include hemato-biochemical alterations, variations in biomarkers of inflammation and bone metabolism during this period, assessment of hepatic fat during this stage, and finally apoptosis of blood leukocytes and hepatic cells during the TP. The following sections discuss different PDs in detail. As most PDs are observed during TP, the initial understanding of the TP pathophysiology will be discussed. The most important PDs are discussed in depth, including fatty liver (FL), ketosis, sub-acute rumen acidosis, liver abscesses, caudal vena cava thrombosis, endocarditis, abomasal ulceration, abomasal displacement, milk fever, downer syndrome, postparturient hemoglobinuria, hypomagnesemia, mastitis, and PT. Transition periodUnderstanding the transition period pathophysiology The TP interval, from 21 days before to 21 days after parturition, is greatly characterized by an increased risk of disease (Lean et al., 2013). During TP, the animal displays a series of physiological, nutritional, and social alterations and is more vulnerable to metabolic and infectious diseases (Ospina et al., 2013). The animal during TP is also in a condition of reserve nutrient mobilization, principally that of fat and protein (Mulligan and Doherty, 2008). Hence, a successful and smooth transition of the animal from the fetal stage to the neonatal stage includes massive physiological readjustments both in the dam and the neonate (Lean et al., 2013). The failure or success of the transition affects the survival of the neonate and the subsequent recovery of the mother (Ospina et al., 2013). In addition, at the PP, several organs, including the nervous system, are greatly mobilized to provide ideal conditions for embryonic growth (Skotnicka et al., 2011). This period is also distinguished by great changes in the endocrine state that are much more observable than at any other stage in the gestation-lactation cycle, and a decrease in feed intake when there is an increase in nutrient requirements for the developing fetus and also for the imitation of lactogenesis (Drackley, 1999). During TP, the animal experiences a rapid several-fold elevation in nutrient intake by the udder associated with milk production compared to the much smaller requirements in late-stage gestation by the developing fetus. Thus, TP is associated with an increased incidence of PD due to insufficient homothetic acclimation in metabolism (Tharwat et al., 2012a,b,c; Tharwat and Al-Sobayil, 2015; Tharwat et al., 2015a,b; Tharwat, 2020; Satoh et al., 2024). These PDs, together with alterations in hormonal balance, progress to an elevation in lipid mobilization with eventual increases in plasma non-esterified fatty acids (NEFAs) and the percentage of fatty acid uptake from the hepatic parenchyma (Grummer, 1995; Mulligan and Doherty, 2008). When the amount of hepatic triglycerides (TG) formation exceeds the rate of TG passing, the cumulation of cholesterol esters and TG in liver cells occurs with a greater risk of fatty infiltration of the liver. TG may vanish through secretion or hydrolysis via the synthesis of very low-density lipoproteins (VLDLs) (Oikawa et al., 2010; Osada et al., 2024). Compared with non-ruminants, ruminants have the same rate of TG formation in the liver, but on the opposite side, they have a very low rate of hepatic secretion of VLDL (Pullen et al., 1990). Fatty liverFL is a known condition that occurs particularly in obese dairy animals and is a principal cause of hepatic-related mortality and morbidity (Bobe et al., 2004). The condition is observed when the liver uptake of fats increases the secretion and oxidation of adipose tissue by the hepatic parenchyma. Excess fats are stored in the liver as TG and it is associated with lowered liver metabolic function. FL occurs mostly in dairy cows during the 1st 4 weeks post-calving (Tharwat, 2012a; Tharwat et al., 2012d). Negative energy balance (NEB), stress, low dry matter intake (DMI), parturition, and hormonal imbalance are serious risk factors for the initiation of hepatic lipidosis in dairy cows (Mohamed et al., 2004a,b). As a result of NEB, fatty acids are mobilized from adipose tissue, leading to an increase in postpartum NEFA concentrations (Grummer, 2008). The severity of FL can be estimated using different methods. It can be reliably measured only by invasive liver biopsy and then analyzed chemically or histologically. The intensity of FL can also be estimated based on hepatic function tests, such as the activity of γ-glutamyl transferase (GGT) and aspartate aminotransferase (AST), and the serum concentration of total bilirubin (Oikawa et al., 2010; Tharwat, 2012b). FL is associated with increased financial costs, decreased milk production, longer calving intervals, and higher culling rates in dairy cows. The estimated cost of FL is somewhat difficult to estimate, as FL can only be diagnosed through a liver biopsy (Tharwat, 2012a; Tharwat et al., 2012d). In cows with FL, the DNA integrity of hepatocytes differs significantly from that of healthy hepatocytes, reflecting apoptosis due to excessive accumulation of TG. This explanation is supported by the elevated GGT and AST levels and total bilirubin. Figure 1 illustrates DNA damage in the hepatic cells of healthy cows with FL. Parallel to the results of the comet assay, apoptosis was also detected immunohistochemically (Fig. 2). Therefore, comet assays and immunohistochemical staining of ssDNA and caspase-3 are good means of detecting apoptosis in animals with FL (Tharwat et al., 2012d). Ketosis (Acetonemia)Ketosis or acetonemia is a metabolic defect of high-lactating animals that manifests as decreased appetite, loss of body condition, decreased milk production, and, occasionally, nervous symptoms. High-producing dairy cattle, for example, exhibit NEB immediately after parturition. The NEB originated from a quick increase in energy demands for milk production, while the intake capacity of feed in early lactation was restricted (Steeneveld et al., 2020). Periparturient intense NEB is an important predisposition to ketosis (Shen et al., 2021). Moreover, dairy cattle with acetonemia are manifested by hepatic damage and oxidative stress, and in some circumstances; affected cows show partially mediated apoptotic changes in the liver (Du et al., 2017).
Fig. 1. Representative comet images of hepatocytes in a dairy cow with FL. In control cow (A), DNA is tightly compressed and the circular disposition of the normal nucleus is maintained. Bobe-damaged DNA in the diseased cow (B) migrates from the core toward the anode, forming the tail of a comet (arrow). Ketotic cows also exhibit decreased DMI, reduced milk production, reduced milk fat, and alterations in lipid and carbohydrate metabolism compared with healthy cows. Immediately before the onset of ketosis, serum lactate and interleukin-6 (IL-6) are the most common biomarkers between ketotic animals and healthy animals, thus making these biomarkers as predictors for the disease in suspected animals (Zhang et al., 2016). Acetonemia is principally detected depending on the levels of ketones in the milk, blood, or urine. Although these tests are rapid and extensively validated, their long-term effectiveness has not been evaluated (Serrenho et al., 2022). In the primary type of ketosis, animals show a gradual reduction in appetite and milk production for a period of 3–4 days with a moderate loss of body condition and depression (Fig. 3). In the nervous system, nervous symptoms include hyperesthesia, depraved appetite, head-pressing, bellowing, walking in circles, compulsive chewing and licking, and blindness (Constable et al., 2017).
Fig. 2. Immunohistochemical examinations by liver staining of ssDNA (A) and caspase-3 (B) in cows with FL. Numerous ssDNA- and caspase-3-positive cells were detected. Subacute rumen acidosisAcute rumen acidosis is the result of excessive consumption of carbohydrate-rich foods. The overfeeding syndrome usually follows, and it may result in acute rumen acidosis and lead to death (Reed et al., 1995). Ruminal acidosis is therefore considered a bovine disorder that influences dairy animals and feedlot cattle. It can be represented in various forms, extending from per-acute form to chronic long-standing illness, which is difficult to recognize. Sub-acute rumen acidosis (SARA), on the other side, is one of the most critical metabolic disorders in the dairy industry that impairs animal health and performance even in well-managed and high-yielding dairy animals. SARA has complex and diverse consequences that may include depression of feed intake, reduction in diet digestibility, feed intake inconstancy, reduction in milk production and fat percentage, lameness, hepatic abscesses, and increasing veterinary care costs (Abdela, 2016). Sub-acute rumen acidosis is often manifested as a herd problem with general signs, disturbed digestion, depressed ruminal motility, reduced feed intake, decreased milk production, and reduced fat% causing wasting in high-producing animals. SARA, also known as chronic acidosis, is a well-established gastrointestinal disorder found principally in modern dairy herds (Selim and Tharwat, 2006; Enemark, 2008; Gao and Oba, 2014). Feeding on a diet relatively deficient in long fiber or a large proportion of concentrated feed may result in acidosis in dairy animals (Goff, 2006). Thus, the continuous ingestion of excessive quantities of concentrates with low levels of well-structured fibrous ration over a prolonged period is the principal cause of SARA (Morgante et al., 2007; Plaizier et al., 2008). The propagation of SARA is not well known; a study came from the U.S. revealed a prevalence of 19% in early lactating cattle and 26% in animals deep into lactation (Garrett et al., 1997).
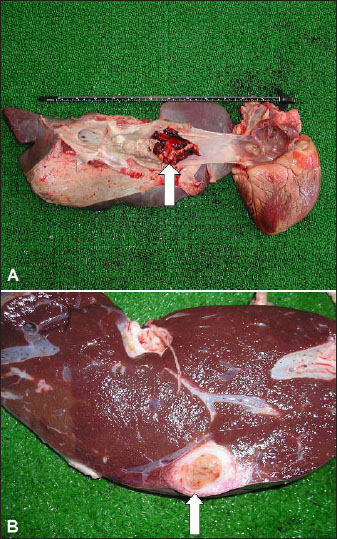
Fig. 3. Gradual loss of body conditions in two female camels during the first 2 months of lactation. The pH of rumen content influences rumen function, and total volatile fatty acids (VFA) and ammonia nitrogen contribute to maintaining rumen pH. Low pH in the rumen is linked with ruminal atony and indigestion symptoms (Fouda, 1995). Declined ruminal pH from 5.5 to 5 with increased VFA concentrations associated with increases in propionate and butyrate, whereas decreases in acetate and normal lactate along with reductions in total ciliates are the major changes at the onset of SARA. Overfeeding with a carbohydrate-rich diet in ruminants is often associated with degenerative changes in the gastrointestinal tract, destruction of the rumen microbial population, and excessive fluid accumulation in the rumen (Constable et al., 2017). It is evident that SARA is a significant economic concern for livestock industries. These financial crises result from decreased milk production efficiency, premature culling, and increased death losses (Krause and Oetzel, 2005). Liver abscessesIn feedlot cattle, liver abscessation originates from intensive grain-feeding regimes, and are affected by several management as well as dietary factors. These conditions have a great financial influence on the industry of feedlots due to the condemnation of the liver, decreased animal performance, and reduction in carcass yield reduction (Nagaraja and Lechtenberg, 2007). Hepatic abscesses are the major liver alterations in feedlots. The incidence is highly changing, but overall, it ranges from 10% to 20% (Amachawadi and Nagaraja, 2016). Hepatic abscesses are usually due to rumenitis and ruminal acidosis in cattle-fed diets with low roughage and an increased proportion of readily fermentable carbohydrates. The lesions of hepatic abscessation are of bacterial origin, and factors focusing on virulence and pathogenicity have been studied extensively (Amachawadi and Nagaraja, 2022). Organisms from the portal circulation are refined by the liver, leading to infection and abscess formation (Mohamed et al., 2004c; Nagaraja and Lechtenberg, 2007; Tharwat, 2010). Fusobacterium necrophorum (F. necrophorum), a ruminal bacterium, is the principal etiologic agent of liver abscesses. The virulence of F. necrophorum participates in the avoidance of host defense approaches and triggers tissue damage that can lead to liver infection (Amachawadi and Nagaraja, 2022). Arcanobacterium pyogenes, an anaerobic microorganism, is a secondary causative agent of hepatic abscesses (Tadepalli et al., 2009). Generally, the control of liver abscesses depends on the implementation of antimicrobials. F. necrophorum is sensitive to penicillins, macrolides, and tetracyclines, but resistant to ionophores and aminoglycosides. The sensitivity of A. pyogenes to antimicrobials is similar to that of Gram-positive bacteria. In feedlots, chlortetracycline, bacitracin, tylosin, oxytetracycline, and virginiamycin are advisable to control hepatic abscesses (Nagaraja and Lechtenberg, 2007). Caudal vena cava thrombosisThe syndrome of caudal vena cava thrombosis (CVCT) is a distinct condition in cattle with different presentations, including pulmonary thrombosis, sudden death due to ruptured hepatic abscesses, congestive right-sided heart failure, suppurative pneumonia, hemoptysis, and/or epistaxis and endocarditis. It may be a sequel to different septic conditions, including mastitis, thrombophlebitis, and metritis (Mohamed et al., 2004c; Tharwat, 2010). The most common cause of CVCT is hepatic abscess (Constable et al., 2017). Abscesses adjacent to CVC might rupture into it. The eventual “white” thrombus is connected to the vein intima and may completely or partially close the lumen. Thrombi are mostly found in the hepatic portion of the CVC, but they are occasionally found in the subphrenic, perirenal, or intrathoracic portion (Braun et al., 2002; Braun, 2008). The most serious microorganisms associated with CVCT are F. necrophorum, A. pyogenes, Streptococci, Staphylococci, and E. coli (Ikawa et al., 1987). A diagnosis of CVCT has been reported in a Holstein cow, which underwent ultrasonography and had a history of lethargy and poor milk production. Medical treatment for the later cow was unsuccessful, and the cow was euthanized. The diagnosis of CVCT was confirmed at necropsy, and F. necrophorum was isolated from the thrombus (Simpson et al., 2012). On several occasions after initial admission, epistaxis and/or hemoptysis are observed, leading to sudden death (Fig. 4). Feces are dark and contain blood. Upon auscultation of the lungs, all animals exhibit mild to intense lung breath sounds, rough breath sounds, moist rales, and grunting on thoracic percussion. Necropsy revealed a friable thrombus tightly connected to the CVC wall extending between the liver and right atrium. Thrombus formation is associated with hepatic abscessation (Fig. 5). In some cases, pulmonary arteries may contain adhered to the walls in patients with multifocal suppurative pneumonia. Intense diffuse interstitial edema and hemorrhage may occasionally be seen surrounding pulmonary tissue, and fresh blood may be observed in the trachea and bronchi. Other postmortem findings include hemorrhagic renal infarction, abomasal ulceration, and bloody rumen contents (Tharwat, 2010). EndocarditisBacterial endocarditis (BE) is the ultimate endocardial disease in cattle (Bexiga et al., 2008; Buczinski et al., 2010a,b,c). It is defined as infection of at least one or more of the endocardial surfaces (Bayer and Scheld, 2000). In cows like humans, a long-standing bacteremia is a must for the pathophysiology of BE (Post et al., 2003). The pathogenesis of BE is not clearly defined; however, chronic infection resulting in recurrent or sustained bacteremia is considered a risk factor (Buczinski et al., 2012; Buczinski et al., 2013). In cattle, BE is noted most commonly on the tricuspid valve, followed by the mitral valve (Constable et al., 2017). In cows, the pulmonic valve is the most common location of the BE, followed by the tricuspid valve, and finally the mitral valve (Tharwat and Buczinski, 2011). Embolism of plaque may follow, resulting in pulmonary, skeletal, hepatic, or splenic involvement (Post et al., 2003). In the absence of clinical signs of heart failure or cardiac murmur, clinical detection of BE may be difficult (Karchmer, 2005). Clinical symptoms of heart failure are not definitive in cattle. With a total of 26 cases, only 7 (27%) had clinical symptoms of heart failure (Buczinski et al, 2010a,b). In addition to BE, a heart murmur may also be auscultated in cases of congenital cardiac defects (Bexiga et al., 2008) and in healthy animals (Rezakhani and Zarifi, 2007).
Fig. 4. Bilateral nasal bleeding and hemoptysis of fresh arterial blood leading to sudden death in a cow with caudal vena cava thrombosis.
Fig. 5. Postmortem findings of cattle with caudal vena cava thrombosis. A: Friable thrombi tightly adhered to the wall of the caudal vena cava (white arrow). Thrombus almost entirely occluded the caudal vena cava and extended between the right atrium and the liver. B: hepatic abscessation (white arrow). Other tests, such as CBC and blood biochemistry analysis, may also lack the sensitivity to detect cardiac diseases (Bexiga et al., 2008). On the other hand, echocardiography provides a useful tool for observing the heart, and valves can be seen (Hallowell et al., 2007; Buczinski et al., 2010a,b,c). Without the use of echocardiography, a confirmatory diagnosis of mitral, tricuspid, or pulmonic vegetation. In most animals, the procedure permits an antemortem verification of BE that can be readily useful in those with a poor outcome to avoid ineffective therapy (Tharwat and Buczinski, 2011; Buczinski et al., 2012; Buczinski et al., 2013). Prognosis depends mainly on the chronicity of the disease, the early detection, and the value of the animal (Braun, 2009; Buczinski et al., 2010). Early detection by ultrasonography will therefore prevent more animal suffering and unnecessary costly medicines (Tharwat and Buczinski, 2011). Abomasal ulcerationUlceration of the abomasum is a general clinical problem in dairy cattle (Tharwat and Ahmed, 2012; Braun et al., 2020). Abomasal ulcers occur most commonly in dairy cows during the 1st 6 weeks of milking (Constable et al., 2017). Intensive management and high-acid diets predispose to the pathogenesis of this condition. Changing the diet from milk to roughage is also a predisposing factor for weaning calves (Blowey, 2004; Constable et al., 2005). Shunting a higher level of cardiac output to the gravid uterus or to the highly lactating udder with a concomitant decrease in abomasal perfusion and an increase in plasma cortisol may lead to abomasal ulceration (Constable et al., 1992). Prolonged usage of non-steroidal anti-inflammatory drugs (NSAIDs) as therapy for musculoskeletal disorders may lead to abomasal ulceration (Dirksen, 1994). Helicobacter pylori and hair balls are present in some cases of abomasal ulceration (Constable et al., 2017). In principle, the pathophysiological etiology is the alteration of the equilibrium between aggressive and protective mechanisms in the abomasal mucosa owing to stress. Clinical signs differ but are generally nonspecific. Hematology, fecal occult blood tests, and chemistry profile, as well as abdominocentesis, and sonographic examination, can assist in diagnosis. Ulceration can be treated medically, symptomatically, or surgically. Medical treatment approaches included blood transfusion, local coagulants, antibiotics, and oral or systemic anti-antacids (Ahmed et al., 2002, 2005). Stressful management practices, including commingling and transport, must be avoided (Hund et al., 2016; Hund and Wittek, 2017). Abomasal ulceration occurs commonly in cattle and can represent an additional therapeutic challenge when present with other surgically manageable conditions (Constable et al., 2017). Abomasal displacementDisplacement of the abomasum (DA) is a common abdominal disorder in dairy cattle and is the most common cause of abdominal surgery. DA may occur on the left side (LDA) or the right side (RDA). The peak occurrence of this disease occurs during the 1st 6 weeks of lactation, but it may also be found sporadically at any time of gestation or lactation (Constable et al., 2017). Several studies have investigated DA in cattle; however, a thorough elucidation of the pathogenesis has not yet been achieved. The exact etiology of DA is unclear. Numerous factors may predispose patients to DA. Excessive VFA formation is caused by intensive diets comprising highly acidic rations, such as corn silage, and readily fermentable grains, such as high-moisture corn. Other causes may include gut stasis due to infectious diseases or metabolic problems, such as ketosis, hypocalcemia, mastitis, metritis, retained placenta, and indigestion. Increased gas accumulation and altered abomasal motility are additional prerequisites for DA in cows (Zerbin et al., 2015). According to many studies, laboratory variables in conjunction with clinical signs offer the potential to objectively assess the prognosis of animals with DA (Sickinger, 2017). Milk feverMilk fever (MF), or parturient paresis (PP), is a complex metabolic disorder in dairy animals that occurs around parturition and is reported to be initiated by a calcium (Ca) metabolism imbalance. Hence, MF is usually observed immediately before, during, or after parturition, although it has also been reported in dry animals and during mid-lactation. Cattle with MF are at high risk of dystocia and metritis and are also much more susceptible to other infectious diseases and metabolic disorders, such as mastitis, displaced abomasum, ketosis, uterine prolapse, retained placenta, and high postpartum culling rates (DeGaris and Lean, 2008). Age increases the risk of MF by almost 9% per lactation (DeGaris and Lean, 2008). Heritability and repeatability for MF within this population are low, although significant (Saborío-Montero et al., 2018). At parturition, MF is manifested by hypocalcemia as a result of an abrupt increase in Ca requirement and delay in Ca metabolism adaptability. Both subclinical and clinical hypocalcemia act as transitional “gateway diseases,” as the presence of either form is associated with other transition disorders. The diseases associated with MF include uterine prolapse, dystocia, endometritis, retained placenta, mastitis, infertility, ketosis, DA, and immunosuppression (Goff, 2008). Cows with MF exhibit changes in metabolites related to carbohydrates and some innate immunity reactants several weeks before the appearance of MF. Tumor necrosis factor and serum amyloid A are the strongest differentials between MF and healthy cows, and they might be valuable predictors of MF (Zhang et al., 2018). Typically, MF has three stages. During the first or excitation stage, anorexia, lethargy, and hyperesthesia occur, and the temperature is increased by 0.5°C. The recumbent or second stage is the most commonly observed stage by veterinarians. The animal will be recumbent in the sternal position, usually with a noticeable curvature in the neck, developing to the phase where the head is resting on the shoulder (Fig. 6). The third or comatose stage is characterized by rumen tympany, lateral recumbency, and total disappearance of the pupillary light reflex (Fig. 7). Downer syndromeHigh-yielding dairy animals are susceptible to several peripartum diseases, including DA, ketosis, MF, and Down syndrome. Downer cow syndrome (DCS) is characterized by sternal or lateral recumbency lasting for more than 24 hour or persisting longer than 2 weeks despite medication (Harwood, 2003). A multitude of infectious, metabolic, degenerative, toxic, and traumatic effects may result in a downer animal. The metabolic causes of DCS include FL, hyperketonemia, hypomagnesemia, hypophosphatemia, and hypocalcemia. Blood ketones, NEFA, glycemia, and minerals can be used to assess the ratio and energetic metabolism, which is a major risk factor for the occurrence of the syndrome (Tharwat and El Magawry, 2008; Tharwat, 2012a,b). In downer cows, the rectal temperature, pulse rate, and respiratory rate values usually include a rectal temperature of 37.5°C and rates of 65 and 27 times/minutes, respectively. Some animals exhibit mild-to-moderate depressed behavior and decreased ruminal motility. Most of the recumbent cows became recumbent postpartum in the sternal position. In advanced and prolonged recumbent cases, severe muscular damage is observed, especially in the hind legs.
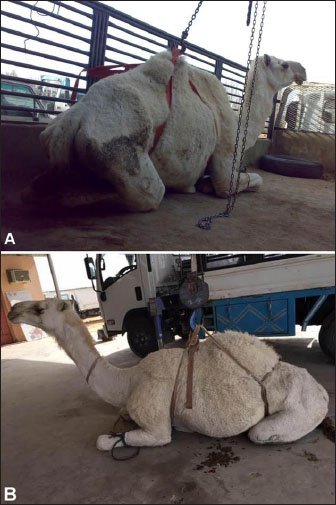
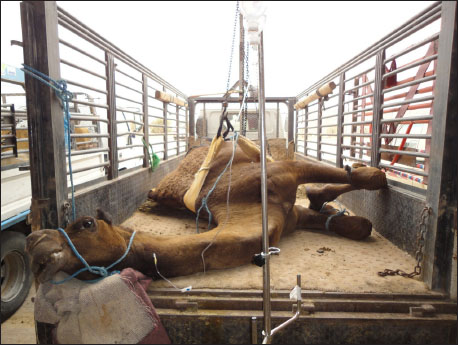
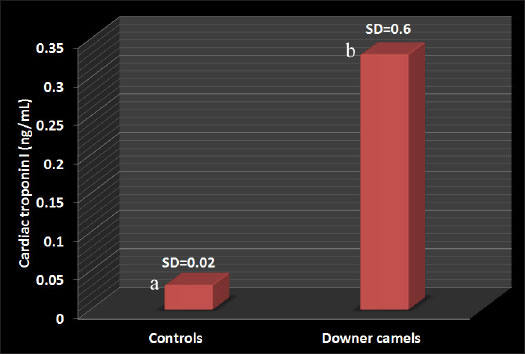
Fig. 6. Second stage or sternal recumbency phase in a cow due to hypocalcemia 72h after parturition. Some clinical signs associated with poor prognosis included the following: (1) the animal is unable to maintain sternal recumbency without support or return to lateral recumbency after being placed in sternal recumbency; (2) there is pronounced abduction of one or both hindlegs; (3) the animal is attempting to rise using the forelimbs only; (4) the hindlimbs are rigidly extended forward; (5) the animal has made no attempts to rise, despite repeated encouragement; and (6) the animal has been recumbent on a bare concrete floor for several days (Tharwat and Magawry, 2008). In the downer camels, the most prominent clinical signs during physical examination are the inability to stand. The inability to climb the recumbent camels was divided into 4 classes (1 to 4) (Fig. 8). In advanced cases, lateral recumbency may occur (Fig. 9). Other clinical findings included inappetence and weak, irregular ruminal contractions. Slinging is often recommended for treatment (Fig. 10). Compared with healthy animals, serum cardiac troponin I (cTnI) concentrations in recumbent camels differed significantly. The serum cTnI concentrations in recumbent camels are almost 10-fold higher than those in healthy camels (Fig. 11) (Tharwat, 2012a). Similar results were reported for downer dairy cows (Labonté et al., 2018; Puerto-Parada et al., 2021). Concerning biochemical variables, the most important changes in downer camels were the elevation of serum AST and CK (Tharwat, 2012b).
Fig. 7. Third stage or lateral recumbency phase in a due to hypocalcemia in a cow.
Fig. 8. Clinical presentation of four downer camels that admitted for examination in a setting position. During lifting by a winch, camels were classified as having mild (A), moderate (B), severe (C), and advanced (D) degrees of inability to stand.
Fig. 9. Clinical presentation of a downer camel that was admitted for examination of lateral recumbency.
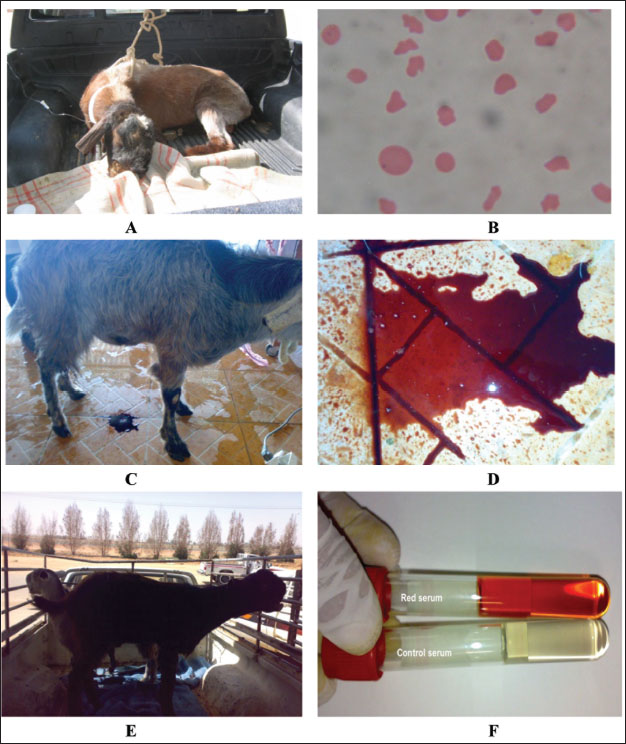
Fig. 10. Slinging of a downer female camel and pushing it to walk away. Postparturient hemoglobinuriaHypophosphatemia or postparturient hemoglobinuria (PPH) is a common condition observed early in anorectic and lactating and dairy cows (Grunberg, 2014). It appears that insufficient dietary phosphorus (P) may have triggered intense hemoglobinuria in cows, but the actual cause is not readily clear (Stockdale et al., 2005). The clinical relationship of PPH in diseased and peri-parturient animals is still under debate, but P reduction has been experimentally implicated as a predisposing or causative factor for typical peri-parturient disorders of dairy animals, such as PPH and Downer cow syndrome. The condition is characterized in early lactating dairy cows by massive intravascular red blood cell lysis and hemoglobinuria as sporadic signs (Grunberg et al., 2015; Resum et al., 2017). Syrian goat breeds are usually susceptible to PPH (Fig. 12). Hemoglobinuria is often a premonitory clinical manifestation before depression, inappetence, anemia, or decreased milk yield is noted (Resum et al., 2017). When anemia is detected, the mucous membranes appear pale and may become yellowish. Additionally, when the heart rate increases to 80–130 beats/minutes, respiration becomes shallow and rapid, diseased cows are weak and recumbent, and forced training at this time may be lethal. The feces may be dry, firm, and bile-stained or diarrheic and fetid. An elevated rectal temperature of up to 40°C in the early stages and intense thirst are variable features. The acute condition (3–5 days) can end in death or be followed by long convalescence of 2–8 weeks. Depraved appetite and ketonuria may occur during the recovery stage (Saed Albayati and Luaibi, 2020). Sloughing of the extremities and gangrene are sequelae (Saed Albayati and Luaibi, 2020).
Fig. 11. Cardiac troponin I values in downer camels compared with control healthy camels. a,b different letters indicate a significant difference (p=0.019). SD=standard deviation.
Fig. 12. Red urine of Syrian goats (A, C, and E). Figure B: Hemolytic RBCs. Image D clearly shows red urine and image F shows red serum in these cases. HypomagnesemiaHypomagnesemia or grass tetany (GT) is a nutritional and/or metabolic disorder of ruminant animals that is found in temperate regions with wet and cold climates, where grazed pastures are the principal source of diets for beef cattle (Tharwat, 1999). GT is linked to upgraded pastures and occurs when animals under intensive practices feed on fast-growing grass (Doncel et al., 2021). Despite the considerable advances in the prevention and pathogenesis of GT, several questions remain unanswered (Martens et al., 2018). The disease mainly affects lactating (lactation tetany) (Doncel et al., 2019) and pregnant dairy cattle fed lush, rapidly-growing grasses such as wheat, oat, or ryegrass. GT also affects pregnant and lactating beef cattle (Dutra, 2009, 2010). Weaned or suckling calves may rarely be influenced by a deficiency of dietary magnesium (Doncel et al., 2021).
Fig. 13. Hypomagnesemic tetany in one sheep and three goats from the core toward the anode, forming the tail of a comet (B). The primary clinical signs of hypomagnesemia include incoordination, hyperexcitability, and nervous seizures. The animals are observed grazing and abruptly adopting an abnormal posture and appear restless with following facial muscle spasms, hyperesthesia, tetany at the base of the ears, aggressiveness, spasticity, erected ears, and raised head. The signs quickly progress to tonic-colonic convulsions with prolonged relaxation. During seizure episodes, it is common to observe jaw chewing locomotion with grinding of the teeth, mouth, and nose foaming, opisthotonus, exaggerated movements of the eyeballs, and violent pedaling movements (Fig. 13). The animal may enter a coma between 6 and 10 hours after the primary clinical signs and eventually collapse (Dewell and Ensley, 2016). In subclinical types, most herds are affected even if severe cases have not been detected. The signs in calves with milk tetany are more intense than those in cows with subacute, acute, and per acute cases (Constable et al., 2017). MastitisAnimals with elevated milk yield are at high risk of mastitis (Ranjan et al., 2021). Mastitis primarily occurs in response to intramammary bacterial invasion, but also to intramammary fungal, mycoplasmal, and algal infections. Thermal and mechanical trauma and chemical insult may predispose the mammary gland to infection. The prevalence of disease in dairy cattle is comparatively high. Acute mastitis in bovines involves a primary phase that includes an inflammatory response followed by a resolution stage (Tharwat, 2011; Tharwat and Selim, 2015). In industrialized countries, clinical mastitis (CM) is the most common and costly disease. Progressive and moderate CMs always require antibiotic treatment and should be managed with consideration of animal well-being (Constable et al., 2017). The subclinical form of mastitis is the principal type in intensive dairy herds, reaching 20%–50% of dairy cows in a given herd (El-Zamkan and Mohamed, 2021; Alhafiz et al., 2022). Staphylococcus aureus and E. coli are the most serious pathogens causing mastitis in dairy animals (Abril et al., 2020; Ranjan et al., 2021). Tearing of the suspensory ligaments occurs commonly in adult dairy cows and evolves progressively over several years. The initial etiology is thought to be intense mammary edema at parturition, with severe weight on the udder leading to the breakdown of the mammary attachments, especially the median suspensory ligament. Thus, the teats on diseased cows are not perpendicularly aligned but present more laterally (Tharwat and Selim, 2015). When the tear of the suspensory ligament occurs in an acute manner, immediately before or after calving, the mammary gland drops sharply and is hard and swollen, the teats are directed laterally, and serum is discharged through the skin (Fig. 14). Pregnancy toxemiaPT is a metabolic disease of pregnant ewes and does, initiated by an altered metabolism of carbohydrates as well as fats, and is observed during the last phase of pregnancy (Tharwat and Al-Sobayil, 2014; Weaver et al., 2021; Tharwat et al., 2025). The disease has a higher incidence rate in those carrying more than one fetus, and it occurs more frequently in lean ewes than in those with a body condition score (BCS) of less than 2 or in obese animals with a BCS of 4 or over. Clinically, cases are typically limited to older female sheep and goats during second or subsequent pregnancies. In susceptible animals, PT generally follows a period of NEB that results in increased fat catabolism, hypoglycemia, ketonuria, and ketonemia (Rook, 2000; Scott, 2020). The disease manifests clinically as hypoglycemic encephalopathy. Affected animals show depression, anorexia, blindness, and neurological signs, followed by recumbency and coma (Menzies and Bailey, 1997). Laboratory findings of hypoketonemia/hyperketonuria and hypoglycemia (Brozos et al., 2011). Thus, increased serum ketone body concentrations and NEFA are associated with the disease, but the clinical signs are not apparent unless hypoglycemia is evident (Tharwat et al., 2025).
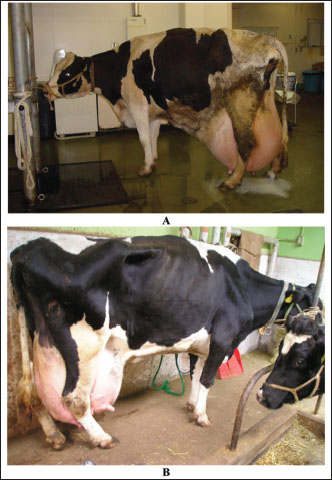
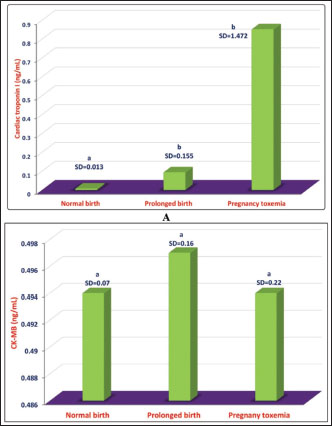
Fig. 14. Rupture of suspensory ligaments in two Holstein cows (A and B). Diagnosis of PT is based principally on history, clinical manifestations, and the appearance of ketones in the urine. In addition, various metabolic alterations develop following hyperketonemia and hypoglycemia. Important secondary differences include dehydration acidosis and renal insufficiency. Treatment is frequently unsuccessful, but repeated injection of small doses of glucose seems to be beneficial if other alterations, such as dehydration and acidosis, are controlled (Marteniuk, 1988; Tharwat et al., 2025). In healthy goats, the cardiac biomarker cTnI is not raised during pregnancy, but its concentration may be raised in a number of animals during vaginal or even cesarean delivery. On the other hand, the concentration of cTnI is sharply elevated in goats with PT; therefore, cTnI may be used as a prognostic marker in these cases. Only a slight elevation in the other cardiac biomarker, creatine kinase-myocardial band, was reported in goats with delayed birth is reported (Fig. 15) (Tharwat, et al., 2012e).
Fig. 15. Comparative mean ± standard deviation (SD) of cTnI in does with normal birth and prolonged birth and in those with PT. a,bdifferent letters indicate a significant difference (p < 0.05) (A). Image B shows comparative mean ± standard deviation (SD) values of CK-MB in does with normal birth, prolonged birth, and in those with PT. asame letter indicate a non-significant difference (p > 0.05). In conclusion, in farm animals, several PDs are encountered principally during TP, leading to decreased producer income, reduced consumer product quality, and impaired animal welfare. This review discusses several PDs that affect farm animals, including cattle, sheep camels, and goats. These diseases included FL, ketosis, sub-acute rumen acidosis, liver abscesses, CVCT, endocarditis, abomasal ulceration, abomasal displacement, milk fever, downer syndrome, postparturient hemoglobinuria, hypomagnesemia, mastitis, and PT. Because of the significant economic losses caused by PD, future directions should focus on the early detection and prevention of these diseases in farm animals. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Author contributionMT: conceptualization, data collection, and writing of the original manuscript draft. AA: sharing in the writing and editing of manuscript drafts. SO: review and editing. All authors have approved the final manuscript for publication. Data availabilityAll data supporting the findings of this review are available in the manuscript, and no additional data sources are required. ReferencesAbdela, N. 2016. Sub-acute ruminal acidosis (SARA) and its consequence in dairy cattle: a review of past and recent research at global prospective. Achiev. Life Sci. 10, 187–196. Abril, G.A., Villa, G.T., Barros-Velázquez, J., Cañas, B., Sánchez-Pérez, A., Calo-Mata, P. and Carrera, M. 2020. Staphylococcus Aureus exotoxins and their detection in the dairy industry and mastitis. Toxins 12, 537. Ahmed, A.F., Constable, P.D. and Misk, N.A. 2002. Effect of an orally administered antacid agent containing aluminum hydroxide and magnesium hydroxide on abomasal luminal pH in clinically normal milk-fed calves. J. Am. Vet. Med. Assoc. 220, 74–79. Ahmed, A.F., Constable, P.D., and Misk, N.A. 2005. Effect of orally administered omeprazole on abomasal luminal pH in dairy calves fed milk replacer. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52, 238–243. Alhafiz, G.A., Alghatam, F.H., Almohammed, H. and Hussen, J. 2022. Milk immune cell composition in dromedary camels with subclinical mastitis. Front. Vet. Sci. 9, 885523. Amachawadi, R.G. and Nagaraja, T.G. 2016. Liver abscesses in cattle: a review of incidence in Holsteins and of bacteriology and vaccine approaches to control in feedlot cattle. J. Anim. Sci. 94, 1620–1632. Amachawadi, R.G. and Nagaraja, T.G. 2022. Pathogenesis of liver abscesses in cattle. Vet. Clin. North Am. Food Anim. Pract. 38, 335–346. Bayer, A.S. and Scheld, W.M. 2000. Endocarditis and intravascular infections. In Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 5th ed. Eds., Mandell, G.L., Bennett, J.E. and Dolin, R. Philadelphia, PA: Churchill Livingstone. pp: 857–902. Bexiga, R., Mateus, A., Philbey, A.W., Ellis, K., Barrett, D.C. and Mellor, D.J. 2008. Clinicopathological presentation of cardiac diseases in cattle and its impact on decision making. Vet. Rc. 162, 575–580. Blowey, R.W. 2004. Digestive disorders of calves. In Bovine medicine: diseases and husbandry of cattle. 2nd ed. Eds., Andrews, A.H., Blowey, R.W., Boyd, H. and Eddy, R.G. Hoboken, NJ: Blackwell Science, pp: 231–238. Bobe, G., Young, J.W. and Beitz, D.C. 2004. Invited review: pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 87, 3105–3124. Braun, U. 2008. Clinical findings and diagnosis of thrombosis of the caudal vena cava in cattle. Vet. J. 175, 118–125. Braun, U. 2009. Traumatic pericarditis in cattle: clinical, radiographic and ultrasonographic findings. Vet. Rec. 182, 176–186 Braun, U., Fluckiger, M. and Pospischil, A. 2002. Diagnosis by ultrasonography of congestion of the caudal vena cava secondary to thrombosis in 12 cows. Vet. Rec. 150, 209–213. Braun, U., Nuss, K., Warislohner, S., Reif, C., Oschlies, C. and Gerspach, C. 2020. Diagnostic reliability of clinical signs in cows with traumatic reticuloperitonitis and abomasal ulcers. BMC Vet. Res. 16, 359. Brozos, C., Mavrogianni, VS. and Fthenakis, G.C. 2011. Treatment and control of peri-parturient metabolic diseases: pregnancy toxemia, hypocalcemia, hypomagnesemia. Vet. Clin. North Am. Food Anim. Pract. 27, 105–113. Buczinski, S., Francoz, D., Fecteau, G. and Difruscia, R. 2010a. A study of heart diseases without clinical signs of heart failure in 47 cattle. Can. Vet. J. 51, 1239–1246. Buczinski, S., Francoz, D., Fecteau, G. and Difruscia, R. 2010b. Heart disease in cattle with clinical signs of heart failure: 59 cases. Can. Vet. J. 51, 1123–1129. Buczinski, S, Rezakhani, A. and Boerboom, D. 2010c. Heart disease in cattle: diagnosis, therapeutic approaches and prognosis. Vet. J. 184, 258–263. Buczinski, S., Tolouei, M., Rezakhani, A. and Tharwat, M. 2013. Echocardiographic measurement of cardiac valvular thickness in healthy cows, cows with bacterial endocarditis, and cows with cardiorespiratory diseases. J. Vet. Cardiol. 15, 253–261. Buczinski, S., Tsuka, T. and Tharwat, M. 2012. The diagnostic criteria used in bovine bacterial endocarditis: a meta-analysis of 460 published cases from 1973 to 2011. Vet. J. 193, 349–357. Constable, P.D., Ahmed A.F. and Misk, N.A. 2005. Effect of suckling cow’s milk or milk replacer on abomasal luminal pH in dairy calves. J. Vet. Intern. Med., 19, 97–102. Constable, P.D., Hinchcliff, K.W., Done, S.H. and Gruenberg, W. 2017. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats, 11th ed. Philadelphia, PA: W.B Saunders Co Ltd. Constable, P.D., Miller, G.Y., Hoffsis, G.F., Hull, B.L. and Rings, D.M. 1992. Risk factors for abomasal volvulus and left abomasal displacement in cattle. Am. J. Vet. Res., 53, 1184–1192. DeGaris, P.J. and Lean, I.J. 2008. Milk fever in dairy cows: a review of pathophysiology and control principles. Vet. J. 176, 58–69. Dewell G. and Ensley S. 2016. Hypomagnesaemia (grass tetany) in beef cattle. PMR1020, Extension and Outreach, Iowa State University. Available via https://store.extension.iastate.edu/product/14522 Dirksen, G.U. 1994. Ulceration, dilatation, and incarceration of the abomasum in calves: clinical investigations and experiences. Bov. Pract. 28, 127–135. Doncel, B., Capelesso, A., Giannitti, F., Cajarville, C., Macías, M., Silveira, C., Costa, A.R. and Riet-Correa, F. 2019. Hipomagnesemia en ganado lechero en Uruguay. Pesq. Vet. Bras. 39, 564–572. Doncel, B., Puentes, J.D., Caffarena, R.D., Riet-Correa, F., Costa, R.A. and Giannitti, F. 2021. Hypomagnesemia in beef cattle1. Pesq. Vet. Bras. 41, e06826. Drackley, J.R. 1999. Biology of dairy cows during the transition period: the final frontier? J. Dairy Sci. 82, 2259–2273. Du, X., Chen, L., Huang, D., Peng, Z., Zhao, C., Zhang, Y., Zhu, Y., Wang, Z., Li, X. and Liu, G. 2017. Elevated apoptosis in the liver of dairy cows with ketosis. Cell Physiol. Biochem. 43, 568–578. Dutra, F. 2009. Tetania del transporte. Arch. Vet. Este. 2, 6–7. Dutra, F. 2010. Tetania del destete. Arch. Vet. Este. 2, 10–11. El-Zamkan, M.A. and Mohamed, H.M.A. 2021. Antimicrobial resistance, virulence genes and biofilm formation in Enterococcus species isolated from milk of sheep and goat with subclinical mastitis. PLoS One 16, e0259584. Enemark, J.M. 2008. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): a review. Vet. J. 176, 32–43 Fouda, T.A.H. 1995. Clinical and biochemical studies on the primary ruminal dysfunction in sheep. Ph. D. thesis, Ismailia, Egypt: Suez Canal University. Gao, X. and Oba, M. 2014. Relationship of severity of subacute ruminal acidosis to rumen fermentation, chewing activities, sorting behavior, and milk production in lactating dairy cows fed a high-grain diet. J. Dairy Sci. 97, 3006–3016. Garrett, E.F., Nordlund, K.V., Goodger, W.J. and Oetzel, G.R. 1997. A cross-sectional field study investigating the effect of periparturient dietary management on ruminal pH in early lactation dairy cows. J. Dairy Sci. 80(Suppl. 1), 169. Goff J.P. 2006. Major advances in our understanding of nutritional influences on bovine health. J. Dairy Sci. 8, 1292–1301 Goff, J.P. 2008. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 176, 50–57. Grummer, R.R. 1995. Impact of changes in organic nutrient metabolism on feeding the transition cow. J. Anim. Sci 73, 2820–2833. Grummer, R.R. 2008. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 176, 10–20 Grunberg, W. 2014. Treatment of phosphorus balance disorders. Vet. Clin. North Am. Food Anim. Pract. 30, 383–408. Grunberg, W., Mol, J.A. and Teske, E. 2015. Red blood cell phosphate concentration and osmotic resistance during dietary phosphate depletion in dairy cows. J. Vet. Intern. Med. 29, 395–399. Hallowell, G.D., Potter, T.J. and Bowen, I.M. 2007. Methods and normal values for echocardiography in adult dairy cattle. J. Vet. Cardiol. 9, 91–98. Harwood, J.P.P. 2003. Tackling the problem of the downer cow: cause, diagnosis and prognosis. Cattle Practice 11, 89–92. Hund, A. and Wittek, T. 2017. Abomasal ulcers in cattle. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere. 45, 121–128. Hund, A., Beer, T. and Wittek, T. 2016. Abomasal ulcers in slaughtered cattle in Austria. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere. 44, 279–285. Ikawa, H., Narushima, T. and Kohno, T. 1987. Bacteriology of caudal vena caval thrombosis in slaughter cattle. Vet. Rec. 120, 184–186. Karchmer, A.W. 2005. Infective endocarditis. In Braunwald’s heart disease a textbook of cardiovascular medicine. 7th edn. Eds., Zipes, D.P., Libby, P., Bonow, R.O. and Braunwald, E. Philadelphia, PA: Elsevier-Saunders, pp: 1633–1655. Krause, K.M. and Oetzel, G.R. 2005. Inducing subacute ruminal acidosis in lactating dairy cows. J. Dairy Sci. 88, 3633–3639. Labonté, J., Dubuc, J., Roy, J.P. and Buczinski, S. 2018. Prognostic value of cardiac troponin I and L-lactate in blood of dairy cows affected by downer cow syndrome. J. Vet. Intern. Med. 32, 484–490. Lean, I.J., Van Saun, R. and Degaris, P.J. 2013. Energy and protein nutrition management of transition dairy cows. Vet. Clin. North Am. Food Anim. Pract. 29, 337–366. Marteniuk, J.V. 1988. Pregnancy toxemia and ketosis of ewes and does. Vet. Clin. North Am. Food Anim. Pract. 4, 30. Martens, H., Leonhard-Marek, S., Röntgen, M. and Stumpff, F. 2018. Magnesium homeostasis in cattle: absorption and excretion. Nut. Res. Rev. 31, 114–130. Menzies, P.I. and Bailey, D. 1997. Diseases of the periparturient ewe. In Current therapy in large animal theriogenology. Ed. Youngquist, R.S. Philadelphia, PA: WB Saunders; pp: 639–643. Mohamed, T., Oikawa, S., Iwasaki, Y., Mizunuma, Y., Takehana, K., Endoh, D., Kurosawa, T. and Sato, H. 2004a. Metabolic profiles and bile acid extraction rate in the liver of cows with fasting-induced hepatic lipidosis. J. Vet. Med. Sci. A 51, 113–118. Mohamed, T., Oikawa, S., Kurosawa, T., Takehana, K., Hosaka, Y., Okada, H., Koiwa, M. and Sato, H. 2004b. Focal fatty liver in a heifer: utility of ultrasonography in diagnosis. J. Vet. Med. Sci. 66, 341–344. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2004c. Ultrasonographic localisation of thrombi in the caudal vena cava and hepatic veins in a heifer. Vet. J. 168, 103–106. Morgante, M., Stelletta, C., Berzaghi, P., Gianesella M. and Andrighetto I. 2007. Subacute rumen acidosis in lactating cows: an investigation in intensive Italian dairy herds. J. Anim. Physiol. Anim. Nutr. 91, 226–234. Mulligan, F.J. and Doherty, M.L. 2008. Production diseases of the transition cow. Vet J. 176, 3–9. Nagaraja, T.G. and Lechtenberg, K.F. 2007. Liver abscesses in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23, 351–369. Oikawa, S., Mizunuma, Y., Iwasaki, Y. and Tharwat, M. 2010. Changes of very low-density lipoprotein concentration in hepatic blood from cows with fasting-induced hepatic lipidosis. Can. J. Vet. Res. 74, 317–320. Osada, S., Chisato, K., Fukumori, R. and Oikawa, S. 2024. Comparison of serum very low-density lipoprotein concentrations during transition in primiparous and multiparous cows. J. Vet. Med. Sci. 86, 358–362. Ospina, P.A., McArt, J.A., Overton, T.R., Stokol, T. and Nydam, D.V. 2013. Using nonesterified fatty acids and β-hydroxybutyrate concentrations during the transition period for herd-level monitoring of increased risk of disease and decreased reproductive and milking performance. Vet. Clin. North Am. Food Anim. Pract. 29, 387–412. Plaizier, J.C., Krause, D.O., Gozho, G.N. and Mc Bride, B.W. 2008. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet. J. 176, 21–31 Post, K.W., Rushton, S.D. and Billington, S.J. 2003. Valvular endocarditis associated with Helcococcus ovis infection in a bovine. J. Vet. Diagn. Invest. 15, 473–475. Puerto-Parada, M., Bilodeau, M.È., Francoz, D., Desrochers, A., Nichols, S., Babkine, M., Arango-Sabogal, J.C. and Fecteau, G. 2021. Survival and prognostic indicators in downer dairy cows presented to a referring hospital: a retrospective study (1318 cases). J. Vet. Intern. Med. 35, 2534–2543. Pullen, D.L., Liesman, J.S. and Emery, R.S. 1990. A species comparison of liver slice synthesis and secretion of triacylglycerol form nonesterified fatty acids in media. J. Anim. Sci. 68, 1395–1399. Ranjan, R., Narnaware, S.D. and Prakash, V. 2021. Incidence, risk factors and economic impact of clinical mastitis in dromedary camel (Camelus dromedarius). Trop. Anim. Health Prod. 54, 31. Reed, W.D.C., Elliot, R.C. and Topps, J.H. 1995. Phosphorus excretion of cattle fed on high energy diets. Nature 208, 953–954. Resum, N.S., Kour, P., Singh, H. and Sharma, N. 2017. Post-partum hemoglobinuria (PPH) in bovine. Theriogenol. Insight. 7, 51. Rezakhani, A. and Zarifi, M. 2007. Auscultatory findings of cardiac murmurs in clinically healthy cattle. Online J. Vet. Res. 11, 62–66. Rook, J.S. 2000. Pregnancy toxemia of ewes, does, and beef cows. Vet. Clin. North Am. Food Anim. Pract. 16, 293–317. Saborío-Montero, A., Vargas-Leitón, B., Romero-Zúñiga, J.J. and Camacho-Sandoval, J. 2018. Additive genetic and heterosis effects for milk fever in a population of Jersey, Holstein × Jersey, and Holstein cattle under grazing conditions. J. Dairy Sci. 101, 9128–9134. Saed Albayati, O.A.S. and Luaibi, O.K. 2020. Study of hemato-biochemical changes in post-parturient hemoglobinurea in Iraqi local cows. Plant Arch. 20, 584–588. Satoh, H., Chisato, K., Fukumori, R., Tharwat, M. and Oikawa, S. 2024. A case–control study on the usefulness of serum lecithin:cholesterol acyltransferase activity as a predictor of retained placenta in close-up dairy cows. Animals 14, 3640. Scott, P.R. 2020. Sheep medicine, 2nd ed. Boca Raton, FL: CRC Press. Selim, H. and Tharwat, M. 2006. Clinical and laboratory investigations in dairy cows affected with sub-acute ruminal acidosis. In Proceedings of the 12th Veterinary Medical Conference, Faculty of Veterinary Medicine, Assiut University, Asyut, Egypt. pp: 337–355. Serrenho, R.C., Williamson, M., Berke, O., LeBlanc, S.J., DeVries, T.J., McBride, B.W. and Duffield, T.F. 2022. An investigation of blood, milk, and urine test patterns for the diagnosis of ketosis in dairy cows in early lactation. J. Dairy Sci. 105, 7719–7727. Shen, T., Xu, F., Fang, Z., Loor, J.J., Ouyang, H.O., Chen, M., Jin, B., Wang, X., Shi, Z., Zhu, Y, Liang, Y., Ju, L., Song, Y., Wang, Z., Li, X., Du, X. and Liu, G. 2021. Hepatic autophagy and mitophagy status in dairy cows with subclinical and clinical ketosis. J. Dairy Sci. 104, 4847–4857. Sickinger, M. 2017. Abomasal displacement in cattle short overview of recent research results. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere. 45, 187–190. Simpson, K.M., Streeter, R.N., Cramer, S., Lamm, C.G. and Love, B.C. 2012. Caudal vena caval thrombosis following treatment of deep digital sepsis. Can. Vet. J. 53, 182–186. Skotnicka, E., Muszczyński, Z. and Suska, M. 2011. Effect of the periparturient period on serum lipid and cholesterol lipoprotein concentrations in goats (capra hircus). Acta Vet. Hung. 59, 445–454. Steeneveld, W., Amuta, P., van Soest, F.J.S., Jorritsma, R. and Hogeveen, H. 2020. Estimating the combined costs of clinical and subclinical ketosis in dairy cows. PLoS One 15, e0230448. Stockdale, C.R., Moyes, T.E. and Dyson, R. 2005. Acute post-parturient haemoglobinuria in dairy cows and phosphorus status. Aust. Vet. J. 83, 362–366. Tadepalli, S., Narayanan, S.K., Stewart, G.C., Chengappa, M.M. and Nagaraja, T.G. 2009. Fusobacterium necrophorum: a ruminal bacterium that invades liver to cause abscesses in cattle. Anaerobe 15, 36–43. Tharwat, M. 1999. Some studies on hypomagnesemia in calves under natural and experimental conditions. Master Thesis, Zagazig, Egypt, Zagazig University. Tharwat, M. 2010. Diagnostic ultrasonography in cattle with caudal vena cava thrombosis. J. Agri. Vet. Sci. 3, 111–125. Tharwat, M. 2011. Accelerated neutrophil apoptosis in cows affected with acute mastitis. J. Agri. Vet. Sci. 4, 125–134. Tharwat, M. 2012a. The cardiac biomarker troponin I and other hematological and biochemical variables in downer camels (Camelus dromedarius). J. Camel Pract. Res. 19, 123–128. Tharwat, M. 2012b. Ultrasonography as a diagnostic and prognostic approach in cattle and buffaloes with fatty infiltration of the liver. Pol. J. Vet. Sci. 15, 83–93. Tharwat, M. 2020. Serum concentration of bone metabolism biomarkers in goats during the transition period. Vet. Med. Intern. 2020, 4064209. Tharwat, M. and Ahmed, A.F. 2012. Abomasal ulceration in buffaloes and cattle: clinico-biochemical and pathological findings. J. Anim. Vet. Adv. 9, 1327–1331. Tharwat, M. and Al-Sobayil, F. 2014. Cord and jugular blood acid–base and electrolyte status and haematobiochemical profiles in goats with naturally occurring pregnancy toxaemia. Small Rumin. Res. 117, 73–77. Tharwat, M. and Al-Sobayil, F. 2015. Serum concentrations of acute phase proteins and bone biomarkers in female dromedary camels during the periparturient period. J. Camel Pract. Res. 22, 271–278. Tharwat, M. and Buczinski, S. 2011. Clinicopathological findings and echocardiographic prediction of the localisation of bovine endocarditis. Vet. Rec. 169, 180. Tharwat, M. and El Magawry, S. 2008. Assessment of serum biochemical parameters for predicting whether downer cows will recover. In Proceedings of Veterinary Medical Conference, Faculty of Veterinary Medicine, Port Said, Egypt, Zagazig University. Tharwat, M. and Selim, L. 2015. Apoptosis of blood polymorphonuclear leukocytes in cows with acute mastitis. Global Vet. 14, 112–117. Tharwat, M., Ali, A., Al-Sobayil, F. and Abbas, H. 2015a. Hematobiochemical profiles in female camels (Camelus dromedaries) during the periparturient period. J. Camel Pract. Res. 22, 101–106. Tharwat, M., Ali, A. and Al-Sobayil, F. 2015b. Hematological and biochemical profiles in goats during the transition period. Comp. Clin. Pathol. 24, 1–7. Tharwat, M., Alkhedhairi, S., Saadeldin, I.M. and Gomaa, N. 2024a. Metabolic and hematological biomarkers alterations during the transition period in healthy farm animals: a review. Int. J. Vet. Sci. 13, 962–969. Tharwat, M., Alkhedhairi, S. and El Tigani-Asil, E.T.A. 2024b. Clinical predictive significance of biomarker molecules elevation during the transition period in cattle suffering from different pathological states: a review. Open Vet. J. 14, 1345–1357. Tharwat, M., Alkheraif, A.A. and Marzok, M. 2025. Pregnancy toxemia in small ruminants: clinical, sonographic, hematobiochemical and pathologic findings. Int. J. Vet. Sci.14, 204–211. Tharwat, M., Endoh, D. and Oikawa, S. 2012a. DNA damage in peripheral blood mononuclear cells and neutrophils of dairy cows during the transition period. Open Vet. J. 2, 65–68. Tharwat, M., Oikawa, S. and Buczinski, S. 2012b. Ultrasonographic prediction of hepatic fat content in dairy cows during the transition period. J. Veterinar. Sci. Technolo. 3, 1. Tharwat, M., Takamizawa, A., Hosaka, Y.Z., Endoh, D. and Oikawa, S. 2012c. Hepatocyte apoptosis in dairy cattle during the transition period. Can. J. Vet. Res. 76, 241–247. Tharwat, M., Endoh, D. and Oikawa, S. 2012d. Hepatocyte apoptosis in dairy cows with fatty infiltration of the liver. Res. Vet. Sci. 93, 1281–1286. Tharwat, M., Al-Sobayil, F. and Al-Sobayil, K. 2012e. The cardiac biomarkers troponin I and CK-MB in nonpregnant and pregnant goats, goats with normal birth, goats with prolonged birth, and goats with pregnancy toxemia. Theriogenology 78, 1500–1507. Weaver, L.F., Boileau, M.J., Gilliam, L.L. and Taylor, J.D. 2021. Characterization of short- and long-term morbidity and mortality of goat kids born to does with pregnancy toxemia. J. Vet. Intern. Med. 35, 1155–1163. Zerbin, I., Lehner, S. and Dist, O. 2015. Genetics of bovine abomasal displacement. Vet. J. 204, 17–22. Zhang, G., Dervishi, E. and Ametaj, B.N. 2018. Milk fever in dairy cows is preceded by activation of innate immunity and alterations in carbohydrate metabolism prior to disease occurrence. Res. Vet. Sci. 117, 167–177. Zhang, G., Hailemariam, D., Dervishi, E., Goldansaz, S.A., Deng, Q., Dunn, S.M. and Ametaj, B.N. 2016. Dairy cows affected by ketosis show alterations in innate immunity and lipid and carbohydrate metabolism during the dry off period and postpartum. Res. Vet. Sci. 107, 246–256. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Alkheraif AA, Oikawa S. Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview. Open Vet. J.. 2025; 15(1): 18-34. doi:10.5455/OVJ.2025.v15.i1.3 Web Style Tharwat M, Alkheraif AA, Oikawa S. Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview. https://www.openveterinaryjournal.com/?mno=227512 [Access: September 04, 2025]. doi:10.5455/OVJ.2025.v15.i1.3 AMA (American Medical Association) Style Tharwat M, Alkheraif AA, Oikawa S. Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview. Open Vet. J.. 2025; 15(1): 18-34. doi:10.5455/OVJ.2025.v15.i1.3 Vancouver/ICMJE Style Tharwat M, Alkheraif AA, Oikawa S. Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview. Open Vet. J.. (2025), [cited September 04, 2025]; 15(1): 18-34. doi:10.5455/OVJ.2025.v15.i1.3 Harvard Style Tharwat, M., Alkheraif, . A. A. & Oikawa, . S. (2025) Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview. Open Vet. J., 15 (1), 18-34. doi:10.5455/OVJ.2025.v15.i1.3 Turabian Style Tharwat, Mohamed, Abdulrahman A. Alkheraif, and Shin Oikawa. 2025. Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview. Open Veterinary Journal, 15 (1), 18-34. doi:10.5455/OVJ.2025.v15.i1.3 Chicago Style Tharwat, Mohamed, Abdulrahman A. Alkheraif, and Shin Oikawa. "Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview." Open Veterinary Journal 15 (2025), 18-34. doi:10.5455/OVJ.2025.v15.i1.3 MLA (The Modern Language Association) Style Tharwat, Mohamed, Abdulrahman A. Alkheraif, and Shin Oikawa. "Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview." Open Veterinary Journal 15.1 (2025), 18-34. Print. doi:10.5455/OVJ.2025.v15.i1.3 APA (American Psychological Association) Style Tharwat, M., Alkheraif, . A. A. & Oikawa, . S. (2025) Production diseases in farm animals: A comprehensive and illustrated clinical, laboratory, and pathological overview. Open Veterinary Journal, 15 (1), 18-34. doi:10.5455/OVJ.2025.v15.i1.3 |