| Research Article | ||
Open Vet. J.. 2025; 15(1): 348-387 Open Veterinary Journal, (2024), Vol. 15(1): 348-387 Research Article Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interfaceAlbert Neil G. Dulay1, Richelle Mae E. Padilla2, Nyzar Mabeth O. Odchimar1 and Fredmoore L. Orosco1,3,4*1Department of Science & Technology, Virology and Vaccine Research Program, Industrial Technology Development Institute, Taguig, Philippines 2National Institute of Molecular Biology and Biotechnology, College of Science, University of the Philippines – Diliman, Quezon City, Philippines 3Department of Science and Technology, S&T Fellows Program, Taguig, Philippines 4Department of Biology, University of the Philippines, Manila, Philippines *Corresponding Author: Fredmoore L. Orosco. Department of Science & Technology, Virology and Vaccine Research Program, Industrial Technology Development Institute, Taguig, Philippines. Email: orosco.fredmoore [at] gmail.com Submitted: 04/11/2024 Accepted: 09/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
AbstractBackground: African swine fever (ASF) continues to hamper pork production worldwide. In addition to vaccines, there is a need to develop curative therapeutics, such as highly-specific antibodies or nanobodies (NB), against immunogenic proteins with important functions such as the viral CD2 (CD2v)-like protein. However, further characterization of CD2v, such as its binding with cellular partners, is needed for the rational development of such biologics. Aim: We aimed to analyze the binding interaction of CD2v from pandemic genotypes I and II and swine CD58 using molecular simulations and optimize the NB to target the conserved epitope around the identified binding site using a computational pipeline. Methods: CD2v-CD58 complexes and equivalents from swine and humans were generated and simulated to characterize their binding. Mapping of residue contributions to the binding, variability, epitope propensity, and epitope multiplicity allowed us to select a conserved region near the site. Optimized NB were developed from sequences deposited in the NanoLAS repository, following screening for physicochemical and reactogenic parameters, docking, iterative optimization, and molecular dynamics simulations compared to controls. Results: Simulations report poor affinity of CD2v to CD58 in the “canonical” pose and present a novel pose backed by docking, simulation data, and recent observations. The new site might have developed due to immunological considerations and may reproduce observed lymphocyte non-proliferation by hijacking CD58-CD2 ruler functions in immunological synapse formation. Two NB were also developed targeting genotype I and II CD2vs with stable binding and better affinity, solubility, allergenicity, and antigenicity than the controls NB22 and 18a3. Conclusion: Herein, we showcase the power of computational biology techniques to elucidate ASF virus (ASFV)-host interactions and simultaneously design therapeutics to target the identified novel binding site. Further validation is needed to confirm these findings; however, future work on other relevant ASFV proteins may lead to potent cocktails with therapeutic potential. Keywords: CD2v, Computational biology, Site-directed mutagenesis, Antiviral, ASFV. IntroductionAfrican swine fever (ASF) is a fatal hemorrhagic disease that affects swine. The causative agent, ASF virus (ASFV), is an icosahedral nucleocytoplasmic large dsDNA virus belonging to the family Asfarviridae. The disease is highly contagious, causes high mortality, and has impacted pork production worldwide (Simbulan et al., 2024). Thus far, therapeutics against ASF have mostly focused on vaccines, although there is still no widely approved candidate for use (Guarascio et al., 2023). Immunity against ASFV is partially mediated by neutralizing antibodies, delaying disease onset, and offering some protection against viral challenges (Schlafer et al., 1984; Onisk et al., 1994; Escribano et al., 2013; Gaudreault and Richt, 2019). Several ASFV proteins are antibody-inducing (Gaudreault and Richt, 2019), with antibodies mainly raised against p22/pKP177R (outer envelope protein), p54/pE183L (dynein-binding virion trafficking protein), and p30/pCP204L (inner membrane RNAse phosphoprotein) (Escribano et al., 2013; Xu et al., 2023; Nandy et al., 2024). However, only antibodies against viral attachment and internalization confer neutralization. Antibodies against p54, CD2v/pEP402R (hemagglutinin), and p72/pB646L (major capsid protein) block viral attachment, while antibodies against p30 block internalization (Escribano et al., 2013). CD2v is one of the few outer-membrane proteins identified in ASFV in the early 1990s (Rodríguez et al., 1993; Borca et al., 1994; Gao et al., 2023). In mammals, CD2 is a conserved co-stimulatory glycoprotein for T-cell (TC) activation and differentiation, previously identified as one of the etiological causes of erythrocyte rosette formation by T-lineage cells (Meuer et al., 1984; Peterson and Seed, 1987; Plunkett et al., 1987; Recny et al., 1990; Binder et al., 2020). The glycoprotein primarily functions in TC antigen-presenting cell (APC) sensing and the formation of immunological synapses (IS) (Binder et al., 2020). CD2 appears to stabilize IS formation in TCs and NK cells by either directly supporting inter-cell adhesion, recruiting relevant IS proteins, or restricting the access of inhibitory molecules to the IS (Binder et al., 2020). Activation of CD2 appears to affect similar downstream signaling pathways of TCR/CD3 and CD28, additively contributing to TC stimulation (Binder et al., 2020). In ASFV, viral CD2 (CD2v) has also been associated with erythrocyte rosette formation but is considered to primarily function in the invasion of the virus to lymphoid tissue (Rodríguez et al., 1993; Borca et al., 1994; Borca et al., 1998; Rowlands et al., 2009; Gao et al., 2023; Pérez-Núñez et al., 2023). CD2v also appears to inhibit lymphocyte proliferation, directly contributes to delayed apoptosis, and indirectly leads to upregulation of interferon-stimulated genes in macrophages (Borca et al., 1998; Chaulagain et al., 2021; Gao et al., 2023). CD2v also seems indispensable for the efficient reproduction of ASFV in soft-tick vectors (Rowlands et al., 2009). The viral protein has a molecular weight similar to that of non-glycosylated human CD2 (hCD2) (~40 kDa) (Binder et al., 2020; Chaulagain et al., 2021; Pérez-Núñez et al., 2023). However, N-glycosylation appears to be more extensive in CD2v than in hCD2, which can increase the mass of the former by more than 100% (Borca et al., 1994; Chaulagain et al., 2021; Pérez-Núñez et al., 2023). Similar to many virally co-opted cellular proteins, ASFV CD2v shares low sequence homology with mammalian CD2s (~25%), although the single-pass transmembrane two-domain immunoglobulin (Ig) with a long unstructured cytoplasmic tail structure appears to be conserved (Borca et al., 1994). Despite low homology, as in the case of hCD2, CD58 or LFA-3 still seems to be the main target of the viral protein (Borca et al., 1998; Chaulagain et al., 2021). Development of biologic-based therapeutics, such as antibodies targeting CD2v, may help in the treatment of ASFV. Further adaptation of biologics for various effector functions aside from simply targeting CD2v may also be an avenue for further research. In addition to monoclonal antibodies, single-domain antibodies [or nanobodies (NB)] from sharks or camelids promise a greater potential for development due to their compactness, enhanced hydrophilicity, stability, and improved tissue penetration, especially in the brain and tumors (Del Rio et al., 2019; Pothin et al., 2020; Bathula et al., 2021; Sun et al., 2021). To facilitate nanobody development against ASFV CD2v, this study used relatively inexpensive computational methods to characterize CD2v-CD58 binding for the two pandemic genotypes of ASFV (I, II) (Simbulan et al., 2024). After defining the target region on the viral protein, potential nanobody scaffolds were then screened, and simultaneous docking with the genotype I & II CD2vs revealed the top scaffold for consideration. Combined optimization led to two designs that specifically targeted either genotype I or II CD2vs with improved binding affinity and physicochemical or reactogenic profiles compared to controls. Materials and MethodsRetrieval of sequences, alignment, conservation mappingAnnotated ASFV genomes (259) were retrieved from the National Center for Bioinformatics Information (https://www.ncbi.nlm.nih.gov/datasets/genome/) (Sayers et al., 2022). Proteins were clustered in cd-hit (Li and Godzik, 2006) to determine CD2v sequences, especially those from genotypes I and II (249) (see Table S1 for full list). Human (P06729) and swine CD2 (Q76N43) sequences were similarly acquired from UniProt (https://www.uniprot.org/) (The UniProt Consortium et al., 2023). Sequences were aligned through MUSCLE (Edgar, 2004) in MPI Bioinformatics Toolkit (https://toolkit.tuebingen.mpg.de/tools/muscle) (Gabler et al., 2020). Residue variability, such as Shannon Entropy, was retrieved from ProteoVision (https://proteovision.chemistry.gatech.edu/) (Penev et al., 2021). AlignmentViewer (https://alignmentviewer.org/) (Reguant et al., 2020) was used to visualize the alignment. The signal peptide and transmembrane regions were predicted using SignalP 6.0 (https://services.healthtech.dtu.dk/services/SignalP-6.0/) (Nielsen et al., 2024) and DeepTMHMM 1.0 (https://services.healthtech.dtu.dk/services/DeepTMHMM-1.0/) (Hallgren et al., 2022) respectively. Protein domains were annotated using InterPro (https://www.ebi.ac.uk/interpro/) (Paysan-Lafosse et al., 2023), whereas unidentified domains within the protein were matched to homologous proteins in FoldSeek (https://search.foldseek.com/) (Van Kempen et al., 2024), which were then used for annotation. For protein structural clustering, additional CD2 homolog sequences were retrieved for D. rerio ZGC113293 (A0A0R41WF6), and CD2s of E. europaeus (A0A1S2ZW88), O. degus (A0A6P3FP77), M. musculus (P08920), R. norvegicus (P08921), A. melanoleuca (G1LEY7), E. caballus (P37998), A. trivirgatus (A0A7D7B5Y4), and M. fascicularis (Q6SZ61). These were homologous to the ASFV I CD2v structure, with z-scores > 10, as used in a previous study (Nomburg et al., 2024). Representatives of ASFV VII (Malawi Lil-20/1: P0C9V9) and XXII (RSA-2-2008: QRW44656.1) were also included. For multimeric complex prediction, proteins known to interact with CD2v were putatively considered as components of the complex. Co-expressed proteins were also included based on STRING-DB (https://string-db.org/) (Szklarczyk et al., 2023), given the link between interaction and co-expression (Tirosh and Barkai, 2005). Proteins considered included AP-1, CSF2RA (A0A287BFJ1) (Pérez-Núñez et al., 2015; Gao et al., 2023), with CD28 (I3LKR6), CD48 (F1RJY9), CD84 (F1RJY5), and SAMSN identified through STRING-DB. Further analysis focused on proteins that will likely interact in the extracellular space limiting the use of AP-1 and SAMSN. An alternative set of proteins only co-expressed with CD58 from STRING-DB was also considered for multimeric complex prediction, such as ADGRE3 (I3L9N4), ART4 (K7GM93), and CIMAP2/LEXM. However, CIMAP2 was removed from consideration following an intracellular localization prediction from DeepTMHMM. Modelling of CD2, CD58 proteins, and structural clustering of CD2sUsing the reference sequences for CD2v of ASFV genotype 1 or CD2vi (AAA65288.1) and genotype 2 or CD2vii (CAD2068420.1), and known sequences for hCD2 (see above), CD58 (hCD58) (P19256), swine CD2 (sCD2) (see above), and CD58 (sCD58) (Q7YS50), non-glycosylated models were built using AlphaFold (AF) 3 (https://golgi.sandbox.google.com/) (Abramson et al., 2024). For complex prediction, human CD58-CD2, swine CD58-CD2, swine CD58-CD2vi, and swine CD58-CD2vii models were generated using AF 3 (designated AFhCD58-CD2, AFsCD58-CD2, AFsCD58-CD2vi, and AFsCD58-CD2vii, respectively). Alternative swine CD58-CD2vi and CD58-CD2vii models (designated AltCD58-CD2vi and AltCD58-CD2vii, respectively) were also generated to fit the observed canonical CD58-CD2 contact pose. To validate the models, the AFhCD58-CD2 complex was fitted against an experimental structure (PDB ID: 1QA9), while the latter five complexes were validated using ensemble protein-protein docks conducted in ClusPro (https://cluspro.org/home.php) (Kozakov et al., 2017), HawkDock (https://cadd.zju.edu.cn/hawkdock/) (Weng et al., 2019), and HDock (https://hdock.phys.hust.edu.cn/) (Yan et al., 2020) using conformations identified from 300 ns trajectories of the individual proteins in GROMACS [see Molecular dynamics (MD) simulations] following an adapted protocol (Othman et al., 2022). Clustering of the AF model with the top ten models from each server for each conformation combination was performed through pairwise- root-mean-square distance (RMSD) analysis. Additional models were constructed for CD2 structural clustering using AF 3 as described above. These included ZGC133293, the other CD2 homologs, additional CD2vs, and CD2vs already included in the initial analysis: BA71v, Lisbon 1957, NAMP/1995, K49 (I) Georgia 2007 (II), RSA-w1-1999 (IV), Ken06.Bus (IX), Ken05/Tk1, and Uvira B53 (X). Structural clustering of the models was performed using DALI (http://ekhidna2.biocenter.helsinki.fi/dali/) (Holm and Rosenström, 2010). Homology was confirmed when the median score was >8.0, as in a previous study (Nomburg et al., 2024). Multimeric prediction and analysisFor multimeric complex analysis, analysis was done similar for the expanded Izumo-Juno complex (Elofsson et al., 2024). Two sets of pairwise complex predictions were made. One for the CD2-CD58 co-expressed set (CD2, CD2v, CD58, CSF2RA, CD28, CD488, CD84), and the other for the CD58-only co-expressed set (CD2v, CD58, ADGRE3, ART4), with the average of the CD2vi and CD2vii prediction used as the CD2v result in building the interaction network. However, the minimum rank score (0.400 to 0.385) was modified to accommodate CD2v-CD58 interactions while minimizing superfluous interactions. Then, the likely complex interactors were remodeled into respective single complexes to determine their role and importance in the interaction. Rank-scores (rank), iPTM, pTM, and pDockQs from these were then used to compare between multimeric complexes and the original CD58-CD2v binary complexes. Viral and cellular CD2 epitope prediction and co-occurrence analysisFor ASFV II CD2v, known epitopes were sourced from literature (Jia et al., 2022; Jiang et al., 2022; Liu et al., 2022; Ren et al., 2022; Chang et al., 2023; Liu et al., 2023; Song et al., 2023; Fan et al., 2024). Known hCD2 epitopes were also retrieved from previous studies (Meuer et al., 1984; Peterson and Seed, 1987; Damschroder et al., 2004). Nevertheless, continuous and discontinuous B-lymphocyte epitopes have been predicted for human, swine, and mouse CD2, zebrafish ZGC113293, and CD2v from BA71v, Georgia 2007 RSA-w1-1999, Ken05/Tk1, and RSA-2-2004 strains using BepiPred 3.0 (https://services.healthtech.dtu.dk/services/BepiPred-3.0/) (Clifford et al., 2022), SVMTrip (http://sysbio.unl.edu/SVMTriP/) (Yao et al., 2020), ABCpred (https://webs.iiitd.edu.in/raghava/abcpred/index.html) (Saha and Raghava, 2007), BCEpred (https://webs.iiitd.edu.in/raghava/bcepred/bcepred_submission.html) (Saha and Raghava, 2004), LBTope (https://webs.iiitd.edu.in/raghava/lbtope/index.php) (Singh et al., 2015), Discotope (https://services.healthtech.dtu.dk/services/DiscoTope-3.0/) (Høie et al., 2024), and Ellipro (http://tools.iedb.org/ellipro/) (Ponomarenko et al., 2008). Revising a previous method (Assis et al., 2014), we defined epitope propensity (P) by residue (i) as the degree of consensus of hits across the different servers for a CD2 (Eq. 1). We also defined epitope multiplicity by residue (Ei) by calculating the degree of consensus of epitopes available in the literature (Eq. 2).
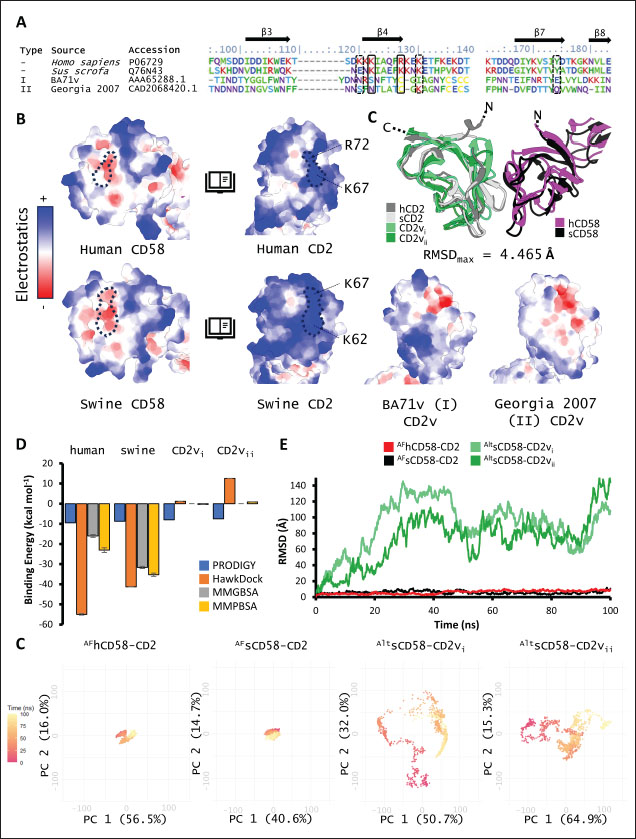
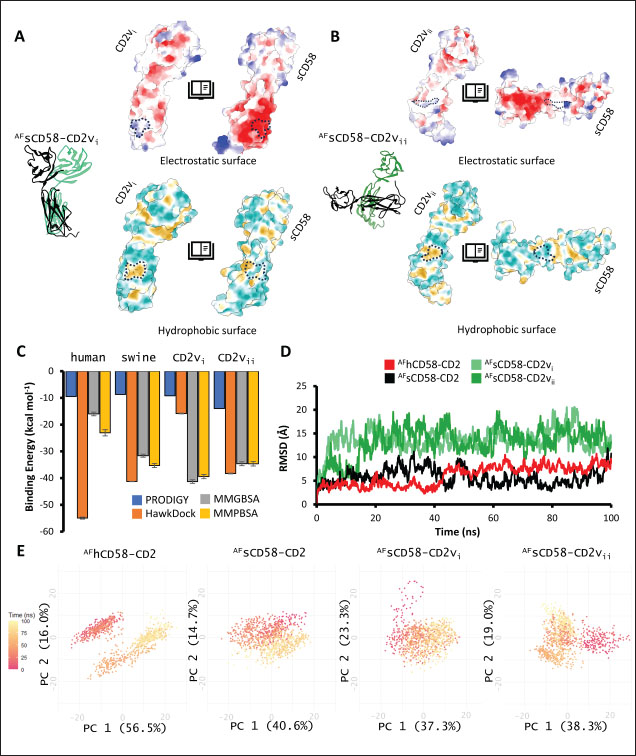
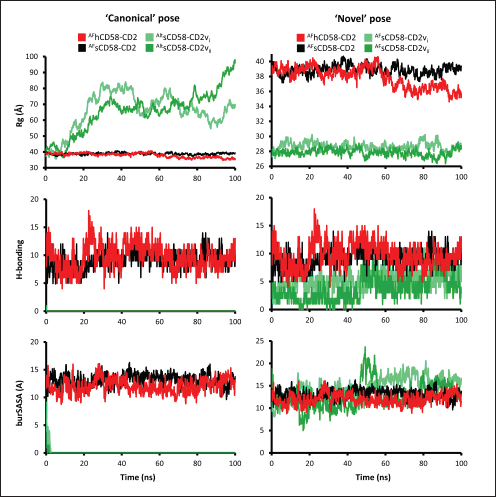
Correlation analysis for variability, epitope propensity, epitope multiplicity, and other data was performed using the Pearson correlation in the PerformanceAnalytics package in R. Clustering of epitope propensity across ZGC113293, selected mammalian and CD2vs was done through hierarchical clustering analysis (HCA) using correlation as distance metric implemented in the pheatmap package in R. Screening of nanobody scaffoldsNanobody sequences (30,667) were retrieved from the NanoLAS repository (https://www.nanolas.cloud/) (Xiong et al., 2024). Sequences were also processed in cd-hit to measure similarity to a nonredundant pool of 346 comparator NB with available PDB structures from the SAbDab-nano repository (https://opig.stats.ox.ac.uk/webapps/sabdab-sabpred/sabdab/nanobodies/) (Schneider et al., 2022). NB that clustered only with comparator NB bound to non-essential targets (e.g., plant and bacterial toxins, viral proteins) were then further filtered, as in previous studies (Panda et al., 2023; Poustforoosh et al., 2023) according to physicochemical parameters such as pI (>8), instability (<40) from ProtParam (https://web.expasy.org/protparam/) (Gasteiger et al., 2005), solubility (>0.45) from Protein-Sol (https://protein-sol.manchester.ac.uk/) (Hebditch et al., 2017), as well as reactogenic parameters such as toxicity (<0.6) from ToxinPred2 (https://webs.iiitd.edu.in/raghava/toxinpred2/batch.html) (Sharma et al., 2022), allergenicity (<0.3) from AlgPred2 (https://webs.iiitd.edu.in/raghava/algpred2/batch.html) (Sharma et al., 2021) and (Probably non-allergenic) from AllerTOP2 (https://www.ddg-pharmfac.net/AllerTOP/index.html) (Dimitrov et al., 2014), and antigenicity from VaxiJen v2.0 (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html) (Doytchinova and Flower, 2007). Only the top 25 NB were considered as scaffolds for model generation and scaffold determination. Determination of top scaffoldModels for the 25 scaffolds were generated in SWISS-MODEL (https://swissmodel.expasy.org) (Waterhouse et al., 2018) using the template method, with the closest comparator nanobody to the scaffold as a template. Model metrics were analyzed using ERRAT (>91), PROCHECK (>95 Ramachandran-favored) (https://saves.mbi.ucla.edu/) (Colovos and Yeates, 1993; Laskowski et al., 1993), and ProSA-web (Pass) (https://prosa.services.came.sbg.ac.at/prosa.php/) (Wiederstein and Sippl, 2007). Models were either refined in GalaxyRefine (https://galaxy.seoklab.org/cgi-bin/submit.cgi?type=REFINE) (Heo, et al., 2013) or re-generated in AF 3 (https://golgi.sandbox.google.com/) (Abramson et al., 2024) when the calculated model quality metrics were sub-optimal. ASFV I and II CD2v models were generated as described above. The scaffold NBs were then checked for simultaneous docking to both CD2v models in the conserved region near the newly identified binding site (ATCXINNTX4NET). Docking was conducted in ClusPro (https://cluspro.org/home.php) (Kozakov et al., 2017) with the docking set to antibody mode, and the analysis was limited to the first ten clustered docks. Docking was also conducted in HADDOCK 2.4 (https://rascar.science.uu.nl/haddock2.4/) (Dominguez et al., 2003), with restraints set to the conserved region and the CDRs identified per scaffold. Scaffolds were primarily filtered by looking at the presence of contacts between the CDR residues and the conserved region and existing clashes of the nanobody to CD58 in UCSF Chimera (Pettersen et al., 2004). The binding affinities of the docks of these candidates to each CD2v were predicted using PRODIGY (https://rascar.science.uu.nl/prodigy/) (Xue et al., 2016). The top scaffold was selected by considering the binding affinity and propensity of epitope-contacting docks in ClusPro and the concurrence of ClusPro and HADDOCK dock poses for both CD2v. Optimization of top scaffoldOptimization involved the identification of mutable residues in nanobody-CD2v (NB-CD2v) docks using (1) DrugScorePPI (https://cpclab.uni-duesseldorf.de/dsppi/main.php) (Krüger and Gohlke, 2010) for in silico alanine screening, (2) HawkDock (http://cadd.zju.edu.cn/hawkdock/) (Weng et al., 2019) for residue decomposition, (3) PDBePISA (https://www.ebi.ac.uk/pdbe/pisa/) (Krissinel and Henrick, 2007), and (4) InterProSurf (https://curie.utmb.edu/pdbcomplex.html) (Negi et al., 2007) for contact and binding interface analysis. The most suitable mutation for a particular mutable residue was determined using in silico site-directed mutagenesis in mCSM-AB2 (https://biosig.lab.uq.edu.au/mcsm_ab2/) (Myung et al., 2020), measuring ΔΔG. Suitable mutations were validated through the generation of nanobody mutants, following the assignment of potential mutations according to the appropriate Taguchi orthogonal array (Poustforoosh et al., 2023). The mutants were generated in UCSF Chimera using the rotamer addition plug-in (Shapovalov and Dunbrack, 2011), followed by repair in FoldX (Delgado et al., 2019) before solvated minimization in Groningen Machine for Chemical Simulations (GROMACS) (Abraham et al., 2015) for 50,000 steps or until convergence of the maximum force below 10 kJ mol−1. The binding energy of each mutant was determined using PRODIGY and HawkDock. The signal-to-noise ratio for each mutation was then calculated (Freddi and Salmon, 2019) for the finalization of mutations to be included in the optimized nanobody. The optimization may either be simple or dock-biased. For simple optimization, analysis of mutable residues was limited to CDR residues to improve binding to the CD2v site, with the top two mutations in terms of conferred ΔΔG selected for each residue prior to validation. For dock-biased optimizations, residues were mainly limited to the CDR regions, but the minimization of alternative docking poses to CD2vs was also considered. Mutable residues in the alternative docking poses were also included in the analysis, and determination of suitable mutations also considered whether the mutation conferred stability to the alternative poses. In this case, suitable mutations per residue were preselected from those that conferred a ΔΔG no lower than −0.5 kcal mol−1 towards the main pose directed to the conserved region, these mutations must also confer the most selective increase in binding affinity toward the main pose against alternative poses prior to validation. The optimized nanobody was then redocked to both CD2vs as before. The minimization of alternative poses by the optimized nanobody was monitored using ClusPro docking. Simple or dock-biased optimizations were repeated to establish an optimization series. The melting temperatures of the optimized NB were monitored against the expected temperatures (>55°C) in SCooP (http://babylone.3bio.ulb.ac.be/SCooP/index.php) (Pucci et al., 2017). MD simulations and binding energy calculationsMD simulations were performed using GROMACS version 2021. For ensemble docking, swine CD2, swine CD58, CD2vi, and CD2vii were prepared for 300 ns all-atom simulations using CHARMM 36 forcefield (Best et al., 2012). The same forcefield was used for 100 ns all-atom simulations of the CD2-CD58 models, NB-CD2v, and two antibody fragment-CD2v complexes of controls NB22, 18a3, and 7e12, respectively (Jia et al., 2022; Zhu et al., 2024), as well as NB-CD2v docks from optimization of the top scaffold. Antibody and nanobody complexes were additionally simulated in 3-µs coarse-grained simulations using the SIRAH 2.2 forcefield (Klein et al., 2023). All simulation boxes were padded as necessary and filled with the appropriate water model and ions (Cl− and Na+) to 0.15 M. For all-atom simulations, minimization was done through steepest descent for 50,000 streps or until the maximum force fell below 10 kJ mol−1 before 100 ps NPT and NVT ensembles and the production run (100 ns). For coarse-grained simulations, minimization was first performed on the backbone of the entire system, both through the steepest descent for 50,000 steps. This was followed by restrained (500 ps) then soft backbone-restrained NVT equilibrations (250 ps) and the production run (3 µs). The RMSD, root-mean-square fluctuation, radius of gyration, buried solvent-accessible surface area (burSASA) (Hollingsworth et al., 2016), number of H-bonds, and PCA of the complex backbone were calculated using the relevant commands in GROMACS. The binding energy was calculated using the trajectory from the 100-ns all-atom simulations using gmx_MMPBSA (Valdés-Tresanco et al., 2021). ResultsASFV CD2v likely has a non-canonical binding site with CD58For ease of docking and to reduce the box dimensions in the simulation, only the extracellular domains without the signal peptides or attached glycans of the protein were included in the analyses. Even then, the AFhCD58-CD2 complex and the experimental structure (PDB ID: 1QA9) agreed very well, within 0.647 A of each other, underscoring the usability of AF models in this study. However, while AFsCD58-CD2 was found to be similar to that of the AFhCD58-CD2 complex, the other AF models were not. To generate canonically bound forms, AltsCD58-CD2vi and AltsCD58-CD2vii were generated by fitting individual proteins to the pose observed in human and swine AF models. These models are presented in Figure 1. Initial analyses using electrostatic surface maps and residue conservation using the AF swine and human models as well as the alternative CD2v models suggested that the canonical CD58 binding site in CD2vi and CD2vii might be non-functional. CD2-CD58 interaction is primarily facilitated by hydrogen bonds and salt bridges formed at the interface, which can be easily disrupted by the intrusion of water molecules to the site (Wang et al., 2015). The alignment of residues that mediate these interactions in hCD2 with swine CD2 showed good conservation, which was not the case for CD2vs. The effects of non-conservation can be seen in the electrostatic maps of CD2vs as a lack of positively charged groups (i.e., equivalent to Lys62, Lys65, Lys67, and Arg72) that can accommodate the corresponding negative patch seen in human and swine CD58, which would be crucial in forming the necessary salt bridges for interaction (Fig. 2b). Given previous reports on the sensitivity of human CD58 to residues at the CD2 interface (Damschroder et al., 2004), extensive substitutions in CD2v compared to swine CD2 may not favor the former’s contact with swine CD58. Binding energy estimates from PRODIGY and HawkDock further indicated unfavorable binding in the fitted CD2v complexes (Fig. 2d). Specifically, PRODIGY and HawkDock results of the fitted CD2v complexes were weaker (PRODIGY CD2vi/CD2vii: −8.0/−7.5 kcal mol−1; HawkDock CD2vi/CD2vii: 1.2/12.6 kcal mol−1) than the human and swine models (PRODIGY hCD2/sCD2: −9.5/−8.7 kcal mol−1; HawkDock hCD2/sCD2: −55.0/−41.3 kcal mol−1), and were even positive in some cases. These results were confirmed in 100 ns MD simulations as shown in Figure. 2E and F. Results within the 100 ns simulation window replicated previous findings of stable hCD2-CD58 interactions, even without stabilizing glycan groups (Wang et al., 2015) as evidenced by a relatively stable H-bonding profile (Fig. S1). Furthermore, the residues that contributed at least −1 kcal mol−1 to the binding interaction were basic residues Lys75 and Arg72, and aromatic Tyr110, which were also considered crucial for binding (Wang et al., 2015).
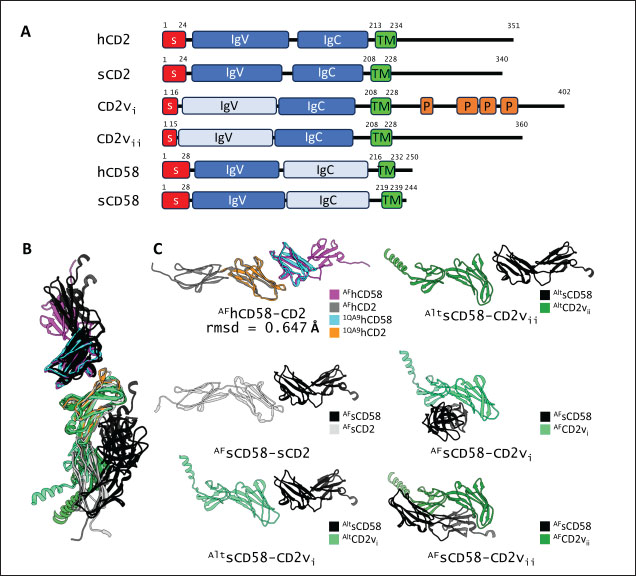
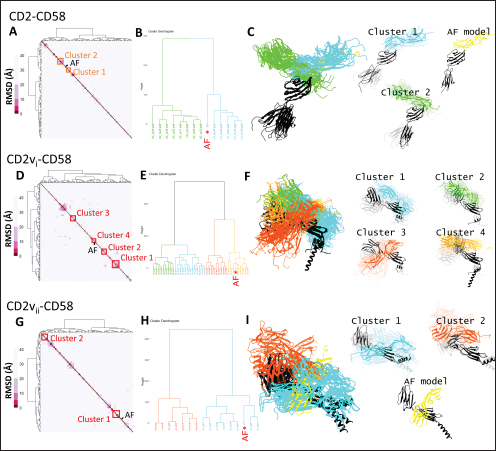
Fig. 1. CD58-CD2 models used in this study. (A) Annotation of CD2, CD58 proteins showing signal peptide (red), Ig domains (blue), transmembrane region (green), and proline-rich region (orange). (B) Superimposed models. (C) Models of CD58-CD2 complexes as generated in AF 3 (AFhCD58-CD2, AFsCD58-CD2, AFsCD58-CD2vi, AFsCD58-CD2vii) and based on refitting to the canonical CD58-CD2 complex (AltsCD58-CD2vi, AltsCD58-CD2vii). Concurrence between AF hCD58-CD2 model and experimental structure (PDB ID: 1QA9) is noted. Swine CD2-CD58 was also observed to stably interact within the time frame and similarly utilized equivalent residues Lys62, R67, and Tyr105 in binding to swine CD58. As expected, swine CD58 was observed to detach from the CD2vs early in the simulation (<2.0 ns). It has previously been noted that hCD2 binds to CD58 with low affinity (Binder et al., 2020). Using the simulation binding energy results from the corresponding MD simulations as a proxy for transient interactions, it may be that swine CD2 to CD58 binding is transient or slightly stronger than that observed in the human case. Furthermore, the observed results for CD2v binding in the canonical pose with swine CD58 further make it less likely to be correct as the calculated binding energies for these poses are almost negligible. To determine the likely CD58-CD2v interaction, an independent method was also used by adapting ensemble protein-protein docking (Othman et al., 2022) with three servers. The conformation ensemble for swine CD2, swine CD58, ASFV CD2vi, and ASFV CD2vii as generated through 300 ns all-atom MD simulations revealed 4, 1, 6, and 4 conformation(s) for the proteins respectively which were then used for docking. The top ten models from each server for each conformation combination were then compared to the respective AF models in HCA using pairwise RMSD as distances (Fig. S2).
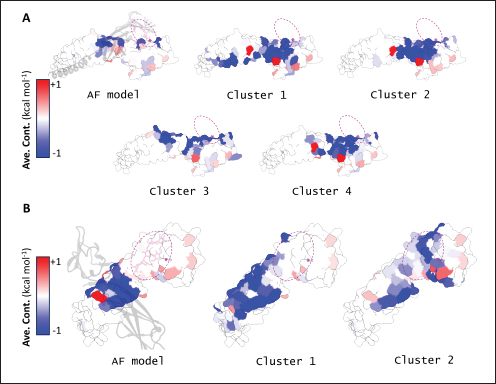
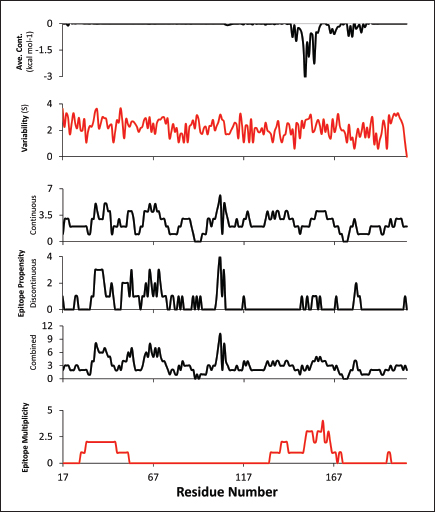
Fig. 2. Comparison of CD58-CD2 models’ binding in the context of canonical binding. (A) Alignment of CD2 sequences. Interacting residues of CD2 in interface bordered, with Lys67 and R72 in solid lines (B) Electrostatic maps of contacting surfaces, complementarily charged grooves highlighted in broken lines. (C) Fitting of CD58-CD2 domain in the interface. (D) Binding energy measurements of complexes in canonical binding poses. (E) Complex RMSD profiles of complexes in canonical binding poses. (F) Principal component plots based on 100 ns trajectories of complexes in canonical binding poses. Fitted complexes using CD2vi and CD2vii show weaker binding energies, bigger RMSD profiles, and increased spreading in PCA. For sCD58-CD2vi and sCD58-CD2vii, the AF models were found to group within corresponded dock clusters - composed of CD58-CD2 poses recapitulated across all three servers (Fig. S2d and g). Generally, in CD2v models, the sCD58 protein binds to the opposite side of the canonical binding site with considerable contact with the second Ig domain of CD2v. Meanwhile, AFsCD58-CD2 only grouped near one such cluster (Fig. S2a). The AF model was seen to be an outlier even when analyzed with the group closest to it (Cluster 1). In this sense, docking methods seem less likely to reproduce the canonical pose. Given the consistency between the AF model and experimental structure in the case of hCD58-CD2 and the poor matching observed for docking-generated models in sCD58-CD2, we considered the AF models to be more informative than those obtained from protein-protein docking. Nevertheless, the confluence of the separate methods for the novel poses seen in CD2vs strongly suggests the potential biological significance of these new binding poses. Further confirmation in 100 ns MD simulations showed that the proposed sCD58-CD2v poses were stable, as opposed to the canonical poses, as discussed previously (Fig. 3). The RMSD profiles of the AF swine CD58-CD2v complexes were delimited to approximately 15 Å compared with the observed 200 Å range for canonical poses (Fig. 3d). The scaling observed in the PC plots of the CD2v complexes was also more similar to the human and swine models (−20 to 20) than before (−100 to 100) (Fig. 3e). Finally, binding energy estimates from MMGBSA and MMPBSA (Fig. 3c) of the new poses - AFsCD58-CD2vi (MMGBSA/MMPBSA: −41.32/−39.45 kcal mol−1) and AFsCD58-CD2vii (−34.63/−34.65 kcal mol−1) - were also more comparable to the human (−16.01/−23.05 kcal mol−1) and swine models (−31.65/−35.31 kcal mol−1) as opposed to what was observed for the fitted CD2vi (0/−0.34 kcal mol−1) and CD2vii (0/0.88 kcal mol−1) complexes. The new binding poses appeared to be facilitated by non-specific interactions between complementarily charged patches and adjacent hydrophobic patches, as shown in Figure 3A and B. Specifically, for CD2vi, binding with CD58 appears to be more motivated by hydrophobic contacts, such as Tyr144, Ile146, Thr150, and Ile151 from CD2vi and Tyr131, Leu144, Leu146, and Ala204 from sCD58 contributing to binding. Meanwhile, hydrogen bonding and salt bridges through residues Tyr140, Asp145, Thr146, Tyr150, and Asn152 from CD2vii and residues Lys41, Tyr34, Ser159, and Ser194 from CD58 appeared to be major contributors to binding in the sCD58-CD2vii system. As noted previously, much of the binding can be seen to occur through the second domain of CD2vs and swine CD58. This is in contrast to what is seen in the canonical pose that only utilizes the N-terminal domains of CD2 and CD58 proteins. Novel binding site might have evolved to distance away from antigenic N-terminal domain, disrupt CD2-CD58 ruler functionsWe further analyzed the novel binding site in terms of immunogenicity, particularly with regards to B-cell responses to identify any evolutionary pressure in its development. Similarly, since CD2-CD58 is crucial for proper lymphocyte activation and differentiation as part of the immunologic synapse (Binder et al., 2020), we also analyzed whether the new binding site could explain the lack of lymphocyte proliferation attributed to CD2v (Borca et al., 1998; Chaulagain et al., 2021). To further refine the location of the novel binding site, residue decomposition (ENE) of the sCD58-CD2v AF model was conducted using 100 ns all-atom trajectories. The variability of the residues (VAR) and their propensity to be B-cell epitopes (PROP) for genotype I and II cases were similarly mapped. As noted previously, the novel binding site is mostly contributed by residues in the second Ig domain of CD2v (Fig. 4b). The face of the interface was composed of a β-sheet composed of β-strands 11, 12, 14, and 15 (Fig. 4a). Notably, residues in β-strands 11 and 12 seem to contribute more to binding and have a higher epitope propensity compared to residues from β-strands 14 and 15 (Fig. 4e), forming a distinct region. However, as seen in the variability map and the sequence alignment, the more conserved residues are in β-strands 14 and 15 which are farther away. Interestingly, B-cell epitope propensity maps seem to indicate another high epitope propensity region in the first Ig domain. Previous studies on ASFV CD2v have also identified B-cell epitopes (Jia et al., 2022; Jiang et al., 2022; Liu et al., 2022; Ren et al., 2022; Chang et al., 2023; Liu et al., 2023; Song et al., 2023; Fan et al., 2024). As of this writing, 14 epitopes have been discovered (Table 1), with most epitopes determined using mouse studies. Most of the epitopes were also elicited using the full CD2v protein, although other preparations involving only the extracellular or intracellular domains, and even one preparation as a fusion protein, have been performed previously. Nevertheless, all studies to date have used an ASFV genotype II strain. Further mapping of the known epitopes to obtain the multiplicity per residue (EPI) (Fig. 4c) showed some similarity with epitope propensity data (Fig. 4f; Fig. S4). Indeed, epitope multiplicity seemed to be significantly correlated with epitope propensity (p < 0.01, Rs=0.24), albeit weakly. Furthermore, the correlation was more aligned with linear epitope propensity (CONT) than with discontinuous epitope propensity (DISC) (Rs=0.28 vs. Rs=0.09). This may reflect the methodology used in epitope studies that verified the epitopes via short peptide fragments. A slight correlation (Rs=0.25) was also observed for epitope propensity and variability, which may suggest difficulty in assigning pan-ASFV epitopes to CD2v. Furthermore, the epitope multiplicity data further support the existence of multiple sites in the CD2v protein that are targeted by antibodies, as suggested by the propensity data. At least two immunogenic sites, one in each Ig domain were observed.
Fig. 3. Non-canonical binding of sCD58 with CD2vs. Surface maps (electrostatics, hydrophobicity) between sCD58 and (A) CD2vi or (B) CD2vii from AF models. Complementary sites marked. (C) Binding affinity of CD2v AF models compared to human and swine models. (D) RMSD profile of AF models. (E) Principal component plots of AF model trajectories.
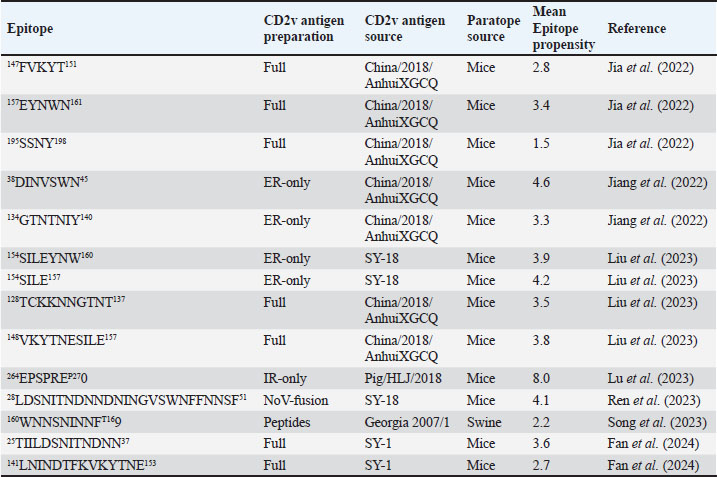
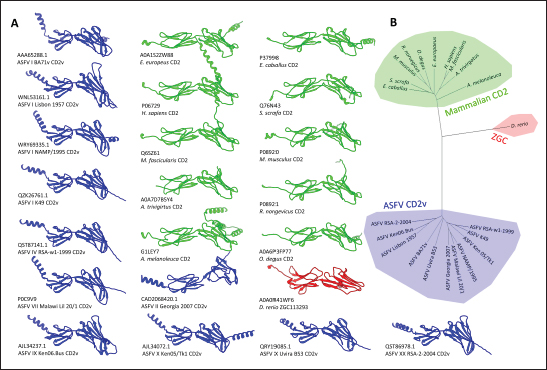
Fig. 4. Characterization of novel CD58 binding site of ASFV CD2v. (A) Structure of ASFV CD2v of genotype I (CD2vi) and II (CD2vii). The four β-strands contributing to the binding site colored accordingly. Visualization of (B) residue contribution to binding energy of CD2v with CD58, (C) epitope multiplicity, (D) residue variability, and (E) aggregate epitope propensity for both CD2vi and CD2vii based on continuous, discontinuous and combined predictions. (F) Correlation plot between average residue contribution to binding (ENE), residue variability (VAR), epitope propensity (CONT, DISC, PROP), & epitope multiplicity (EPI). (G) Alignment of ASFV CD2v sequences in binding site region. Most conserved residues in the site appear near β14 (dashed box). Metrics were primarily mapped onto CD2vi surface. Targeted conserved region marked in dotted lines. We further analyzed the conservation of the two immunogenic sites by comparing ASFV CD2v with other CD2 homologs (Fig. S5). First, results from structural clustering of CD2vs with mammalian CD2s and a CD2 homolog from Danio rerio (ZGC123293) show that ASFV CD2vs form a separate clade more distant than either the mammalian CD2s or zebrafish homolog. This reflects previous trends reported for cellularly-derived viral proteins exhibiting significant divergence from host sequences (Ravantti et al., 2020; Boys et al., 2023; Lomoio et al., 2023; Nomburg et al., 2024). An interesting result obtained from sequence clustering was the preservation of taxonomic relationships between mammals through CD2 homology. Specifically, ungulates, rodents, and primates clustered with each other. Nevertheless, broader relationships between these groups have not been recovered, with members from Euarchontoglires interspersed with those from Laurasiatheria (Upham et al., 2019). Taking this to mean that the structural clustering was more sensitive to closely allied species, it was surprising that (1) CD2vs from disparate genotypes were as diverse as those from different mammalian species, and that (2) several CD2vs from genotype I were clustered with more disparate strains than with each other. Notably, Lisbon 1957 clustered with genotype IX Ken06.Bus, NAMP1/1995 clustered with genotype II Georgia 2007 and genotype VII Malawi-Lil 20/1, and K49 clustered with genotypes IV (RSA-w1-1999) and X (Ken05/Tk1). As shown in Figure 8, the strains Lisbon 1957, NAMP1/1995, and K49 with Liv 5-40 and L60 also vary in sequence with the ASFV I representative CD2v sequence from BA71v. Of the 95 ASFV I CD2v sequences, 85 were identical to the BA71v sequence. In addition to the de novo emergence of new CD2v structures due to environmental pressures, recombination may be a means for how these ASFV I CD2vs have appeared, especially because they are more closely related to CD2vs from other genotypes than to each other. Of the two pandemic strains, ASFV I has been reported more frequently in sub-Saharan Africa, where most of the other genotypes have also been discovered (Njau et al., 2021). Nevertheless, even with co-infection by virtue of geographic proximity, viral recombination is more likely to occur when disparate strains are close to each other in terms of reproductive fitness (Chaikeeratisak et al., 2021). Table 1. Identified B-lymphocyte epitopes of CD2v from literature. Antigen preparation (Full protein or full, extracellular domain only or ER-only, intracellular domain only or IR-only, or as fusion to NoV capsid or NoV-fusion), source, paratope source, and mean epitope propensity indicated.
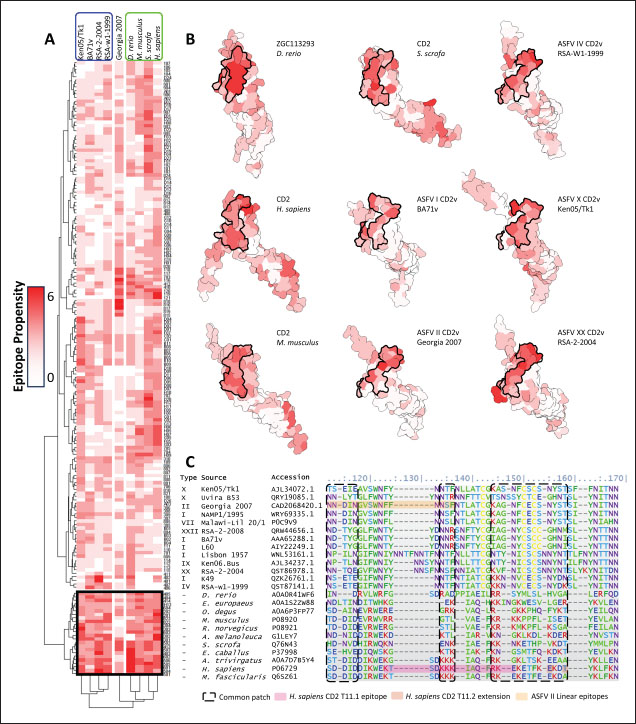
Subsequent hierarchical clustering of predicted epitope propensity in the core aligned residues of some mammalian CD2s, ASFV CD2vs, and ZGC113293 showed a common highly immunogenic patch in the most N-terminal domain (Fig. 5). The “conserved” sequences in the cellular and CD2vs were different, but aligned to the same location. This patch overlapped with the location of a known epitope of hCD2, T11.1, which corresponds to Lys71-Glu74 in the source sequence of Lys44-Glu53 in PDB ID 1QA9 (Meuer et al., 1984; Peterson and Seed, 1987; Borca et al., 1998; Wang et al., 2015). Additional extension epitopes to human T11.2 antibodies and several epitopes for ASFV II CD2v also coincide with the location (Damschroder et al., 2004; Jiang et al., 2022; Ren et al., 2022; Fan et al., 2024). One likely explanation for the migration of the CD58 site from the more distal N-terminal domain to the more proximal domain is the avoidance of this conserved antigenicity. Given the conservation of this predicted B-cell epitope propensity, this site might contain a key structural feature for proteins of this kind that are naturally exploited in affinity maturation. It is also tempting to speculate that leaving the conserved site may also allow the new binding site to be masked to some extent; similarly, many antibodies against SARS-CoV-2 spike proteins are raised against the receptor binding domain (RBD) domain, but most neutralizing antibodies typically target other sites in the spike protein (Magazine et al., 2024). A secondary consideration for the development of a new binding site would be its effect on host CD2-CD58 interactions. Since the alternative binding site does not lead to the competition of CD2v with the host CD2 for CD58, but lymphocyte proliferation is still disrupted, the binding site must still induce an alternative mechanism to prevent proper CD2-CD58 binding or its effects. A noticeable consequence of the binding of CD2vs to CD58, facilitated by the more proximal Ig domains, is that binding occurs at an angle. CD2vii-CD58 exacerbates this in Fig. 6, leading to the inclination of CD58 and a consequent shift of host CD2 position downwards. This led to a decrease of approximately 30 Å in the vertical length of the complex, resulting in a complex length that was considerably shorter than that of the TCR-MHC 1 complex. This may affect the formation of IS given the “kinetic segregation” mechanism which would affect lymphocyte activation and proliferation (Binder et al., 2020). The change in orientation of the distal domain may also affect binding especially in the context of membranes even without interference on the interface, as seen in the case of B7-1 to CD28 (Evans et al., 2006).
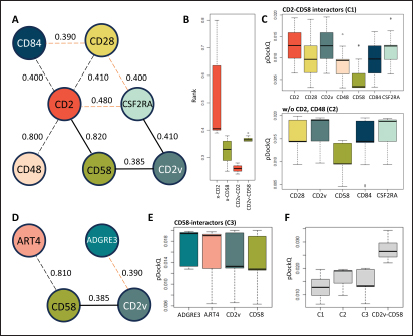
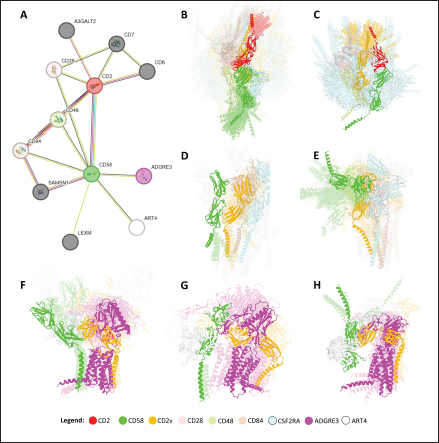
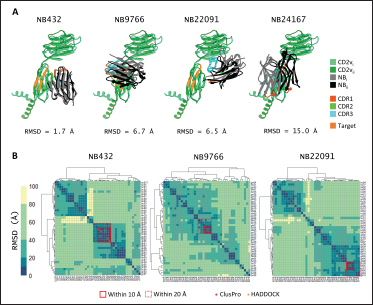
Fig. 5. Clustering of “core” residues across CD2 proteins and analogs using epitope propensity. (A) Hierarchical clustering of “core” CD2 proteins show conserved high propensity residues. (B) Location of high propensity residues mapped to CD2s and analogs. (C) Alignment of sequences from D. rerio ZGC113293, mammalian, and CD2vs show high propensity residues (marked by dashed lines) overlapping with known epitopes.
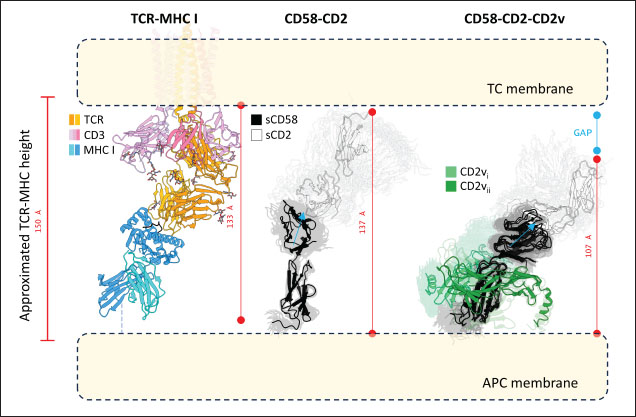
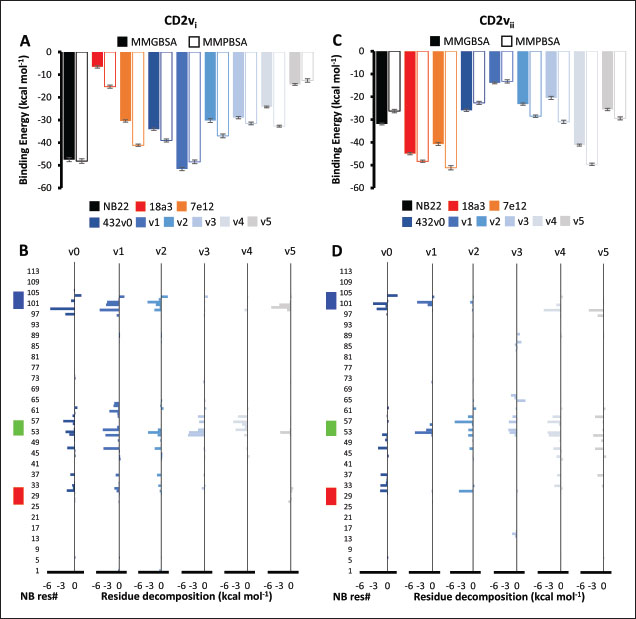
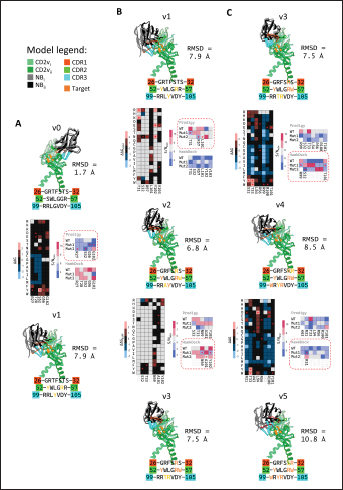
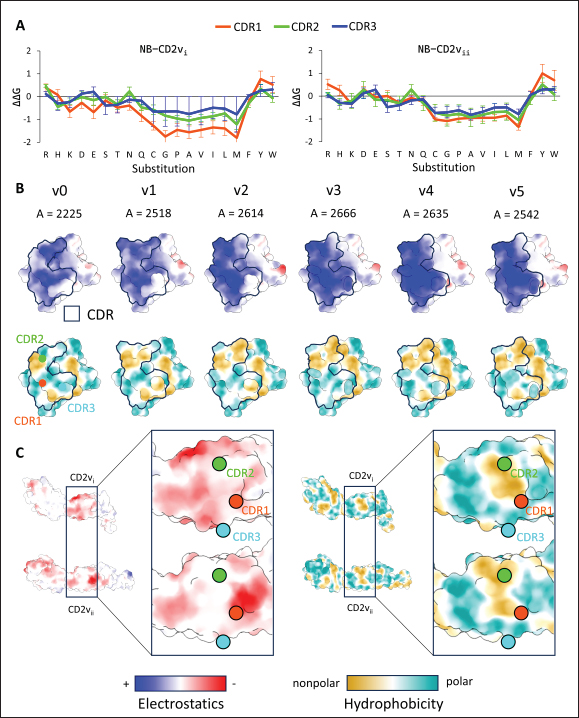
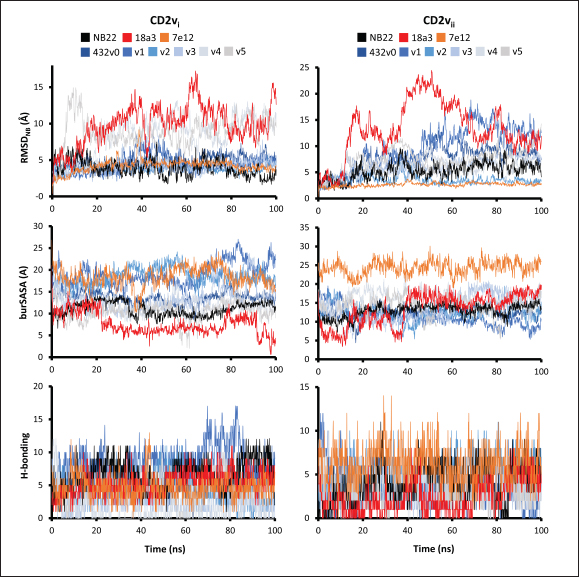
Fig. 6. Model of effect of CD2v binding to CD58 on host CD58-CD2 interactions. Shown side by side are TCR-MHC I complex (PDB ID: 8ES9), unconstrained CD58-CD2 complex, and constrained CD58-CD2 complex upon CD2v binding. Binding of CD2vs on proximal domain of CD58 forces an angle on swine CD58-CD2 pose that reduces the length of the complex, modifying the “ruler” functions of the proteins. Angle of face of CD58 also pivoted away from normal (blue arrows). The 150 Å estimate for the height of the IS core at leftmost. Spans of complexes labeled, plasma membranes for APC and TC in pale yellow. Additional components appear unlikely for CD2v-CD58 complexesPrevious studies have utilized a wide variety of metrics to evaluate AF complexes (Bryant et al., 2022; Gao et al., 2022; Yin et al., 2022; Xia et al., 2023; Zhu et al., 2023). These include metrics based on the global-topology sensitive TM-score such as pTM and ipTM, and DockQ-based metrics suggested to be more sensitive to the accuracy of predicted interfaces (Zhu et al., 2023). We note that a recent metric, the rank-score (or rank), has also been used previously (Elofsson et al., 2024). The thresholds generally set for complex predictions (pDockQ > 0.23, pTM >0.75, ipTM > 0.75) are often used as cut-offs for confidently predicted complexes. Nevertheless, these metrics have also been used in the relative ranking of complexes and for inference, the likelihood of complex components (Elofsson et al., 2024). Even if the human and swine CD2-CD58 metrics were found to be highly similar to the available PDB (RMSD1QA9: 0.647–0.785), the calculated metrics did not reach the thresholds for any of the three metrics mentioned above (pTM: 0.57, ipTM: 0.64–0.66, pDockQ: 0.0195–0.0201). This was observed for the other predicted binary complexes (pTM: 0.36–0.70, ipTM: 0.10–0.68, pDockQ: 0.02–0.032), and even in the multimeric complexes (pTM: 0.31–0.45, ipTM: 0.16–0.36). We nevertheless, utilized the scores as a relative measure to determine the most supported interactions and complexes. Initial pairwise complex predictions for the first complex set (C1): swine CD2, CD58, CD8, CD48, CD84, CSF2RA, and ASFV CD2v revealed that for all interactors except CD2v, CD2 was the more preferred binding partner compared to CD58 (Fig. S6b). Nevertheless, based on the interaction diagram according to rank score (≥0.385), CD2v appears to be more confidently connected to CSF2RA (0.410) than to CD58 (0.385) (Fig. S6a). The putative interaction network also suggests that among CD2-CD58 co-expressed proteins, none directly mediate CD2v-CD58 interaction, with most proteins more likely mediating CD2-CD2v interactions (Fig. S6c). Subsequent complex predictions reproduced the result with CD2 connected to CD84, close to a CSF2RA associated with CD2v. The CD58 protein also consistently contacted CD2 but modeled as if isolated in a separate cell membrane leaflet also leading to a reduced pDockQ for CD58 compared to the other proteins in the complex (Fig. S6c). This is similar to the case of CD9 in the predicted Izumo-Juno complex (Elofsson et al., 2024). Repeating complex prediction without CD2 and CD48 (C2), did not change the low pDockQ seen for CD58 (Fig. S6c), and indeed CD58 was still relatively isolated from the rest of the complex, but now mostly found to be modeled in the same leaflet and contacting CD2v directly, with CSF2RA on the opposite side from CD58 (Fig. S7d). This further reinforced the earlier finding that co-expressed proteins to CD2 and CD58, and even an interactor of CD2v do not appear to mediate CD2v-CD58 binding. An alternative set using only CD58 co-expressed proteins was also analyzed (C3), composed of CD58, CD2v, ART4, and ADGRE3. The pairwise interaction network similarly suggested that the other proteins do not mediate CD2v-CD58 interaction (Fig. S6d). Multimer prediction strongly modeled the new interactors bound to their respective main pair partners, ART4 with CD58 and ADGRE3 with CD2v, the latter consistent even across differing complex topologies. Furthermore, unlike in C2, CD2v-CD58 interactions in C3 appear to be mediated by the interacting partners, mostly through ADGRE3 (Fig. S7f–h). ADGRE3 was found to also be co-expressed with CD58 in horses in STRING-DB, qualifying as a more likely interactor based on conserved orthologous co-expression (Tirosh and Barkai, 2005). Based on complex predictions, higher-ranking interaction partners to one protein that do not necessarily contact the other associated protein can prevent the latter two’s contact, either by sequestering it to a more associated complex (CD2v-CSF2RA-CD2) or through its direct contact (CD2v-ADGRE3). Nevertheless, comparing the mean pDockQ for CD2v for all the complex set-ups clearly present the original CD2v-CD58 binary complex as superior to the alternative cases with additional binding partners (Fig. S6f). Current prediction metrics suggest a low chance for additional components in the CD2v-CD58 complex. Similarly, previous experimental results also make additional components hard to accommodate. Many IgSF or CD2-like proteins that would be co-expressed in myeloid or lymphoid tissues are specific even with low-affinity (i.e., CD28, CD84), with the potential exception of CD48 (Davis et al., 1998; Acuto and Michel, 2003; Evans et al., 2006; Hünig et al., 2015; Siokis et al., 2018; Cuenca et al., 2019). However, as seen in both the interaction network and predicted complexes these proteins did not seem to contact CD2v or CD58. Furthermore, recent co-immunoprecipitation and reverse co-immunoprecipitation of CD2v and CD58 in PK15 cells (Chaulagain et al., 2021) further reduce the diversity of plausible co-interactors. Based on transcriptomic data of this cell line (Yang et al., 2017; Jiang et al., 2019; Lv et al., 2022), of the described potential interactors, only CD48 is additionally expressed in such cells, which again does not seem to interact with CD2v and CD58 in the predicted complexes. Candidate NB432 was discovered as top nanobody scaffoldThe NanoLAS depository collected 30,667 nanobody sequences. Of these, 3,505 sequences were found to cluster with 346 comparator NB with known structures. Only 2,663 NB were clustered with comparators that targeted non-essential targets. Further screening of physicochemical and reactogenic parameters reduced the number of candidates to 196, while antigenicity was used to further determine the top 25 NB (see Table S2). The antigenic scores of the top 25 candidates ranged from 0.410 to 0.606 for bacteria, 0.386–0.550 for viruses, and 0.369–0.628 for tumors. After model production and quality assessment, targeted docking in ClusPro determined four scaffolds (NB432, NB1215, NB22091, and NB24167) that both made contact with the target conserved region and had clashes with CD58 bound to CD2v. Of these, only three (NB432, NB1215, NB22091) were observed to have corresponding nanobody poses within 10 Å for CD2vi and CD2vii. Finally, of the three scaffolds, only NB432 was validated in HADDOCK models, with available HADDOCK poses within 10 Å of ClusPro CD2vi and CD2vii docks (Fig. S8). Optimization of NB 432 leads to two best NBs, one for either CD2vi or CD2viiOptimization of the scaffold focused on modification of the CDR residues to stably target the conserved residues. This led to an optimization series as shown in Fig. S9. Optimization was performed in two modes: simple binding optimization (v0–v1, v3–v5), which only focused on improving residues for binding to the target region, and dock-biased binding optimization, which focused on reducing alternative binding modes (v1–v3). Single point mutations in mCSM-AB2 for residues in CDRs were found to follow a curve that was similar for all CDRs (Fig. S10a). In general, small and nonpolar residues were not favored for substitution in all CDRs for binding to both CD2v from ASFV genotypes 1 and 2. Meanwhile, another general result observed is the slight preferability for Tyr and Trp substitutions into the CDRs, with Tyr being preferable than Trp. Some CDRs also showed specific additional preferences, such as Arg in CDR1, Asn in CD2, and Asp and Glu in CDR3. Nevertheless, as seen in the optimization series, the co-opted mutations still veered mostly to bulky residues, such as Arg, Tyr, and Trp. The effect of the mutations on the CDR surface properties of the NB is shown in Figure S10B vis-à-vis the target surface of CD2vs (Fig. S10c). The surface area of the CDRs increased from an initial value of 2,225 to as high as 2,666. This was accompanied by an increase in positive charge in the CDRs which complements the negative electrostatic potential found on the CD2v target surfaces. Meanwhile, the local CDR3 surface was also observed to become more hydrophobic, which may improve binding to the corresponding hydrophobic patch on CD2v that it contacts. Thus, optimization seems to have rationalized the properties of the CDR of the NB in the context of the CD2v target region, given the more favorable electrostatic and hydrophobic interactions. Nevertheless, considering the stronger negatively charged patch in ASFV CD2vii compared to CD2vi, the optimized NB may have a stronger affinity for CD2vii than for CD2vi. Initial 100 ns simulations of the NB and the three controls: nanobody NB22 developed for cELISA (Zhu et al., 2024), and the variable domains fragments (FV) from antibodies 18a3 and 7e12 (Jia et al., 2022), showed some stability for the complexes, especially in the last 10 ns of the simulation (Fig. S11), as well as elucidated mixed trends in binding affinities of the NB vis-à-vis the controls (Fig. 7). Against ASFV CD2vi, the optimization series peaked relatively early in terms of the binding energy (Fig. 7a). The binding energy from v0 (MMGBSA/MMPBSA: −34.05/−39.03 kcal mol−1), increased for v1 (−51.66/−48.47 kcal mol−1) then dropped off with subsequent optimizations in v2 (−30.23/−37.04 kcal mol−1), v3 (−29.00/−31.51 kcal mol−1), v4 (−24.21/−32.72 kcal mol−1), and v5 (−14.31/−12.55 kcal mol−1) with no recovery. Meanwhile, the binding affinity for CD2vii generally increased (Fig. 7c). The affinity to CD2vii of v0 was (MMGBSA/MMPBSA) −25.93/−22.67 kcal mol−1, which dipped in v1 (−13.90/−3.23 kcal mol−1), increasing again in v2 (−23.20/−28.53 kcal mol−1), v3 (−20.49/−31.02 kcal mol−1), and v4 (−41.19/−49.65 kcal mol−1), before finally decreasing in v5 (−25.56/−29.47 kcal mol−1).
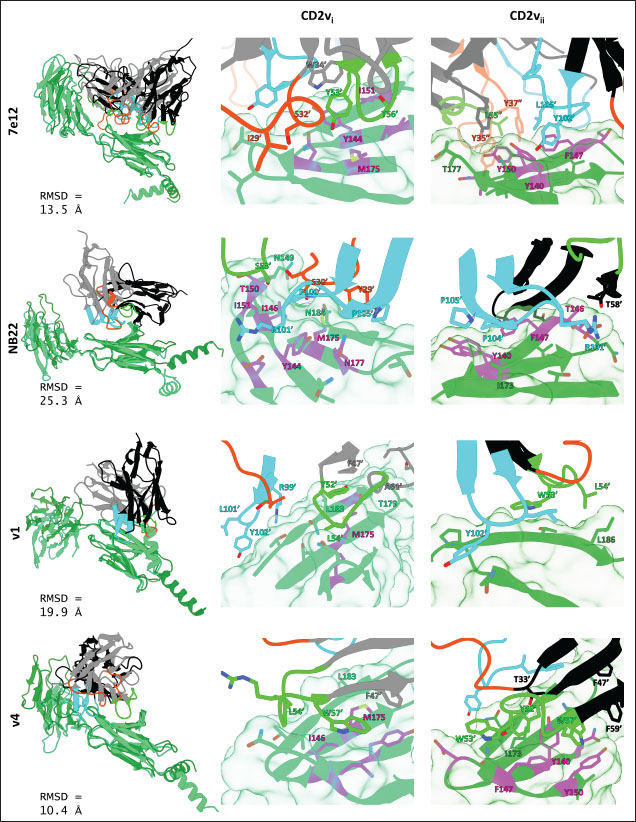
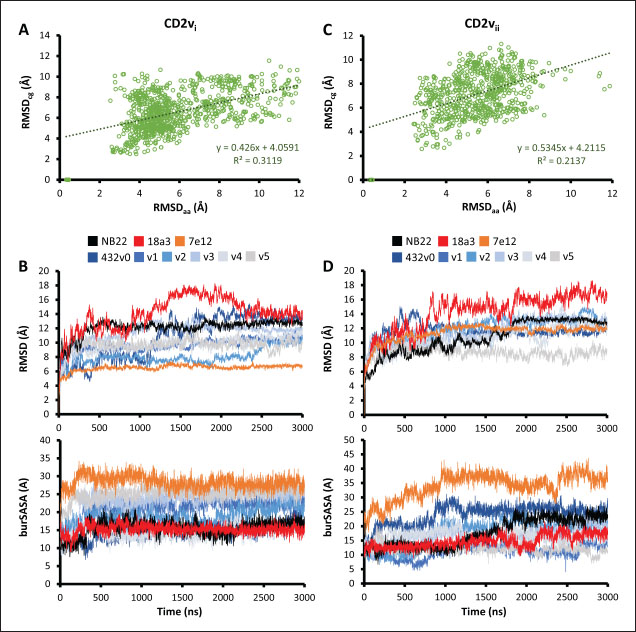
Fig. 7. Binding affinities from MD simulations of controls and optimization series. Shown are (A) binding of controls and optimization series to ASFV genotype I CD2 (CD2vi), (B) residue decomposition of optimization series in binding to CD2vi, (C) binding of controls and optimization series to ASFV genotype II CD2 (CD2vii), (D) residue decomposition of optimization series in binding to CD2vii. The trends observed for the optimization series seem to be captured in the residue decompositions of the nanobody series, particularly in the contribution of CDR2 to binding (Fig. 7b and d). This can be seen as a “spread” of residues in or near the CDR with significant binding energy. In cases where the nanobody has the lowest affinity to CD2v, the contribution from CDR2 also appears to be the lowest, and the same was seen when the affinity is highest; the contribution from CDR2 appears to be greater. Thus, for the optimized series, CDR2 appeared to be a key determinant of binding to ASFV CD2v. We also noticed that for all controls, there was more affine CD2v. These were CD2vii for the antibody fragments 7e12 and 18a3, and surprisingly, CD2vi for NB22. The difference in affinity of the controls to either CD2vi or CD2vii was found to range from 10.32 to 38.38 kcal mol−1, which was similar to what was observed for the nanobody series (8.12–37.76 kcal mol−1). While CDR2 being a key determinant for affinity to CD2vi or CD2vii in the optimized series might be expected given the optimization procedure, this is not necessarily the case for the controls that were developed only in the context of CD2vii (Jia et al., 2022; Zhu et al., 2024). Therefore, we explored the binding of the top NB in the series (v1 and v4) and controls (NB22 and 7e12), as shown in Fig. 8. Binding to CD2vi appears to require a broad interacting surface that cuts across the β-sheet. For NB22 and v1, this appeared to be achieved through a core (CDR3, CDR2) and accessory CDR (CD1, CDR3) for latching on the surface. Residues in these CDRs also seem to contribute to fewer nonpolar interactions. Meanwhile, in the case of binding to CD2vii, binding is mostly supported by targeting the aromatic residues present in strands β11–12. This is contrary to what was observed in CD58 binding, which emphasizes the role of short polar groups in interacting with Tyr residues. The 7e12 antibody and v4 seem to contribute to the swath of complementary aromatic residues through either a combination of or a single CDR. Therefore, optimization for both CD2vi and CD2vii may need to take these into consideration. While antibody fragments may be able to switch key interacting CDRs like in the case of 7e12 for this effect, with CDRs from the heavy fragment for CD2vi and CDRs from both fragments for CD2vii, this might be more difficult for NB. Nevertheless, using a long, flexible CDR as the core may be more useful as observed for NB22. NB and controls were then subjected to a longer coarse-grained simulation to determine whether the binding was stable for up to 3 µs (Fig. 9). Regression analysis of the complex RMSD of the all-atom and coarse-grained simulations in the first 100 ns suggests a correlation between the two ( p < 0.01, RCD2vi: 0.3119, RCD2vii: 0.2137), which may prove the utility of this approach. RMSDs of the NB generally appear to be bounded by the controls (18a3 and NB22) in the case of binding to CD2vi, while some controls (18a3) appear to be the maximal case in the case of binding to CD2vii. A general flattening behavior was also observed for most NB suggesting stability. Meanwhile, burSASA plots of the NB were generally seen to maintain or exceed the level observed in the first 100 ns throughout the rest of the 3 µs simulations. This implies that the levels of binding and affinity may have also been maintained throughout that time (Hollingsworth et al., 2016). Finally, comparing the physicochemical properties and reactogenicity of the NB to the controls suggests an overall better profile for the optimized NB (Table 2). In particular, v1 is predicted to be more soluble, more stable, has no allergenicity alerts, and has reduced antigenicity scores compared to the NB22 control (Zhu et al., 2024), which is more affine to CD2vi. Meanwhile, v4 is also likely to be more soluble and more stable, has reduced allergenicity alerts, and has lower antigenicity scores than the 18a3 control, which is more affine to CD2vii (Jia et al., 2022). The computed isoelectric points for the two NB were also above 9.4, which makes the NB potentially cell-penetrating (Li et al., 2012). This may further increase the scope of NB to target not only extracellular CD2v but also those distributed intracellularly, which has previously been shown to be a considerable fraction (Goatley and Dixon, 2011). Overall, our results point to a comparative advantage in the optimized NB v1 and v4 against controls NB22 and 18a3, respectively, towards their more affine CD2vs. DiscussionDeep-learning based protein modeling has revolutionized the study of protein dynamics and biomolecular interaction even leading to predictions of multimeric complexes that may be difficult to achieve through experimental methods (Bryant et al., 2022; Gao et al., 2022; Yin et al., 2022; Xia et al., 2023; Zhu et al., 2023; Elofsson et al., 2024). In this study, our initial results report an untenable “canonical” ASFV CD2v-swine CD58 interaction specifically due to the non-conservation of important residues (Wang et al., 2015), especially those that maintain proper charges at the interface, leading to the dismal binding seen even in short simulations. Indeed, a previous report on human CD58 binding to almost-identical primate CD2s showed cases of non-affinity, with only a few mutations in regions of the interface not previously implicated in binding (Damschroder et al., 2004). One mechanism to overcome the weak binding of viral proteins to host ligands has been observed in influenza, in which numerous hemagglutinin receptors are located on the viral surface to compensate for the poor affinity to host sialic acid (Gamblin et al., 2021). However, CD2v is generally expressed at low to moderate levels during infection (Cackett et al., 2022) and is not abundant in the ASFV virion (Alejo et al., 2018), which precludes this strategy.
Fig. 8. Binding residues between selected controls and NB and ASFV genotype I or II CD2vs (CD2vi in spring green, CD2vii in forest green). RMSD between nanobody or antibody in CD2vi and CD2vii pose noted. Binding residues contributing at least −0.5 kcal mol−1 to binding affinity are shown, and those contributing at least −1.5 kcal mol−1 are labeled (nanobody or antibody residues with “or”). CD2v residues implicated in CD58 binding are in magenta. CDRs from 1, 2, 3 are colored orange-red, green, and cyan respectively. Other nanobody or antibody residues are colored in gray or black.
Fig. 9. MD simulations of controls and optimization series. Shown are results from regression analysis of RMSD between all-atom (aa) and coarse-grained (cg) simulations within first 100-ns showing some correlation for (A) CD2vi and (C) CD2vii binding. RMSD and buried SASA plots of NBs and controls in 3-µs MD simulations against (B) CD2vi and (D) CD2vii. Following this, using protein-protein docking and AF3 predictions we propose a non-canonical binding pose that utilizes the proximal domains of both CD2v and CD58. This binding pose was found to be stable and has a similar binding affinity to native swine CD2-CD58 (MMGBSA/MMPBSA: −41.32/−39.45, −34.63/−34.65, −31.65/−35.31 kcal mol−1 for CD2vi, CD2vii, and CD2 respectively). We also note that given the primacy of CD58 in CD2v-linked NFκB activation (Chaulagain et al., 2021), the recent study on NFκB-activity blocking epitopes for ASFV CD2v (Fan et al., 2024) seems to also support our finding. An epitope discovered (141LNINDTFVKYTNE153) overlaps the β-sheet of the CD2v proximal domain facing CD58 in the non-canonical binding pose. We also tracked potential pressures that have led to this novel binding site, such as potential immunogenic considerations that either function in protecting the CD58 interaction from the conserved epitope in the distal domain to potential masking functions, as seen in the SARS-CoV-2 spike proteins (Magazine et al., 2024). We further noted that even with the shift in site, CD2v binding to host CD58-CD2 interactions may still impact IS formation by reducing the span of the complex. As previously noted, CD58-CD2 complexes appear to mediate the inclusion of TCR-MHC I complexes to develop IS from the outer regions while restricting the entry of inhibitory phosphatases. Dubbed “kinetic segregation”, the process is thought to occur by the steric exclusion of proteins with larger extracellular domains such as those phosphatases (Binder et al., 2020). Segregation and exclusion of proteins by size at membrane interfaces is spontaneous, but the process is also affected by crowding, protein affinity, and membrane height fluctuations (Schmid et al., 2016). Following that principle, the reduced length of the CD58-CD2 “ruler” to about 100 Å might hinder the development of proper IS of about ~150 Å in width and consequently reduce lymphocyte activation (Dustin et al., 2010). Additionally, the angle introduced by CD2v binding on CD58 also reorients the CD58 canonical interface away from the normal of the cell surface. In the context of surface-surface interactions, a non-optimal orientation of a protein’s binding interface reduces its affinity to the cognate protein even if a low dissociation constant was monitored in solution binding studies (Evans et al., 2006). Through such a mechanism the novel binding pose stays consistent with previous reports of reduced lymphocyte activity associated to CD2v expression alone (Borca et al., 1998) even without directly interfering with CD2-CD58 binding. The novel binding interaction may also be informative on the recent within-cell CD2-CD58 interactions also found to be necessary for lymphocyte activation (Li et al., 2022). Table 2. Physicochemical and reactogenic profiles of NB in controls and optimization series.
Finally, we also analyzed the likelihood of additional binding partners to the CD2v-CD58 complex. Complex predictions suggest that potential interactors are generally not poised to mediate CD2v-CD58 binding, except possibly for ADGRE3 which also features orthologous co-expression that is associated with true protein interactors (Tirosh and Barkai, 2005). Nevertheless, the minimal CD2v-CD58 complex was found to be more accurate compared to alternate arrangements. Compounded with the specificity of IgSF proteins, and transcriptomic data on PK15 cells where co-immunoprecipitation studies on CD2v and CD58 were conducted on, additional binders to CD2v-CD58 appear to be unlikely. Nevertheless, there are still limitations in the analyses. While we were able to posit how changes in CD2v sequence might impact CD58 binding, our current methods were not able to support analysis on the effect of the substantive glycosylation or the signal peptide of CD2vs which have also been linked to hemadsorption (Pérez-Núñez et al., 2023). Similarly, our analysis on additional binding partners were not exhaustive, focused on likely interacting partners based on co-expression and known interactors. Nevertheless, aside from CD2v, further discussion on other potential cellular analogs in ASFV that have also experienced significant drift might be needed as environmental pressures might have also acted on these proteins to develop alternative mechanisms in dealing with host proteins. This study also reports novel nanobody designs targeting the novel CD2v-CD58 binding site for the development of neutralizing antibodies against ASFV. We found that v1 and v4 were both more stable and affinely bound to the site than controls NB22 and 18a3. The optimized NB also exhibited better overall physicochemical and reactogenic parameters. These optimized NB may soon become part of a much-needed arsenal for the treatment of ASFV infections. A promising avenue for future research would be non-injectable nanobody platforms, such as through feeds or lactic acid bacteria, given positive examples in the protection of mice against enterotoxigenic Escherichia coli and chickens against Clostridium perfingens-mediated necrotic enteritis (Amcheslavsky et al., 2021; Gangaiah et al., 2022; Loutet et al., 2024). The oral application of NB against human Crohn’s has also been used in clinical trials (Sun et al., 2021). Bridging oral delivery to systemic applications, especially for antiviral treatment, may also be supported by the permeability of NB and wide tissue availability, stability, insusceptibility to pepsin, and refolding ability after denaturing conditions (Del Rio et al., 2019; Sun et al., 2021). Transmucosal affinity can also be introduced by fusing NB with carrier proteins or targets of transcytotic receptors (Bakshi et al., 2020; Bern et al., 2020). All of these can help further transition nanobody technologies into real-world applications, bringing the future to here and now. AcknowledgementsThis study was supported by the Philippine Council for Aquaculture Agriculture Research and Development (PCAARRD). We are grateful to the Department of Science and Technology – Advanced Science and Technology Institute (DOST-ASTI) for the use of High-performance Computing Units in the Computational and Archiving Resource environment (COARE). Special thanks to Advanced Materials Technology Laboratory (ADMATEL) for our home, and Mr. Edward Banico, Ms. Ella Sira, & Mr. Wreindt Ora for their help in processing the MD simulations. Conflict of interestThe authors declare no conflicts of interest. FundingThe authors gratefully acknowledge the support of the Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD) of the Philippines’ Department of Science and Technology, which funded the project under the Virology and Vaccine Research Program. Author contributionsANGD, FLO, & RMEP conceptualized the study. ANGD & FLO facilitated the lease of computational resources needed in the study. FLO acquired funding for the study, other resources, supervised, and administered the project. ANGD & NMOO developed the methodology for the study. ANGD and RMEP curated the data for the study. ANGD conducted formal analysis, investigation, wrote scripts used, wrote the original draft, and prepared Figures and visualizations for the manuscript. ANGD & NMOO did validation. ANGD, FLO, RMEP, and NMOO reviewed and edited the paper. All authors approved the submission of the final version of the manuscript. Data availabilityThe primary data supporting this study’s findings are included in the manuscript. Additional supporting data are available from the authors upon reasonable request. ReferencesAbraham, M.J., Murtola, T., Schulz, R., Páll, S., Smith, J.C., Hess, B. and Lindahl, E. 2015. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25. Abramson, J., Adler, J., Dunger, J., Evans, R., Green, T., Pritzel, A., Ronneberger, O., Willmore, L., Ballard, A.J., Bambrick, J., Bodenstein, S.W., Evans, D.A., Hung, C.-C., O’Neill, M., Reiman, D., Tunyasuvunakool, K., Wu, Z., Žemgulytė, A., Arvaniti, E., Beattie, C., Bertolli, O., Bridgland, A., Cherepanov, A., Congreve, M., Cowen-Rivers, A.I., Cowie, A., Figurnov, M., Fuchs, F.B., Gladman, H., Jain, R., Khan, Y.A., Low, C.M.R., Perlin, K., Potapenko, A., Savy, P., Singh, S., Stecula, A., Thillaisundaram, A., Tong, C., Yakneen, S., Zhong, E.D., Zielinski, M., Žídek, A., Bapst, V., Kohli, P., Jaderberg, M., Hassabis, D. and Jumper, J.M. 2024. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630(8016), 493–500. Acuto, O. and Michel, F. 2003. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 3(12), 939–951. Alejo, A., Matamoros, T., Guerra, M. and Andrés, G. 2018. A proteomic atlas of the African swine fever virus particle. J. Virol. 92(23), e01293–e01218. Amcheslavsky, A., Wallace, A.L., Ejemel, M., Li, Q., McMahon, C.T., Stoppato, M., Giuntini, S., Schiller, Z.A., Pondish, J.R., Toomey, J.R., Schneider, R.M., Meisinger, J., Heukers, R., Kruse, A.C., Barry, E.M., Pierce, B.G., Klempner, M.S., Cavacini, L.A. and Wang, Y. 2021. Anti-CfaE nanobodies provide broad cross-protection against major pathogenic enterotoxigenic Escherichia coli strains, with implications for vaccine design. Sci. Rep. 11(1), 2751. Assis, L.M., Sousa, J.R., Pinto, N.F.S., Silva, A.A., Vaz, A.F.M., Andrade, P.P., Carvalho, E.M. and De Melo, M.A. 2014. B-cell epitopes of antigenic proteins in Leishmania infantum: an in silico analysis. Parasite Immunol. 36(7), 313–323. Bakshi, S., Sanz Garcia, R., Van Der Weken, H., Tharad, A., Pandey, S., Juarez, P., Virdi, V., Devriendt, B., Cox, E. and Depicker, A. 2020. Evaluating single-domain antibodies as carriers for targeted vaccine delivery to the small intestinal epithelium. J. Control Release 321, 416–429. Bathula, N.V., Bommadevara, H. and Hayes, J.M. 2021. Nanobodies: the future of antibody-based immune therapeutics. Cancer Biother. Radio. 36(2), 109–122. Bern, M., Nilsen, J., Ferrarese, M., Sand, K.M.K., Gjølberg, T.T., Lode, H.E., Davidson, R.J., Camire, R.M., Bækkevold, E.S., Foss, S., Grevys, A., Dalhus, B., Wilson, J., Høydahl, L.S., Christianson, G.J., Roopenian, D.C., Schlothauer, T., Michaelsen, T.E., Moe, M.C., Lombardi, S., Pinotti, M., Sandlie, I., Branchini, A. and Andersen, J.T. 2020. An engineered human albumin enhances half-life and transmucosal delivery when fused to protein-based biologics. Sci. Transl. Med. 12(565), eabb0580. Best, R.B., Zhu, X., Shim, J., Lopes, P.E.M., Mittal, J., Feig, M. and MacKerell, A.D. 2012. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ 1 and χ 2 dihedral angles. J. Chem. Theory Comput. 8(9), 3257–3273. Binder, C., Cvetkovski, F., Sellberg, F., Berg, S., Paternina Visbal, H., Sachs, D.H., Berglund, E. and Berglund, D. 2020. CD2 immunobiology. Front. Immunol. 11, 1090. Borca, M.V., Carrillo, C., Zsak, L., Laegreid, W.W., Kutish, G.F., Neilan, J.G., Burrage, T.G. and Rock, D.L. 1998. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J. Virol. 72(4), 2881–2889. Borca, M.V., Kutish, G.F., Afonso, C.L., Irusta, P., Carrillo, C., Brun, A., Sussman, M. and Rock, D.L. 1994. An African swine fever virus gene with similarity to the T-lymphocyte surface antigen CD2 mediates hemadsorption. Virology 199(2), 463–468. Boys, I.N., Johnson, A.G., Quinlan, M.R., Kranzusch, P.J. and Elde, N.C. 2023. Structural homology screens reveal host-derived poxvirus protein families impacting inflammasome activity. Cell Rep. 42(8), 112878. Bryant, P., Pozzati, G., Zhu, W., Shenoy, A., Kundrotas, P. and Elofsson, A. 2022. Predicting the structure of large protein complexes using AlphaFold and Monte Carlo tree search. Nat. Commun. 13(1), 6028. Cackett, G., Portugal, R., Matelska, D., Dixon, L. and Werner, F. 2022. African swine fever virus and host response: transcriptome profiling of the Georgia 2007/1 strain and porcine macrophages. J. Virol. 96(5), e01939–e01921. Chaikeeratisak, V., Birkholz, E.A., Prichard, A.M., Egan, M.E., Mylvara, A., Nonejuie, P., Nguyen, K.T., Sugie, J., Meyer, J.R. and Pogliano, J. 2021. Viral speciation through subcellular genetic isolation and virogenesis incompatibility. Nat. Commun. 12(1), 342. Chang, Z., Du, Y., Li, R., Sun, X., Chen, Y., Li, M., Fan, L., Liu, S., Wang, S., Ding, P. and Zhang, G. 2023. Development and characterization of monoclonal antibody against the critical loop structure of African swine fever virus P72 protein. Vet. Microbiol. 283, 109776. Chaulagain, S., Delhon, G.A., Khatiwada, S. and Rock, D.L. 2021. African swine fever virus CD2v protein induces β-interferon expression and apoptosis in swine peripheral blood mononuclear cells. Viruses 13(8), 1480. Clifford, J.N., Høie, M.H., Deleuran, S., Peters, B., Nielsen, M. and Marcatili, P. 2022. BEPIPRED ‐3.0: improved B‐cell epitope prediction using protein language models. Protein Sci. 31(12), e4497. Colovos, C. and Yeates, T.O. 1993. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 2(9), 1511–1519. Cuenca, M., Sintes, J., Lányi, Á. and Engel, P. 2019. CD84 cell surface signaling molecule: an emerging biomarker and target for cancer and autoimmune disorders. Clin. Immunol. 204, 43–49. Damschroder, M.M., Kozhich, A.A., Woods, R.M., Cheng, L., Mullikin, B.A., Wilson, S.D., Ulbrandt, N.D., Bachy, C.M., Wu, H., Suzich, J.A., Kiener, P.A., Dall’Acqua, W.F. and White, W.I. 2004. Analysis of human and primate CD2 molecules by protein sequence and epitope mapping with anti-human CD2 antibodies. Mol. Immunol. 41(10), 985–1000. Davis, S.J., Davies, E.A., Tucknott, M.G., Jones, E.Y. and Van Der Merwe, P.A. 1998. The role of charged residues mediating low affinity protein–protein recognition at the cell surface by CD2. Proc. Natl. Acad. Sci. USA. 95(10), 5490–5494. Del Rio, B., Redruello, B., Fernandez, M., Martin, M.C., Ladero, V. and Alvarez, M.A. 2019. Lactic acid bacteria as a live delivery system for the in situ production of nanobodies in the human gastrointestinal tract. Front. Microbiol. 9, 3179. Delgado, J., Radusky, L.G., Cianferoni, D. and Serrano, L. 2019. FoldX 5.0: working with RNA, small molecules and a new graphical interface. Bioinformatics 35(20), 4168–4169. Dimitrov, I., Bangov, I., Flower, D.R. and Doytchinova, I. 2014. AllerTOP v.2—a server for in silico prediction of allergens. J. Mol. Model. 20(6), 2278. Dominguez, C., Boelens, R. and Bonvin, A.M.J.J. 2003. HADDOCK: a protein−protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 125(7), 1731–1737. Doytchinova, I.A. and Flower, D.R. 2007. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 8(1), 4. Dustin, M.L., Chakraborty, A.K. and Shaw, A.S. 2010. Understanding the structure and function of the immunological synapse. CSH Perspect Biol 2(10), a002311. Edgar, R.C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32(5), 1792–1797. Elofsson, A., Han, L., Bianchi, E., Wright, G.J. and Jovine, L. 2024. Deep learning insights into the architecture of the mammalian egg-sperm fusion synapse. eLife 13, RP93131. Escribano, J.M., Galindo, I. and Alonso, C. 2013. Antibody-mediated neutralization of African swine fever virus: myths and facts. Virus Res. 173(1), 101–109. Evans, E.J., Castro, M.A.A., O’Brien, R., Kearney, A., Walsh, H., Sparks, L.M., Tucknott, M.G., Davies, E.A., Carmo, A.M., Van Der Merwe, P.A., Stuart, D.I., Jones, E.Y., Ladbury, J.E., Ikemizu, S. and Davis, S.J. 2006. Crystal structure and binding properties of the CD2 and CD244 (2B4)-binding protein, CD48. J. Biol. Chem. 281(39), 29309–29320. Fan, R., Wei, Z., Zhang, M., Jia, S., Jiang, Z., Wang, Y., Cai, J., Chen, G., Xiao, H., Wei, Y., Shi, Y., Feng, J., Shen, B., Zheng, Y., Huang, Y. and Wang, J. 2024. Development of novel monoclonal antibodies for blocking NF-κB activation induced by CD2v protein in African swine fever virus. Front. Immunol. 15, 1352404. Freddi, A. and Salmon, M. 2019. Introduction to the Taguchi method. In Design principles and methodologies, Springer tracts in mechanical engineering. Cham: Springer International Publishing, pp: 159–180. Gabler, F., Nam, S., Till, S., Mirdita, M., Steinegger, M., Söding, J., Lupas, A.N. and Alva, V. 2020. Protein sequence analysis using the MPI bioinformatics toolkit. Curr. Protoc. Bioinformatics 72(1), e108. Gamblin, S.J., Vachieri, S.G., Xiong, X., Zhang, J., Martin, S.R. and Skehel, J.J. 2021. Hemagglutinin structure and activities. CSH Perspect Med 11(10), a038638. Gangaiah, D., Ryan, V., Van Hoesel, D., Mane, S.P., Mckinley, E.T., Lakshmanan, N., Reddy, N.D., Dolk, E. and Kumar, A. 2022. Recombinant Limosilactobacillus (Lactobacillus) delivering nanobodies against Clostridium perfringens NetB and alpha toxin confers potential protection from necrotic enteritis. MicrobiologyOpen 11(2), e1270. Gao, M., Nakajima An, D., Parks, J.M. and Skolnick, J. 2022. AF2Complex predicts direct physical interactions in multimeric proteins with deep learning. Nat. Commun. 13(1), 1744. Gao, Q., Yang, Y., Luo, Y., Chen, X., Gong, T., Wu, D., Feng, Y., Zheng, X., Wang, H., Zhang, G., Lu, G. and Gong, L. 2023. African swine fever virus envelope glycoprotein CD2v interacts with host CSF2RA to regulate the JAK2-STAT3 pathway and inhibit apoptosis to facilitate virus replication. J. Virol. 97(4), e01889–e01822. Gasteiger, E., Hoogland, C., Duvaud, S., Wilkins, M.R., Appel, R.D. and Bairoch, A. 2005. Protein identification and analysis tools on the expasy server. In The proteomics protocols handbook, Ed., Walker J. Totowa, NJ: Humana Press, pp: 571–607. Gaudreault, N.N. and Richt, J.A. 2019. Subunit vaccine approaches for African swine fever virus. Vaccines (Bassel) 7(2), 56. Goatley, L.C. and Dixon, L.K. 2011. Processing and localization of the African swine fever virus CD2v transmembrane protein. J. Virol. 85(7), 3294–3305. Guarascio, F., Vu, K., Flores, M. and Guarascio, F. 2023. Exclusive: world animal health body warns of swine fever vaccine risks as Vietnam readies exports. Thomson Reuters, New York, NY 10036, USA. Hallgren, J., Tsirigos, K.D., Pedersen, M.D., Almagro Armenteros, J.J., Marcatili, P., Nielsen, H., Krogh, A. and Winther, O. 2022. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv. [PrePrint] Hebditch, M., Carballo-Amador, M.A., Charonis, S., Curtis, R. and Warwicker, J. 2017. Protein–Sol: a web tool for predicting protein solubility from sequence. Bioinformatics 33(19), 3098–3100. Heo, L., Park, H. and Seok, C. 2013. GalaxyRefine: protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 41(W1), W384–W388. Høie, M.H., Gade, F.S., Johansen, J.M., Würtzen, C., Winther, O., Nielsen, M. and Marcatili, P. 2024. DiscoTope-3.0: improved B-cell epitope prediction using inverse folding latent representations. Front. Immunol. 15, 1322712. Hollingsworth, S.A., Batabyal, D., Nguyen, B.D. and Poulos, T.L. 2016. Conformational selectivity in cytochrome P450 redox partner interactions. Proc. Natl. Acad. Sci. USA. 113(31), 8723–8728. Holm, L. and Rosenström, P. 2010. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38(Web Server issue), W545–W549. Hünig, T., Beyersdorf, N. and Kerkau, T. 2015. CD28 co-stimulation in T-cell homeostasis: a recent perspective. Immunotargets Ther. 4, 111. Jia, R., Zhang, G., Bai, Y., Liu, H., Chen, Y., Ding, P., Zhou, J., Feng, H., Li, M., Tian, Y. and Wang, A. 2022. Identification of linear B cell epitopes on CD2V protein of African swine fever virus by monoclonal antibodies. Microbiol. Spectr. 10(2), e01052–21. Jiang, S., Li, F., Li, X., Wang, L., Zhang, L., Lu, C., Zheng, L. and Yan, M. 2019. Transcriptome analysis of PK-15 cells in innate immune response to porcine deltacoronavirus infection. PLoS One 14(10), e0223177. Jiang, W., Jiang, D., Li, L., Wang, J., Wang, P., Shi, X., Zhao, Q., Liu, B., Ji, P. and Zhang, G. 2022. Identification of two novel linear B cell epitopes on the CD2v protein of African swine fever virus using monoclonal antibodies. Viruses 15(1), 131. Klein, F., Soñora, M., Helene Santos, L., Nazareno Frigini, E., Ballesteros-Casallas, A., Rodrigo Machado, M. and Pantano, S. 2023. The SIRAH force field: a suite for simulations of complex biological systems at the coarse-grained and multiscale levels. J. Struct. Biol. 215(3), 107985. Kozakov, D., Hall, D.R., Xia, B., Porter, K.A., Padhorny, D., Yueh, C., Beglov, D. and Vajda, S. 2017. The ClusPro web server for protein–protein docking. Nat. Protoc. 12(2), 255–278. Krissinel, E. and Henrick, K. 2007. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372(3), 774–797. Krüger, D.M. and Gohlke, H. 2010. DrugScorePPI webserver: fast and accurate in silico alanine scanning for scoring protein-protein interactions. Nucleic Acids Res. 38(Web Server issue), W480–W486. Laskowski, R.A., MacArthur, M.W., Moss, D.S. and Thornton, J.M. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26(2), 283–291. Li, B., Lu, Y., Zhong, M.-C., Qian, J., Li, R., Davidson, D., Tang, Z., Zhu, K., Argenty, J., De Peredo, A.G., Malissen, B., Roncagalli, R. and Veillette, A. 2022. Cis interactions between CD2 and its ligands on T cells are required for T cell activation. Sci. Immunol. 7(74), eabn6373. Li, T., Bourgeois, J., Celli, S., Glacial, F., Le Sourd, A., Mecheri, S., Weksler, B., Romero, I., Couraud, P., Rougeon, F. and Lafaye, P. 2012. Cell‐penetrating anti‐GFAP VHH and corresponding fluorescent fusion protein VHH‐GFP spontaneously cross the blood‐brain barrier and specifically recognize astrocytes: application to brain imaging. FASEB J. 26(10), 3969–3979. Li, W. and Godzik, A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22(13), 1658–1659. Liu, H., Wang, A., Yang, W., Liang, C., Zhou, J., Chen, Y., Liu, Y., Zhou, Y. and Zhang, G. 2023. Expression of extracellular domain of ASFV CD2v protein in mammalian cells and identification of B cell epitopes. Virus Res. 323, 199000. Liu, S., Ding, P., Du, Y., Ren, D., Chen, Y., Li, M., Sun, X., Wang, S., Chang, Z., Li, R. and Zhang, G. 2022. Development and characterization of monoclonal antibodies against the extracellular domain of African swine fever virus structural protein, CD2v. Front. Microbiol. 13, 1056117. Lomoio, U., Puccio, B., Tradigo, G., Guzzi, P.H. and Veltri, P. 2023. SARS-CoV-2 protein structure and sequence mutations: evolutionary analysis and effects on virus variants. PLoS One. 18(7), e0283400. Loutet, S.A., Cheung, S., Zaytsoff, S., Hofacre, C., Jones, M.K., Van Petegem, F. and Abnousi, H. 2024. Research note: clostridium perfringens NetB and CnaA neutralizing nanobodies in feed reduce the incidence of poultry necrotic enteritis. Poultry Sci. 103(4), 103578. Lv, H., Peng, Z., Jia, B., Jing, H., Cao, S., Xu, Z. and Dong, W. 2022. Transcriptome analysis of PK-15 cells expressing CSFV NS4A. BMC Vet Res. 18(1), 434. Magazine, N., Zhang, T., Bungwon, A.D., McGee, M.C., Wu, Y., Veggiani, G. and Huang, W. 2024. Immune epitopes of SARS-CoV-2 spike protein and considerations for universal vaccine development. Immunohorizons 8(3), 214–226. Meuer, S.C., Hussey, R.E., Fabbi, M., Fox, D., Acuto, O., Fitzgerald, K.A., Hodgdon, J.C., Protentis, J.P., Schlossman, S.F. and Reinherz, E.L. 1984. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell 36(4), 897–906. Myung, Y., Rodrigues, C.H.M., Ascher, D.B. and Pires, D.E.V. 2020. mCSM-AB2: guiding rational antibody design using graph-based signatures. Bioinformatics 36(5), 1453–1459. Nandy, S., Bora, N.R., Gaurav, S. and Kumar, S. 2024. The p30 protein of the African swine fever virus behaves as an RNase. Virology 590, 109967. Negi, S.S., Schein, C.H., Oezguen, N., Power, T.D. and Braun, W. 2007. InterProSurf: a web server for predicting interacting sites on protein surfaces. Bioinformatics 23(24), 3397–3399. Nielsen, H., Teufel, F., Brunak, S. and Von Heijne, G. 2024. SignalP: the evolution of a web server. Methods Mol Biol. 2024;2836:331–367. Njau, E.P., Machuka, E.M., Cleaveland, S., Shirima, G.M., Kusiluka, L.J., Okoth, E.A. and Pelle, R. 2021. African swine fever virus (ASFV): biology, genomics and genotypes circulating in Sub-Saharan Africa. Viruses 13(11), 2285. Nomburg, J., Doherty, E.E., Price, N., Bellieny-Rabelo, D., Zhu, Y.K. and Doudna, J.A. 2024. Birth of protein folds and functions in the virome. Nature 633(8030), 710–717. Onisk, D.V., Borca, M.V., Kutish, S., Kramer, E., Irusta, P. and Rock, D.L. 1994. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 198(1), 350–354. Othman, H., Messaoud, H.B., Khamessi, O., Ben-Mabrouk, H., Ghedira, K., Bharuthram, A., Treurnicht, F., Achilonu, I., Sayed, Y. and Srairi-Abid, N. 2022. SARS-CoV-2 spike protein unlikely to bind to integrins via the Arg-Gly-Asp (RGD) motif of the receptor binding domain: evidence from structural analysis and microscale accelerated molecular dynamics. Front. Mol. Biosci. 9, 834857. Panda, M., Kalita, E., Singh, S., Kumar, K. and Prajapati, V.K. 2023. Nanobody-peptide-conjugate (NPC) for passive immunotherapy against SARS-CoV-2 variants of concern (VoC): a prospective pan-coronavirus therapeutics. Mol. Divers. 27(6), 2577–2603. Paysan-Lafosse, T., Blum, M., Chuguransky, S., Grego, T., Pinto, B.L., Salazar, G.A., Bileschi, M.L., Bork, P., Bridge, A., Colwell, L., Gough, J., Haft, D.H., Letunić, I., Marchler-Bauer, A., Mi, H., Natale, D.A., Orengo, C.A., Pandurangan, A.P., Rivoire, C., Sigrist, C.J.A., Sillitoe, I., Thanki, N., Thomas, P.D., Tosatto, S.C.E., Wu, C.H. and Bateman, A. 2023. InterPro in 2022. Nucleic Acids Res. 51(D1), D418–D427. Penev, P.I., McCann, H.M., Meade, C.D., Alvarez-Carreño, C., Maddala, A., Bernier, C.R., Chivukula, V.L., Ahmad, M., Gulen, B., Sharma, A., Williams, L.D. and Petrov, A.S. 2021. ProteoVision: web server for advanced visualization of ribosomal proteins. Nucleic Acids Res. 49(W1), W578–W588. Pérez-Núñez, D., García-Belmonte, R., Riera, E., Fernández-Sesma, M.H., Vigara-Astillero, G. and Revilla, Y. 2023. Signal peptide and N-glycosylation of N-terminal-CD2v determine the hemadsorption of African swine fever virus. J. Virol. 97(10), e01030–23. Pérez-Núñez, D., García-Urdiales, E., Martínez-Bonet, M., Nogal, M.L., Barroso, S., Revilla, Y. and Madrid, R. 2015. CD2v interacts with adaptor protein AP-1 during African swine fever infection. PLoS One 10(4), e0123714. Peterson, A. and Seed, B. 1987. Monoclonal antibody and ligand binding sites of the T cell erythrocyte receptor (CD2). Nature 329(6142), 842–846. Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Greenblatt, D.M., Meng, E.C. and Ferrin, T.E. 2004. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 25(13), 1605–1612. Plunkett, M.L., Sanders, M.E., Selvaraj, P., Dustin, M.L. and Springer, T.A. 1987. Rosetting of activated human T lymphocytes with autologous erythrocytes. Definition of the receptor and ligand molecules as CD2 and lymphocyte function-associated antigen 3 (LFA-3). J. Exp. Med. 165(3), 664–676. Ponomarenko, J., Bui, H.-H., Li, W., Fusseder, N., Bourne, P.E., Sette, A. and Peters, B. 2008. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics 9(1), 514. Pothin, E., Lesuisse, D. and Lafaye, P. 2020. Brain delivery of single-domain antibodies: a focus on VHH and VNAR. Pharmaceutics 12(10), 937. Poustforoosh, A., Faramarz, S., Negahdaripour, M. and Hashemipour, H. 2023. Modeling and affinity maturation of an anti-CD20 nanobody: a comprehensive in-silico investigation. Sci. Rep. 13(1), 582. Pucci, F., Kwasigroch, J.M. and Rooman, M. 2017. SCooP: an accurate and fast predictor of protein stability curves as a function of temperature. Bioinformatics 33(21), 3415–3422. Ravantti, J.J., Martinez-Castillo, A. and Abrescia, N.G.A. 2020. Superimposition of viral protein structures: a means to decipher the phylogenies of viruses. Viruses 12(10), 1146. Recny, M.A., Neidhardt, E.A., Sayre, P.H., Ciardelli, T.L. and Reinherz, E.L. 1990. Structural and functional characterization of the CD2 immunoadhesion domain. Evidence for inclusion of CD2 in an alpha-beta protein folding class. J. Biol. Chem. 265(15), 8542–8549. Reguant, R., Antipin, Y., Sheridan, R., Dallago, C., Diamantoukos, D., Luna, A., Sander, C. and Gauthier, N.P. 2020. AlignmentViewer: sequence analysis of large protein families. F1000Res. 9, 213. Ren, D., Ding, P., Liu, S., Zhang, N., Chen, Y., Li, Q., Fan, L., Chang, Z. and Zhang, G. 2022. Development and characterization of recombinant ASFV CD2v protein nanoparticle-induced monoclonal antibody. Int. J. Biol. Macromol. 209, 533–541. Rodríguez, J.M., Yáñez, R.J., Almazán, F., Viñuela, E. and Rodriguez, J.F. 1993. African swine fever virus encodes a CD2 homolog responsible for the adhesion of erythrocytes to infected cells. J. Virol. 67(9), 5312–5320. Rowlands, R.J., Duarte, M.M., Boinas, F., Hutchings, G. and Dixon, L.K. 2009. The CD2v protein enhances African swine fever virus replication in the tick vector, Ornithodoros erraticus. Virology 393(2), 319–328. Saha, S. and Raghava, G.P.S. 2004. BcePred: prediction of continuous B-cell epitopes in antigenic sequences using physico-chemical properties. In Artificial immune systems, lecture notes in computer science. Vol. 3239, Eds., Nicosia, G., Cutello, V., Bentley, P.J. and J. Timmis. Berlin, Heidelberg: Springer Berlin Heidelberg, pp: 197–204. Saha, S. and Raghava, G.P.S. 2007. Prediction methods for B-cell epitopes. Methods Mol Biol. 2007:409:387–394. Sayers, E.W., Bolton, E.E., Brister, J.R., Canese, K., Chan, J., Comeau, D.C., Connor, R., Funk, K., Kelly, C., Kim, S., Madej, T., Marchler-Bauer, A., Lanczycki, C., Lathrop, S., Lu, Z., Thibaud-Nissen, F., Murphy, T., Phan, L., Skripchenko, Y., Tse, T., Wang, J., Williams, R., Trawick, B.W., Pruitt, K.D. and Sherry, S.T. 2022. Database resources of the national center for biotechnology information. Nucleic Acids Res. 50(D1), D20–D26. Schlafer, D.H., Mebus, C.A. and McVicar, J.W. 1984. African swine fever in neonatal pigs: passively acquired protection from colostrum or serum of recovered pigs. Am. J. Vet. Res. 45(7), 1367–1372. Schmid, E.M., Bakalar, M.H., Choudhuri, K., Weichsel, J., Ann, H.S., Geissler, P.L., Dustin, M.L. and Fletcher, D.A. 2016. Size-dependent protein segregation at membrane interfaces. Nat. Phys. 12(7), 704–711. Schneider, C., Raybould, M.I.J. and Deane, C.M. 2022. SAbDab in the age of biotherapeutics: updates including SAbDab-nano, the nanobody structure tracker. Nucleic Acids Res. 50(D1), D1368–D1372. Shapovalov, M.V. and Dunbrack, R.L. 2011. A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure 19(6), 844–858. Sharma, N., Naorem, L.D., Jain, S. and Raghava, G.P.S. 2022. ToxinPred2: an improved method for predicting toxicity of proteins. Brief Bioinform. 23(5), bbac174. Sharma, N., Patiyal, S., Dhall, A., Pande, A., Arora, C. and Raghava, G.P.S. 2021. AlgPred 2.0: an improved method for predicting allergenic proteins and mapping of IgE epitopes. Brief Bioinform. 22(4), bbaa294. Simbulan, A.M., Banico, E.C., Sira, E.M.J.S., Odchimar, N.M.O. and Orosco, F.L. 2024. Immunoinformatics-guided approach for designing a pan-proteome multi-epitope subunit vaccine against African swine fever virus. Sci Rep. 14(1), 1354. Singh, H., Gupta, S., Gautam, A. and Raghava, G.P.S. 2015. Designing B-cell epitopes for immunotherapy and subunit vaccines. Methods Mol Biol. 2015:1348:327–340. Siokis, A., Robert, P.A., Demetriou, P., Dustin, M.L. and Meyer-Hermann, M. 2018. F-actin-driven CD28-CD80 localization in the immune synapse. Cell Rep. 24(5), 1151–1162. Song, J., Wang, M., Du, Y., Wan, B., Zhang, A., Zhang, Y., Zhuang, G., Ji, P., Wu, Y. and Zhang, G. 2023. Identification of a linear B-cell epitope on the African swine fever virus CD2v protein. Int. J. Biol. Macromol. 232, 123264. Sun, S., Ding, Z., Yang, X., Zhao, X., Zhao, M., Gao, L., Chen, Q., Xie, S., Liu, A., Yin, S., Xu, Z. and Lu, X. 2021. Nanobody: a small antibody with big implications for tumor therapeutic strategy. Int. J. Nanomed. 16, 2337–2356. Szklarczyk, D., Kirsch, R., Koutrouli, M., Nastou, K., Mehryary, F., Hachilif, R., Gable, A.L., Fang, T., Doncheva, N.T., Pyysalo, S., Bork, P., Jensen, L.J. and von Mering, C. 2023. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51(D1), D638–D646. The UniProt Consortium, Bateman, A., Martin, M.-J., Orchard, S., Magrane, M., Ahmad, S., Alpi, E., Bowler-Barnett, E.H., Britto, R., Bye-A-Jee, H., Cukura, A., Denny, P., Dogan, T., Ebenezer, T., Fan, J., Garmiri, P., Da Costa Gonzales, L.J., Hatton-Ellis, E., Hussein, A., Ignatchenko, A., Insana, G., Ishtiaq, R., Joshi, V., Jyothi, D., Kandasaamy, S., Lock, A., Luciani, A., Lugaric, M., Luo, J., Lussi, Y., MacDougall, A., Madeira, F., Mahmoudy, M., Mishra, A., Moulang, K., Nightingale, A., Pundir, S., Qi, G., Raj, S., Raposo, P., Rice, D.L., Saidi, R., Santos, R., Speretta, E., Stephenson, J., Totoo, P., Turner, E., Tyagi, N., Vasudev, P., Warner, K., Watkins, X., Zaru, R., Zellner, H., Bridge, A.J., Aimo, L., Argoud-Puy, G., Auchincloss, A.H., Axelsen, K.B., Bansal, P., Baratin, D., Batista Neto, T.M., Blatter, M.-C., Bolleman, J.T., Boutet, E., Breuza, L., Gil, B.C., Casals-Casas, C., Echioukh, K.C., Coudert, E., Cuche, B., De Castro, E., Estreicher, A., Famiglietti, M.L., Feuermann, M., Gasteiger, E., Gaudet, P., Gehant, S., Gerritsen, V., Gos, A., Gruaz, N., Hulo, C., Hyka-Nouspikel, N., Jungo, F., Kerhornou, A., Le Mercier, P., Lieberherr, D., Masson, P., Morgat, A., Muthukrishnan, V., Paesano, S., Pedruzzi, I., Pilbout, S., Pourcel, L., Poux, S., Pozzato, M., Pruess, M., Redaschi, N., Rivoire, C., Sigrist, C.J.A., Sonesson, K., Sundaram, S., Wu, C.H., Arighi, C.N., Arminski, L., Chen, C., Chen, Y., Huang, H., Laiho, K., McGarvey, P., Natale, D.A., Ross, K., Vinayaka, C.R., Wang, Q., Wang, Y. and Zhang, J. 2023. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 51(D1), D523–D531. Tirosh, I. and Barkai, N. 2005. Computational verification of protein-protein interactions by orthologous co-expression. BMC Bioinformatics 6(1), 40. Upham, N.S., Esselstyn, J.A. and Jetz, W. 2019. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17(12), e3000494. Valdés-Tresanco, M.S., Valdés-Tresanco, M.E., Valiente, P.A. and Moreno, E. 2021. gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory. Comput. 17(10), 6281–6291. Van Kempen, M., Kim, S.S., Tumescheit, C., Mirdita, M., Lee, J., Gilchrist, C.L.M., Söding, J. and Steinegger, M. 2024. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. 42(2), 243–246. Wang, X., Ji, C.G. and Zhang, J.Z.H. 2015. Glycosylation modulates human CD2-CD58 adhesion via conformational adjustment. J. Phys. Chem B. 119(22), 6493–6501. Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., Heer, F.T., de Beer, T.A.P., Rempfer, C., Bordoli, L., Lepore, R. and Schwede, T. 2018. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46(W1), W296–W303. Weng, G., Wang, E., Wang, Z., Liu, H., Zhu, F., Li, D. and Hou, T. 2019. HawkDock: a web server to predict and analyze the protein–protein complex based on computational docking and MM/GBSA. Nucleic Acids Res. 47(W1), W322–W330. Wiederstein, M. and Sippl, M.J. 2007. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 35(Web Server), W407–W410. Xia, Y., Zhao, K., Liu, D., Zhou, X. and Zhang, G. 2023. Multi-domain and complex protein structure prediction using inter-domain interactions from deep learning. Commun Biol. 6(1), 1221. Xiong, S., Liu, Z., Yi, X., Liu, K., Huang, B. and Wang, X. 2024. NanoLAS: a comprehensive nanobody database with data integration, consolidation and application. Database (Oxford). 2024, baae003. Xu, Z., Hu, Y., Li, J., Wang, A., Meng, X., Chen, L., Wei, J., Tong, W., Kong, N., Yu, L., Yu, H., Shan, T., Tong, G., Wang, G. and Zheng, H. 2023. Screening and identification of the dominant antigens of the African swine fever virus. Front. Vet. Sci. 10, 1175701. Xue, L.C., Rodrigues, J.P., Kastritis, P.L., Bonvin, A.M. and Vangone, A. 2016. PRODIGY: a web server for predicting the binding affinity of protein–protein complexes. Bioinformatics 32(23), 3676–3678. Yan, Y., Tao, H., He, J. and Huang, S.-Y. 2020. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 15(5), 1829–1852. Yang, S., Pei, Y. and Zhao, A. 2017. iTRAQ-based proteomic analysis of porcine kidney epithelial PK15 cells infected with Pseudorabies virus. Sci. Rep. 7(1), 45922. Yao, B., Zheng, D., Liang, S. and Zhang, C. 2020. SVMTriP: a method to predict B-cell linear antigenic epitopes. Methods Mol Biol. 2020:2131:299–307. Yin, R., Feng, B.Y., Varshney, A. and Pierce, B.G. 2022. Benchmarking ALPHAFOLD for protein complex modeling reveals accuracy determinants. Protein Sci. 31(8), e4379. Zhu, J., Liu, Q., Li, L., Zhang, R., Chang, Y., Zhao, J., Liu, S., Zhao, X., Chen, X., Sun, Y. and Zhao, Q. 2024. Nanobodies against African swine fever virus p72 and CD2v proteins as reagents for developing two cELISAs to detect viral antibodies. Virol. Sin. 39(3), 478–489. Zhu, W., Shenoy, A., Kundrotas, P. and Elofsson, A. 2023. Evaluation of AlphaFold-multimer prediction on multi-chain protein complexes. Bioinformatics 39(7), btad424. APPENDIXTable S1. Accessions of ASFV CD2vs, CD2 homologs utilized in this study and annotations.
Table S2. Top 25 nanobody candidates as scaffolds with sequences with CDRs underlined.
Fig. S1. Other measures from 100-ns simulations of CD58-CD2v in “canonical” and “novel” poses. Shown are Rg, Hydrogen-bonding (H-bonding), and burSASA profiles of the CD2 complexes compared to the control human and swine CD58-CD2 complexes in each case. Note diverging Rg profile, lack of H-bonding, and fast diminishing interface surface for canonical pose simulations of CD58-CD2v.
Fig. S2. Clustering of CD58-CD2, -CD2vi, or -CD2vii models. HCA of protein-protein docking derived and AF models for CD58 with (A) CD2, (D) CD2vi, and (G) CD2vii. Re-clustering of AF model with annotated clusters. AF model in red star for CD58 with (C) CD2, (E) CD2vi, and (H) CD2vii. Visualization of clusters with AF model in yellow for CD58 with (C) CD2, (F) CD2vi, (I) CD2vii.
Fig. S3. Residue decomposition maps for CD2vs. (A) Residue decomposition map for CD2vi for the four clusters and AF model. (B) Residue decomposition map for CD2vii for the two clusters and AF model. Most contributing residues in blue.
Fig. S4. Coincidence of average residue contribution to binding, residue variability, epitope propensity, & epitope multiplicity.
Fig. S5. Structural clustering of CD2 proteins and analogs. (A) Modeled CD2 proteins and analogs in AF3 for structural clustering in DALI server. ASFV CD2vs in blue, mammalian CD2s in green, D. rerio ZGC113293 in red. (B) Dendrogram showing clustering of CD2 proteins and analogs.
Fig. S6. Pairwise and multimeric complex analysis of CD58-CD2v system with potential interactors. Pairwise networks following the rank cut-off for (A) CD2 and CD58 co-expressed potential interactors, and (D) CD58-only co-expressed potential co-interactors. (B) Preference of interactors in “A” and CD2v towards CD2 (red) or CD58 (green) by rank. (C) pDockQ scores for components in full network in “A” or complex C1, and reduced network without CD2 and poorly connecting CD48 or complex C2. (E) pDockQ scores for components in network in “D” or complex C3. (F) Comparison of pDockQ of CD2v in complexes C1, C2, and C3 against the CD2v-CD58 binary complex.
Fig. S7. STRING-DB result for CD2-CD58 and visualization of multimeric complexes. (A) STRING-DB network for swine CD2-CD58. (B) Multimeric complex of CD2 and CD58 co-expressed potential interactors (C1), and (C) emphasis on CD2v-CSF2RA interaction in multimer. (D, E) Potential states (joined, inverted) for multimeric complex of C1 proteins without CD2 and CD48 (C2). (F–H) Potential states (joined, opposed, inverted)) for multimeric complex of CD58-only co-expressed potential interactors (C3).
Fig. S8. Selection of top scaffold. (A) The four scaffolds with CDRs contacting the target conserved region and clashing with CD58. Components colored as above. (B) Validation of ClusPro docks to HADDOCK models, clustering within 10 and 20 Å highlighted with solid and dotted red lines respectively. ClusPro and HADDOCK poses in within 10 Å clusters marked with red and orange star.
Fig. S9. Development of optimization series for NB432. (A) Original scaffold (v0) optimized to v1. (B) Alternative binding optimization from v1 to v3 followed by (C) simple binding optimization from v3 to v5. Shown are poses of the optimized NBi and NBii (dark gray, black) on CD2vi and CD2vii (spring green, forest green) respectively. CDR1, 2, & 3 in orange-red, green and cyan, and sequences shown with recent mutations in yellow, and previously mutated residues in orange. Heatmaps depict top two individual mutations tested (in star) for Taguchi experiments leading to final mutations introduced (in star).
Fig. S10. Effects of mutations in optimization. (A) Preferability of residue mutation in CDRs. CDR1, CDR2, CDR3 colored in orange-red, green, and blue respectively. (B) Surface mapping of CDRs of source and optimized NB. CDR surface (border) colored according to electrostatics and hydrophobicity. CDR1, CDR2, CDR3 marked in colored circles (CDR1, CDR2, CDR3 colored in orange-red, green, and cyan). (C) Surface mapping of ASFV CD2v according to electrostatics and hydrophobicity, overlay of nanobody CDRs also marked in colored circles (see above).
Fig. S11. Other measures from 100-ns simulations of NB and controls to CD2vi and CD2vii respectively. Shown are root-mean-squared distance centered on the nanobody (RMSDNB), burSASA, and Hydrogen-bonding (H-bonding) profiles. For CD2vi, most NB or antibodies, except 18a3 show some flattening in the RMSD profiles by the last 10 ns of the simulation. The same was observed for all complexes with CD2vii. 18a3 also appears to have reduced interface binding with CD2vi during the sudden RMSD jump near the end. | ||
| How to Cite this Article |
| Pubmed Style Dulay ANG, Padilla RME, Odchimar NMO, Orosco FL. Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface. Open Vet. J.. 2025; 15(1): 348-387. doi:10.5455/OVJ.2025.v15.i1.33 Web Style Dulay ANG, Padilla RME, Odchimar NMO, Orosco FL. Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface. https://www.openveterinaryjournal.com/?mno=227234 [Access: October 20, 2025]. doi:10.5455/OVJ.2025.v15.i1.33 AMA (American Medical Association) Style Dulay ANG, Padilla RME, Odchimar NMO, Orosco FL. Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface. Open Vet. J.. 2025; 15(1): 348-387. doi:10.5455/OVJ.2025.v15.i1.33 Vancouver/ICMJE Style Dulay ANG, Padilla RME, Odchimar NMO, Orosco FL. Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface. Open Vet. J.. (2025), [cited October 20, 2025]; 15(1): 348-387. doi:10.5455/OVJ.2025.v15.i1.33 Harvard Style Dulay, A. N. G., Padilla, . R. M. E., Odchimar, . N. M. O. & Orosco, . F. L. (2025) Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface. Open Vet. J., 15 (1), 348-387. doi:10.5455/OVJ.2025.v15.i1.33 Turabian Style Dulay, Albert Neil Gura, Richelle Mae Evasco Padilla, Nyzar Mabeth Obenio Odchimar, and Fredmoore Legaspi Orosco. 2025. Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface. Open Veterinary Journal, 15 (1), 348-387. doi:10.5455/OVJ.2025.v15.i1.33 Chicago Style Dulay, Albert Neil Gura, Richelle Mae Evasco Padilla, Nyzar Mabeth Obenio Odchimar, and Fredmoore Legaspi Orosco. "Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface." Open Veterinary Journal 15 (2025), 348-387. doi:10.5455/OVJ.2025.v15.i1.33 MLA (The Modern Language Association) Style Dulay, Albert Neil Gura, Richelle Mae Evasco Padilla, Nyzar Mabeth Obenio Odchimar, and Fredmoore Legaspi Orosco. "Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface." Open Veterinary Journal 15.1 (2025), 348-387. Print. doi:10.5455/OVJ.2025.v15.i1.33 APA (American Psychological Association) Style Dulay, A. N. G., Padilla, . R. M. E., Odchimar, . N. M. O. & Orosco, . F. L. (2025) Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface. Open Veterinary Journal, 15 (1), 348-387. doi:10.5455/OVJ.2025.v15.i1.33 |