| Research Article | ||
Open Vet. J.. 2025; 15(1): 314-324 Open Veterinary Journal, (2025), Vol. 15(1): 314-324 Research Article Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, EgyptHadeer Abd El-hak Rashed1, Layla Omran Elmajdoub2*, Eman Fayad3 and Ali Hussein Abu Almaaty11Zoology Department, Faculty of Science, Port Said University, Port Said, Egypt 2Zoology Department, Faculty of Science, Misurata University, Misurata, Libya 3Department of Biotechnology, College of Sciences, Taif University, Taif, Saudi Arabia *Corresponding Author: Layla Omran Elmajdoub. Zoology Department, Faculty of Science, Misurata University, Misurata, Libya. Email: elmajdoublayla [at] sci.misuratau.edu.ly Submitted: 16/10/2024 Accepted: 13/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
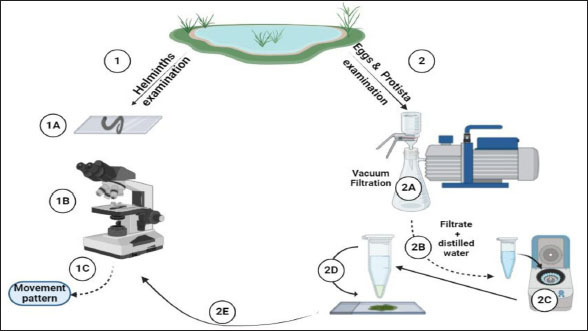
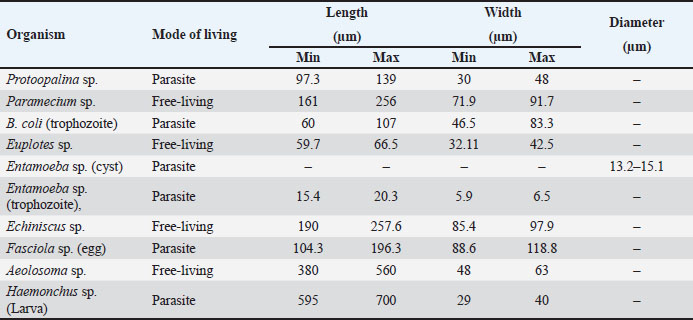
AbstractBackground: Protists and helminths are considered the main organisms invading different cultures, especially aquatic organisms. Morphometric characteristics remain the most trustworthy descriptors of species identification or, more broadly, pattern recognition. Aim: This study aimed to provide morphological descriptions of the most prevalent Protista and helminths found in various street wetlands in Port Said, Egypt. Methods: This study collected three water samples per area each month. The samples were sent to the Parasitology Laboratory at Port Said University, Egypt. The samples were analyzed in two ways according to organism size. Large helminths were observed within 24 hours using a microscope, and their movements were recorded. Protists and helminth eggs were isolated by filtering water through a 0.45 μm cellulose acetate filter, followed by centrifugation. The isolated organisms were counted and imaged. Results: Nine dominant organisms were identified in the collected samples, including five parasitic species (Protoopalina sp., Balantidium coli, Entamoeba sp., Fasciola sp., and Haemonchus sp.) and four free-living species (Paramecium sp., Euplotes sp., Echiniscus sp., and Aeolosoma sp.). Protoopalina sp. was the most abundant parasite (17.6%), exhibiting a cylindrical, elongated shape (97.3–139 µm in length, 30–48 µm in width). Euplotes sp. were the free-living organisms (17%), measuring 59.7–66.5 µm in length and 32.1–42.5 µm in width, featuring cirri and dark bristles. Paramecium sp., with lengths between 161 and 256 µm, was spindle-shaped and had visible contractile vacuoles. B. coli trophozoites measured 60–107 µm in length and 46.5–83.3 µm in width, with a large posterior macronucleus. Haemonchus sp. larvae were about 700 µm long, with a distinct tail filament assisting their complex movement. Conclusion: The street wetlands in the surveyed area contain various free-living and pathogenic taxa. There is a need to link the presence of these organisms to physicochemical analyses. Future studies should prioritize broader geographic sampling, the integration of molecular techniques, and the exploration of host-parasite relationships. Keywords: Free-living, Parasites, Morphometric features, Richness, Movement. IntroductionProtists are found in terrestrial and aquatic environments, where they live freely and as symbionts—including parasites—with a wide variety of species, including humans. These eukaryotes are by far the most diverse; they are often single-celled or colonial microorganisms (Adl et al., 2012). In aquatic systems, tens of thousands of individuals per milliliter are present per gram of bulk soil (Finlay, 2002; Stefan et al., 2014). In water surface systems, Protista plays essential roles in the transfer of energy and nutrients by transforming sunlight to useful chemical power, remineralizing organic compounds, regulating the biomass of microbes, and preserving symbioses (Gadd and Raven, 2010; Worden et al., 2015; Caron et al., 2017). Nematodes are found in soil, water, and sediment environments (Antofica and Poiras, 2009). They are abundant in freshwater sediments, and they play a crucial role in the ecosystem. (Abebe, 2006; Antofica and Poiras, 2009). Trematodes found in the water system either as adult pathogens or as developmental stages (miracidium, rediae, cercariae, and metacercariae) invading the water source itself or the resident fish and invertebrates (Rashied et al., 2016; Jones et al., 2018; Rafiq et al., 2020; Moravec and Prouza, 2024). Shape is considered one of the earliest and still the most trustworthy descriptors for species, or more broadly, pattern identification. The shape is a large-scale expression of multiple organizing, conflicting, and highly regulated biological processes, especially concerning living things (Backes et al., 2012). In contrast to prokaryotes, the Protista are rich in morphological and ultrastructure information. Although many protists can be assigned to cohesive phyletic assemblages because of their remarkable phenotypic diversity, ties between key lineages can occasionally become muddled (Sogin and Silberman, 1998). Numerous ecological processes at the individual, population, community, and ecosystem levels—such as assemblages, species coexistence, invasion spreading, home range, site fidelity, and foraging—are all dependent on the movement of animals (Fryxell et al., 2008; Nathan, 2008; Revilla and Wiegand, 2008). Besides studying the morphometric descriptions and measurements of the different organisms, analyzing their movements contributes to understanding their behavior and response to environmental gradients, which is crucial for understanding nutrient cycles and ecological dynamics and inspiring biomimetic applications (Dickinson et al., 2000; Fenchel, 2002; Berg, 2003; Mo et al., 2020). Hence, the present study aimed to provide some morphological descriptions of the most dominant Protista and helminths invading different street wetlands collected from Port Said, Egypt. Materials and MethodsSamples collectionThree samples per area were collected monthly. The examined areas varied between fifteen and twenty. The collected water samples were directly transported to the Parasitology Laboratory, Zoology Department, Faculty of Science, Port Said University, Egypt. The samples were examined in two pathways according to their sizes (Fig. 1). Tracking large helminthes and movement observationWithin 24 hours, the samples were initially examined under a microscope to separate and count large helminths and track their movements. Using an inverted Optika B5 binocular microscope, the samples’ movements were captured in videos to study their movement pattern (Fig. 1). Isolation of Protista and helminthic eggsAccording to El-Abbassy et al. (2024), every water sample was passed through a 0.45 μm mesh diameter cellulose acetate filter with a suction pump and magnetic funnel. The filtrate was centrifuged for 10 minutes at 3,000 rpm after being diluted in 10 ml of filtered distilled water. Three drops of the precipitate were transferred separately to clean slides, and a coverslip was placed over. The isolated organisms were counted and imaged (Fig. 1). Statistical analysisThe minimum and maximum ranges of the morphometric measurements were recorded. In addition, some data were presented as mean and standard deviation (mean ± SD) using the statistical software SigmaStat (SPSS), version 20. Ethical approvalThe study was approved by the Research Ethics Committee of the Faculty of Science, Suez Canal University (protocol number ERN: PSU.Sci.63. ResultsFrom the collected samples, nine identified organisms were dominant at almost all time points of the sample collection. Five were parasitic forms, and the rest were free-living. Table 1 summarizes the sorting of the collected samples according to mode of living and measurements, including lengths and widths.
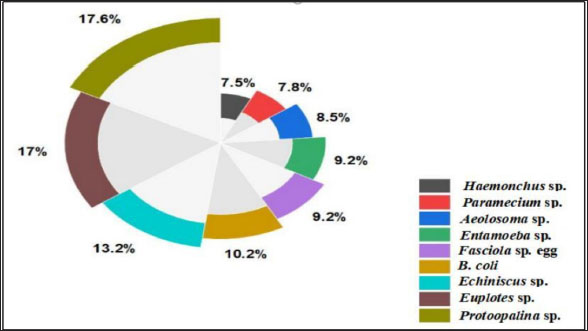
Fig. 1. Experimental design. In addition to the higher number of parasitic species, Protoopalina sp. had the highest richness with a percentage of 17.6%. The highest prevalence of Euplotes sp. among free-living organisms was 17% (Fig. 2). Protoopalina sp. has a cylindrical, elongated shape covered on the outside by numerous small flagella with pointed ends (Fig. 3A). The dimensions range from 97.3 to 139 µm in length and from 30 to 48 µm in width, resulting in a length/width ratio (L/W) of 3.24. Paramecium sp. length varied between 161 and 256 µm, while the recorded width ranged from 71.9 to 91.7 µm. The spindle had a basic elongated shape with a rounded anterior end and a slightly pointed posterior end (Fig. 3B). Paramecium species were easily identified by their two large contractile vacuoles and multiple food vacuoles. The B. coli trophozoites were ovoid in shape, with a blunt, round posterior end and a pointed anterior end. A large macronucleus was identified near the posterior end of the organism (Fig. 3C). Their sizes ranged from 60 to 107 µm in length and from 46.5 to 83.3 µm in width. Table 1. Modes of living and dimensions of the collected organisms.
Fig. 2. Percentage of the different organism’s dominance in water samples.
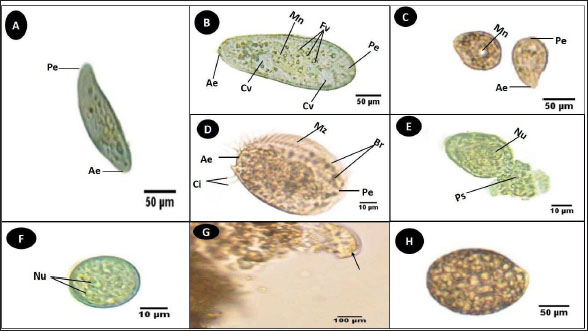
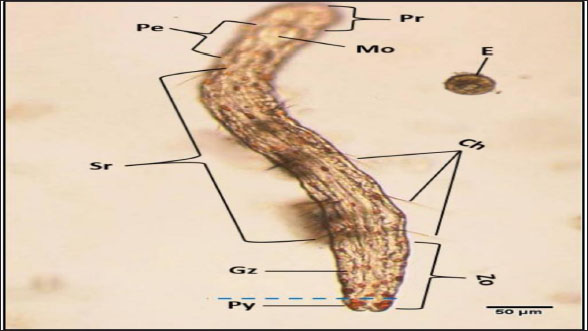
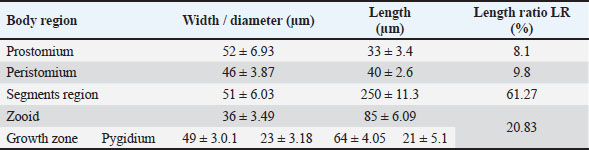
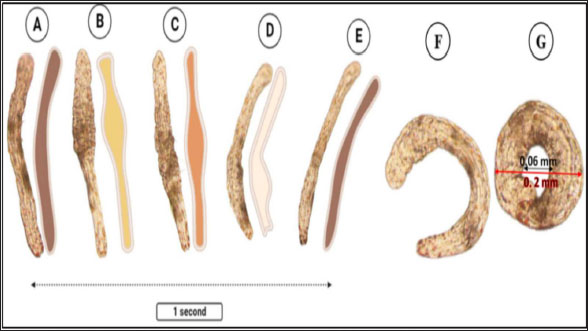
Fig. 3. Photomicrograph of the collected Protozoa and parasitic eggs collected from water samples. (A) Protoopalina sp. (B) Paramecium sp. (C) Trophozoite of B. coli. (D) Euplotes sp. (E) Trophozoite of Entamoeba sp. (F) Cyst of Entamoeba sp. (G) Echiniscus sp. (black arrow) rolling over food particles (H) Egg of Fasciola sp. Ae; anterior end, Pe; posterior end, Cv; contractile vacuole, Fv; food vacuoles, Mn; macronucleus, Ci; cirri, Mz; membranelle zone, Br; bristles, Nu; nucleus, Ps; pseudopodium. Euplotes sp. was characterized by a beige–brownish color. On the dorsal side, the membranelles zone was lined with dark bristles. The external margins of Euplotes sp. also exhibited several cirri (Fig. 3D). The dimensions of the Euplotes sp. ranged from 59.7 to 66.5 µm in length and 32.11 to 42.5 µm in width. The length of Entamoeba sp. trophozoites varied between 15.4 and 20.3µm when pseudopodia were expanded. The width of Protista ranged from 5.9 to 6.5 µm. The diameter of the nucleus was measured between 0.9 and 1.5 µm (Fig. 3E). The cysts were spherical, measuring 13.2 to 15.1 µm in diameter, and four identical nuclei were observed (Fig. 3F). The lengths of the Echiniscus species ranged from 190 to 257.6 µm, while their widths varied between 85.4 and 97.9 µm. The animal swiftly moved around the food particles present in the water samples (Fig. 3G). The primary characteristics of Fasciola sp. eggs include their brown, oval shape, and thin outer shell. The egg dimensions ranged from 104.3 to 196.3 µm in length and from 88.6 to 118.8 µm in width (Fig. 3H). Aeolosoma sp. were dorsoventrally flattened and slender in shape, characterized by a light reddish-brown color with orange pigmented spots, averaging 100 ± 10. These spots were more concentrated in the posterior region than in the anterior. The body was divided into four main regions: the prostomium, peristomium, segmental region, and zooid. The prostomium was club-like in shape, and the mouth was discernible in the peristomium (Fig. 4). The average number of segments was 12 ± 3.72, and they were equipped with chaetae, averaging 40.2 ± 10.3 µm in length. The zooid region consisted of two parts: the growth zone, which constituted most of the area, and a small pygidium (Fig. 4). The average total length of Aeolosoma sp. straight form was 408 ± 70.53 µm, while the average width at the widest region was 52 ± 6.93 µm. Table 2 presents various measurements of the body regions, including width, length, and relative length percentage in relation to the entire body length. The prostomium had the greatest width but the lowest relative length 8.1%. In contrast, the segmental region was the longest, with a relative length of 61.27%. The movement pattern of Aeolosoma sp. can be characterized by a combination of vertical directional (wriggling) and circular bending motions, as illustrated in Fig. 5. When the animal initiated directional movement, it straightened its entire body (Fig. 5A). This movement began in the peristomium and became wider and constricted, while the rest of the body remained straightened (Fig. 5B). The movement then progressed to the segmental region and zooid, where similar contractions occured (Fig. 5C and 5D). Finally, all body regions returned to their original straightened position (Fig. 5E), similar to their position at the start of the movement. The circular bending movement occured in two successive stages: initially, the organism formed a semicircular curvature (Fig. 5F), followed by a complete circular bend with an external bending diameter of approximately 0.2mm (Fig. 5G).
Fig. 4. General description of Aeolosoma sp. Pr; prostomium, Pe; peristomium, M; mouth Sr; segments region, Zo; zooid, Gz; growth zone, Py; pygidium, Ch; Chaetae, E; egg. Table 2. Different measurements of Aeolosoma sp. body regions.
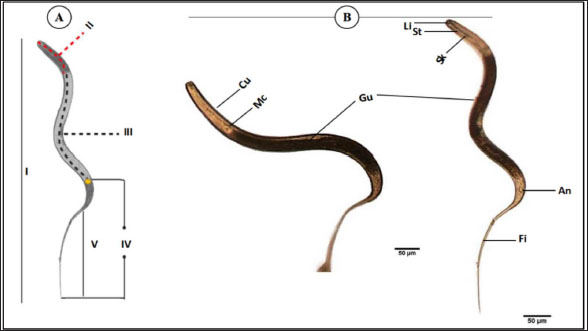
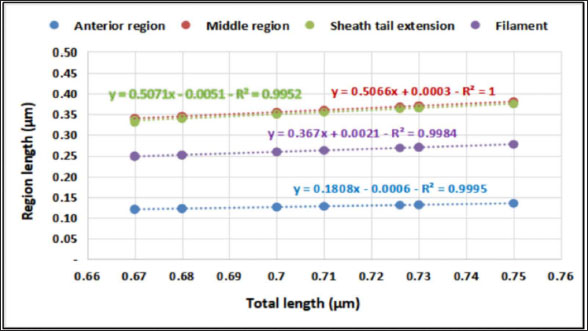
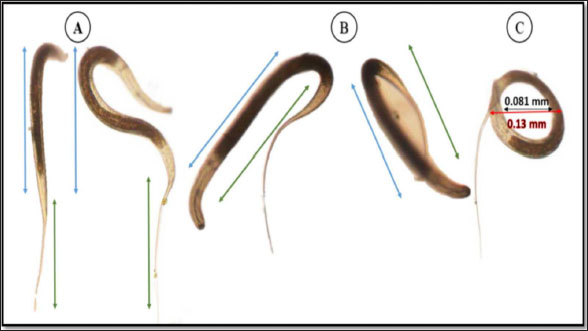
The average total length of the examined Haemonchus sp. larvae was 700 ± 90 µm, with an average width of 32 ± 3.3 µm. The larval body was divided into three regions: semi-rounded anterior, the middle (general viscera), and posterior (sheath, and filament). The anterior region began with a semi-rounded tip surrounded by a lip structure, followed by a stylet that enlarged posteriorly to form a stylet knob. The middle region contained the general viscera, including the reproductive organs and gut, which terminate at the anus. The posterior region extended from the anal area, was referred to as the tail tip, and included the sheath tail extension that forms the filament (Fig. 6A and 6B). The relation between the different body parts and the total length is represented in Figure 7. The regression equations were as follows: y=0.18.8x – 0.0006 → (Anterior region) y=0.5066x + 0.0003 → (Middle region) y=0.5071x + 0.0051 → (Sheath tail extension) y=0.367x + 0.0021 → (Filament) The present research identifies three distinct motion types in Haemonchus sp. The terminal filament in the nematode was the main motor shaft for all motions. The three motion forms of this animal are shown in Figure 8. In the first type of motion (Fig. 8A), the tail was directed in a vertical direction with the rest of the body. Fig. 8B shows the semi-bending motion of the filament and the rest of the body on parallel lines. The complete bending pattern is shown in Fig. 8C, showing an internal bending diameter of 0.081 mm. DiscussionThe dominance of Protoopalina sp. could indicate the presence of its amphibian hosts in the sampling area, as this parasite is transmitted through host feces. Previous studies by Rashed et al. (2023) documented the presence of Protoopalina sp. in Port Said waters, and amphibian species were recorded in the same region by Ibrahim (2008 and 2013). The detection of Entamoeba sp. aligns with the findings of Brožová et al. (2023), who reported this protozoan as one of several parasites that can be transmitted through contaminated food and water. The higher prevalence of Euplotes sp. (17%) in this study compared with the 12.4% found by Marín-Leal et al. (2022) in Lake Maracaibo could be attributed to environmental factors or differences in sampling conditions.
Fig. 5. Aeolosoma sp. movement pattern. (A–E) Vertical movement. (F–G) Circular bending movement.
Fig. 6. General description of Haemonchus sp. larvae. (A) A line drawing showing the main regions of the body. (B) Morphological details of each region. Cu; cuticle, Mc; meta- corpus, Gu; gut, Li; lip region, St; stylet, Sk; stylet knob, An; anus, Fi; filament. I; whole mount, II; anterior region, III; middle region, Iv; sheath tail extension, V; filament. The dimensions of Protoopalina sp. in the present study (97.3–139 µm in length and 30–48 µm in width, with an L/W ratio of 3.24) are notably larger than those reported by Ojeda et al. (2020) for samples collected from Boana pulchella, which ranged from 60 to 77.5 µm in length and 5 to 10 µm in width, yielding a much higher L/W ratio of 9.89. This size difference may reflect variations in environmental factors, host species, and local ecological conditions that influence parasite growth. Paramecium sp. is a single-celled eukaryotic ciliate that ranges in length from 100 to 300 μm, varying according to species (Fokin, 2010). The size range of Paramecium sp. observed in this study (161–256 µm in length and 71.9–91.7 µm in width) indicates that it is a large species within its genus. This range is consistent with earlier reports of ciliate size, suggesting that environmental factors and species variability may play a role in determining the dimensions of Paramecium in different habitats. The rest of the morphological descriptions are consistent with previous studies (Fokin and Chivilev, 2000; Fokin, 2010; Wichterman, 2012).
Fig. 7. Relationship between body region and total length of Haemonchus sp. larva.
Fig. 8. Haemonchus sp. larva movement pattern. (A) Vertical panel. (B) Semi-bending form. (C) Complete bending form. The B. coli trophozoites recorded in the current study differ from those recorded by Ponce-Gordo and Jirků-Pomajbíková (2017), who noted that their lengths ranged from 100 to 150 µm. Morphological variations may be linked to differences in growth conditions (Levine, 1961). A similar nutrient-related variability was observed in in vitro cultures (Barbosa Ada et al., 2015). The genus Euplotes (Ehrenberg, 1830) is found in nearly all types of habitats and includes a large number of recognized species, with over 70 species identified (Wilbert and Song, 2008; Shao et al., 2010; Di Giuseppe et al., 2011, 2013, 2015). The morphometric characteristics both match and differ in some respects from those documented in previous studies (Abraham et al., 2021). This variation could be attributed to differences in the species or sampling areas. Based on the morphological characteristics described in the results section for Entamoeba samples, the isolated sample may correspond to E. histolytica, E. moshkovskii, or E. dispar, as these three species share similar morphological features in both life stages (Brumpt, 1925). However, these distinctions were largely overlooked until 1978 when significant advances in immunology, biochemistry, and genetics demonstrated that E. histolytica actually comprises two distinct species: the invasive pathogen (E. histolytica) and the non-invasive species (E. dispar) (Diamond and Clark, 1993). The body length measurements obtained in this study for Echiniscus sp. are consistent with those reported by Gąsiorek et al. (2021), who found a length range of 192–273 µm for Echiniscus species. The phylum Tardigrada includes Echiniscus species and consumes a wide variety of food types across different habitats, such as bacteria, detritus, algae, plant cell contents, fungi, protozoans, and small metazoans, including rotifers, nematodes, and other tardigrades (Nelson et al., 2015). The presence of the animal was concomitant with the presence of these food particles, as the animal was observed moving around them. Aeolosoma sp. exhibit light-reddish-brown coloration with orange-pigmented spots. Their body was divided into four primary regions: the prostomium, peristomium, segmental region, and zooid. The segmental region was equipped with chaetae. The body color, regions, and presence of chaetae align with previous descriptions (Paxton, 2000; Timm, 2020; Rouse et al., 2022). The chaetae, measuring 40.2 ± 10.3 µm, are classified as short according to Simioni and Garraffoni (2024), who categorized chaetae into long (93–174 µm) and short (32–64 µm) types. Among the body regions, the prostomium had the greatest width (52 ± 6.93 µm) but the lowest length (33 ± 3.4 µm). Simioni and Garraffoni (2024) also noted that the prostomium was wider than it was long and is semi-elliptical in shape, with dimensions of 109–142 µm in length and 147 µm in width. The current study is the first to provide detailed insights into the movement of Aeolosoma sp. The movement pattern of Aeolosoma sp. can be described as a blend of vertical directional (wriggling) and circular bending motions. When Aeolosoma sp. began to move directionally, it straightened its entire body, starting with the peristomium, which widened and constricted. This straightening continued through the segmental region and zooid region, where similar contractions occurred before all body regions returned to their original shape. Gusakov et al. (2023) reported that the family Aeolosomatidae employs wriggling movements for swimming. The worm was able to bend itself to an external bending diameter of approximately 0.2 mm. This remarkable bending efficiency can be attributed to the considerable flexibility of the annelids. The annelid cuticle functions as a flexible exoskeleton that is specifically designed to withstand the internal pressure generated by muscle contractions. The cylindrical or near-cylindrical bodies of annelids are encased in a helical arrangement of cuticle fibers, which traverse the body in alternating layers of left- and right-handed spirals (Picken, 1960). This arrangement is optimal for accommodating variable pressures while also providing the high degree of flexibility necessary for annelids to undergo substantial changes in their resting length (Cowey, 1952). Key morphological features used to identify nematodes include the shape of the head, number of annulus, body length, stylet length, shape of the stylet knob, structure of the lateral fields, and presence or absence of a tail sheath (Handoo et al., 2008; Wyk and Mayhew, 2013). These characteristics were used in our descriptive study, as mentioned in the results section. The average total length of the examined Haemonchus sp. larvae was 700 ± 90 µm, with an average width of 32 ± 3.3 µm. These measurements are consistent with those reported by Abuelwafa et al. (2016), who estimated larval sizes ranging between 600 and 850 µm. The relationships of various parts of Haemonchus sp. with their total length were first characterized. Such descriptive measurements are essential tools for the classification of nematodes (Kornaś et al., 2009; Abuelwafa et al., 2016). The locomotion of Haemonchus sp, as highlighted in previous (Gray and Lissmann, 1964) and current studies, provides valuable insights into the complex mechanics of movement in nematodes. Previous research has primarily focused on the wave dynamics of swimming, noting that Haemonchus sp. generates less than a complete wave during certain phases of its movement cycle. This incomplete wave formation results in unique locomotion characteristics, including the presence of two distinct nodes around which the body glides tangentially. These nodes facilitate yawing motion, allowing the organism to navigate its environment effectively. Interestingly, while one might expect that the yawing motion would decrease swimming speed, the observed speed of Haemonchus was greater than theoretical predictions, indicating a more complex interaction between body dynamics and environmental forces than previously understood. The current study shifts the focus to the structural role of the tail filament as a critical component of Haemonchus locomotion. The tail filament’s role in stabilizing and fine-tuning these movements adds a significant layer to our understanding of Haemonchus locomotion, as it allows for adaptability to different environmental contexts. ConclusionIn this study, nine distinct organisms were identified from the collected samples: five were parasitic or pathogenic, and four were free-living. The dominant parasitic organisms were Protoopalina sp., B. coli, Entamoeba sp., Fasciola sp., and Haemonchus sp., while the free-living species were Paramecium sp., Euplotes sp., Echiniscus sp., and Aeolosoma sp. The parasitic Protoopalina sp. was most abundant (17.6%), suggesting the presence of amphibian hosts in the area, as indicated by previous studies. In contrast, Euplotes spp., a free-living organism, exhibited a prevalence of 17%. Morphological analysis of key species showed variations from previous records, likely due to environmental and biological factors. Additionally, the movement patterns of both parasitic and free-living species, such as Aeolosoma sp. and Haemonchus sp., revealed insights into their adaptability and locomotion mechanisms, further contributing to our understanding of these organisms in aquatic environments. Future research should focus on expanding geographic sampling, incorporating molecular analysis, and investigating host-parasite dynamics. Additionally, long-term monitoring of species and water quality is recommended to better understand environmental impacts and public health risks. AcknowledgmentThe authors express their gratitude to Port Said University. Conflicts of interestThe author declares no conflict of interest. FundingThis study was self-funded by the authors, with no external funding received. Author’s contributionsAll authors made equal contributions to this work. They reviewed, revised, and approved the final version of the manuscript. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesAbebe, E. 2006. Preface. In: Freshwater nematodes: ecology and taxonomy. Eds., Abebe, E., Traunspurger, W. and Andrássy, I. Wallingford, UK: CABI Publisher, pp: 752. Abraham, J.S., Somasundaram, S., Maurya, S., Gupta, R., Makhija, S. and Toteja, R. 2021. Characterization of Euplotes lynni nov. spec., E. indica nov. spec., and descriptions of E. aediculatus and E. woodruffi (Ciliophora, Euplotidae) using an integrative approach. Eur. J. Protistol. 79, 125779. Abuelwafa, S.A., Al-kappany, Y.M. and El-Alfy, E.N. 2016. Identification of nematodes third stage larvae of ruminant animals. Egypt. Vet. Med. Soc. Parasitol. J. 12(1), 60–73. Adl, S.M., Simpson, A.G., Lane, C.E., Lukeš, J., Bass, D., Bowser, S.S., Brown, M.W., Burki, F., Dunthorn, M., Hampl, V., Heiss, A., Hoppenrath, M., Lara, E., Le Gall, L., Lynn, D.H., McManus, H., Mitchell, E.A., Mozley-Stanridge, S.E., Parfrey, L.W., Pawlowski, J., Rueckert, S., Shadwick, L., Schoch, C.L., Smirnov, A. and Spiegel, F.W. 2012. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 59(5), 429–514. Antofica, A. and Poiras, L. 2009. Species diversity of freshwater and soil nematodes of some localities along the Dniester river. Oltenia 25, 51–55. Backes, A.R., Bruno, M.O. and and Florindo, J.B. 2012. Shape analysis using fractal dimension: a curvature based approach. Chaos 22, 043103. Barbosa Ada, S., Bastos, O.M., Uchôa, C.M., Pissinatti, A., Ferreira Filho, P.R., Dib, L.V., Azevedo, E.P., de Siqueira, M.P., Cardozo, M.L. and Amendoeira, M.R. 2015. Isolation and maintenance of Balantidium coli (Malmsteim, 1857) cultured from fecal samples of pigs and non-human primates. Vet. Parasitol. 210(3–4), 240–245. Berg, H.C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72, 19–54. Brožová, K., Jirků, M., Lhotská, Z., Květoňová, D., Kadlecová, O., Stensvold, C.R., Samaš, P., Petrželková, K.J. and Jirků, K. 2023. The opportunistic protist Giardia intestinalis, occurs in gut-healthy humans in a high-income country. Emerg. Microbes. Infect. 12(2), 2270077. Brumpt, E. 1925. Étude sommaire de l’Entamoeba dispar n. sp. Amibe à kystes quadrinucléés, parasite de l’homme. Bull. Acad. Med. 94, 943–952. Caron, D.A., Alexander, H., Allen, A.E., Archibald, J.M., Armbrust, E.V., Bachy, C., Bell, C.J., Bharti, A., Dyhrman, S.T., Guida, S.M., Heidelberg, K.B., Kaye, J.Z., Metzner, J., Smith, S.R. and Worden, A.Z. 2017. Probing the evolution, ecology and physiology of marine protists using transcriptomics. Nat. Rev. Microbiol. 15, 6–20. Cowey, J.B. 1952, The structure and function of the basement membrane muscle system in Amphiporus lactifloreus (Nemertea). Quart. J. Microsc. Sci. 93, 1. Di Giuseppe, G., Barbieri, M., Vallesi, A., Luporini, P. and Dini, F. 2013. Phylogeographical pattern of Euplotes nobilii, a protist ciliate with a bipolar biogeographical distribution. Mol. Ecol. 22, 4029–4037. Di Giuseppe, G., Dini, F., Vallesi, A. and Luporini, P. 2015. Genetic relationships in bipolar species of protist ciliate, Euplotes. Hydrobiologia 761, 71–83. Di Giuseppe, G., Erra, F., Dini, F., Alimenti, C., Vallesi, A., Pedrini, B., Wuthrich, K. and Luporini, P. 2011. Antarctic and Arctic populations of the ciliate Eupotes nobilii show common pheromone-mediated cell-cell signaling and cross-mating. Natl. Acad. Sci. U.S.A. 108, 3181–3186. Diamond, L.S. and Clark, C.G. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911)separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40, 340–344. Dickinson, M.H., Farley, C.T., Full, R.J., Koehl, M.A., Kram, R. and Lehman, S. 2000. How animals move: an integrative view. Science 288(5463), 100–106. Ehrenberg, C.G. 1830. Organisation, Systematik und geographisches Verhaltniss der Infusionsthierchen. Zwei Vortrage. In Commission bei Dummler. El-Abbassy, S.A., Reda, E.S. and Marzouk, A.M. 2024. Isolation and identification of some fresh Protozoa from surface water sources and taps in Dakahlyia Governorate, Egypt. J. Med. Life. Sci. 6(2), 115–131. Fenchel, T. 2002. Microbial behavior in a heterogeneous world. Science 296(5570), 1068–1071. Finlay, B.J. 2002. Global dispersal of free-living microbial eukaryote species. Science 296, 1061–1063. Fokin, S.I. 2010. Paramecium genus: biodiversity, some morphological features and the key to the main morphospecies discrimination. Protistol 6, 227–235. Fokin, S.I. and Chivilev, S.M. 2000. Paramecium morphometric analysis and taxonomy. Acta. Protozool. 39(1), 1–14. Fryxell, J.M., Hazell, M., Börger, L., Dalziel, B.D., Haydon, D.T. and Morales, J.M. 2008. Multiple movement modes by large herbivores at multiple spatiotemporal scales. Proc. Natl. Acad. Sci. U.S.A. 105, 19114–19119. Gadd, G.M. and Raven, J.A. 2010. Geomicrobiology of eukaryotic microorganisms. Geomicrobiol. J. 27, 491–519. Gąsiorek, P., Bochnak, M., Vončina, K. and Michalczyk, Ł. 2021. Phenotypically exceptional Echiniscus species (Heterotardigrada: Echiniscidae) from Argentina (Neotropics). Zool. Anz. 294, 210–228. Gray, J. and Lissmann, H.W. 1964. The locomotion of nematodes. J. Exp. Biol. 41(1), 135–154. Gusakov, V., Dien, T.D., Tran, H.Q., Thanh, N.T.H., Huan, P.T., Ha, V.T. and Dinh, C.N. 2023. An annotated checklist of the main representatives of meiobenthos from inland water bodies of central and southern Vietnam—ii—annelid worms (Oligochaeta and Aeolosomatidae). Water 15(12), 2273. Handoo, Z.A., Carta, L.K. and Skantar, A.M. 2008. Taxonomy, morphology and phylogenetics of coffee-associated root-lesion nematodes, Pratylenchus spp. Plant-parasitic nematodes of coffee. Dordrecht, Netherlands: Springer, pp: 29–50. Ibrahim, A.A. 2008. Amphibians and reptiles of the Suez Canal University campuses, Egypt. Herpetol. Bull. 105, 1–9. Ibrahim, A.A. 2013. Amphibians of Egypt: a troubled resource. Basic Appl. Herpetol. 27, 107–117. Jones, R.A., Brophy, P.M., Davis, C.N., Davies, T.E., Emberson, H., Rees Stevens, P. and Williams, H.W. 2018. Detection of Galba truncatula, Fasciola hepatica, and Calicophoron daubneyi environmental DNA within water sources on pasture land, a future tool for fluke control? Parasit. Vectors. 11, 1–9. Kornaś, S., Gawor, J., Cabaret, J., Molenda, K., Skalska, M. and Nowosad, B. 2009. Morphometric identification of Equid cyathostome (Nematoda: Cyathostominae) infective larvae. Vet. Parasitol. 162(3–4), 290–294 Levine, N.D. 1961. Protozoan parasites of domestic animals and of man. Washington, DC: Smithsonian Institution. Marín-Leal, J.C., Rincón-Miquilena, N.J., Díaz-Borrego, L.C. and Pire-Sierra, M.C. 2022. Toxicidade aguda de elementos potencialmente tóxicos em protozoários ciliados do Lago de Maracaibo (Venezuela). Acta. Limnol. Bras. 34, e21. Mo, X., Ge, W., Miraglia, M., Inglese, F., Zhao, D., Stefanini, C. and Romano, D. 2020. Jumping locomotion strategies: from animals to bioinspired robots. Appl. Sci. 10(23), 8607. Moravec, F. and Prouza, A. 2024. Some trematodes including three new species from freshwater fishes of Venezuela. Folia. Parasitol. 71, 1–19 Nathan, R. 2008. An emerging movement ecology paradigm. Proc. Natl. Acad. Sci. U.S.A 105, 19050–19051. Nelson, D., Guidetti, R. and Rebecchi, L. 2015. Phylum Tardigrada. In: Ecology and general biology: Thorps and Covich´s freshwater invertebrates. Eds., Rogers, T.J.D.C. Cambridge, MA: Academic Press Publisher, pp: 347–380. Ojeda, M.A.V., Guagliardo, S.E. and Tanzola, R.D. 2020. New species of Protoopalina (Opalinida) and first record of Zelleriella hylaxena (Opalinida) in tadpoles of Boana pulchella (Anura, Hylidae) from Argentina. Pan-Am. J. Aquat. Sci. 15(4), 252–257. Paxton, H. 2000. Family Aeolosomatidae. In: Polychaetes and allies: the southern synthesis. Eds., Beesley, P.L. and Ross, G.J.B. Clayton, Victoria: CSIRO Publisher, pp: 321–322. Picken, L.E.R. 1960. The organization of cells and other organisms. Oxford, UK: Clarendon Press. Ponce-Gordo, F. and Jirků-Pomajbíková, K. 2017. Balantidium coli water and sanitation for the 21st century: health and microbiological aspects of excreta and wastewater management (global water pathogen project). UNESCO-international hydrological programme. East Lansing, MI: Michigan State University, pp: 10. Rafiq, N., Ayaz, S., Niaz, S., Haleem, S., Ullah, R., Bari, A., Bourhia, M. and Ali, E.A. 2022. Changes in the prevalence of natural Paramphistomum cercariae infection in Indoplanorbis and Lymnaea intermediate hosts influenced by meteorological factors. J. Trop. Med. (1), 8719834. Rashed, H.A., Fayad,E., AlMaliki, N.A.M., Abu Almaaty, A.H. and Tawfik, M.M. 2023. Comparative screening of the possible activity of Rhopilema nomadica jellyfish venom against different Protista and invertebrates. Egypt. J. Aquat. Biol. Fish. 27(5), 689–701. Rashied, H.A., Abu Almaaty, A.H., Hassan, E.A. and Soliman, F.M. 2016. Comparative Study of the effect of biological factors on helminthes occurrence in Oreochromis niloticus and Tilapia zilli from Lake Manzala, Port Said, Egypt. Egypt. Acad. J. Biol. Sci. B Zool. 8(1), 25–32. Revilla, E. and Wiegand, T. 2008. Individual movement behavior, matrix heterogeneity, and the dynamics of spatially structured populations. Proc. Natl. Acad. Sci. U.S.A. 105, 19120–19125. Rouse, G., Pleijel, F. and Tilic, E. 2022. Annelida. New York, NY: Oxford University Press. Shao, C., Ma, H., Gao, S., Khaled, A.A. and Song, W. 2010. Reevaluation of cortical developmental patterns in Euplotes (s.l.), including a morphogenetic redescription of E. charon (Protozoa, Ciliophora, Euplotida). Chin. J. Oceanol. Limn. 28, 593–602. Simioni, N.D.G. and Garraffoni, A.R.S. 2024. In the footsteps of Prof. Ernst Marcus: redescription and lectotypes/paralectotypes designations of Aeolosoma species (Annelida, Aeolosomatidae) from original material. Ocean. Coast. Res. 72(suppl 1), e24047. Sogin, M.L. and Silberman, J.D. 1998. Evolution of the protists and protistan parasites from the perspective of molecular systematics. Int. J. Parasitol. 28(1), 11–20. Stefan, G., Cornelia, B., Jörg, R. and Michael, B. 2014. Soil water availability strongly alters the community composition of soil protists. Pedobiologia 57(4–6), 205–213. Timm, T. 2020. Class Aphanoneura. In: freshwater invertebrates: keys to neotropical and Antarctic fauna. Eds. Rogers, C., Damborenea, C. and Thorp, J. Cambridge, MA: Academic Press Publisher, pp: 475–479. Wichterman, R. 2012. The biology of Paramecium. Berlin, Germany: Springer Science and Business Media. Wilbert, N. and Song, W. 2008. A further study on littoral ciliates (Protozoa, Ciliophora) near King George Island, Antarctica, with description of new genus and seven new species. J. Nat. Hist. 42, 979–1012. Worden, A.Z., Follows, M.J., Giovannoni, S.J., Wilken, S., Zimmerman, A.E. and Keeling, P.J. 2015. Rethinking the marine carbon cycle: factoring in the multifarious lifestyles of microbes. Science 347, 1257594. Wyk, J.V. and Mayhew, E. 2013. Morphological identification of parasitic nematode-infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res. 80(1), 1–14. | ||
| How to Cite this Article |
| Pubmed Style Rashed HAE, Elmajdoub LO, Fayad E, Almaaty AHA. Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt. Open Vet. J.. 2025; 15(1): 314-324. doi:10.5455/OVJ.2025.v15.i1.30 Web Style Rashed HAE, Elmajdoub LO, Fayad E, Almaaty AHA. Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt. https://www.openveterinaryjournal.com/?mno=224888 [Access: October 20, 2025]. doi:10.5455/OVJ.2025.v15.i1.30 AMA (American Medical Association) Style Rashed HAE, Elmajdoub LO, Fayad E, Almaaty AHA. Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt. Open Vet. J.. 2025; 15(1): 314-324. doi:10.5455/OVJ.2025.v15.i1.30 Vancouver/ICMJE Style Rashed HAE, Elmajdoub LO, Fayad E, Almaaty AHA. Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt. Open Vet. J.. (2025), [cited October 20, 2025]; 15(1): 314-324. doi:10.5455/OVJ.2025.v15.i1.30 Harvard Style Rashed, H. A. E., Elmajdoub, . L. O., Fayad, . E. & Almaaty, . A. H. A. (2025) Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt. Open Vet. J., 15 (1), 314-324. doi:10.5455/OVJ.2025.v15.i1.30 Turabian Style Rashed, Hadeer Abd El-hak, Layla Omran Elmajdoub, Eman Fayad, and Ali Hussein Abu Almaaty. 2025. Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt. Open Veterinary Journal, 15 (1), 314-324. doi:10.5455/OVJ.2025.v15.i1.30 Chicago Style Rashed, Hadeer Abd El-hak, Layla Omran Elmajdoub, Eman Fayad, and Ali Hussein Abu Almaaty. "Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt." Open Veterinary Journal 15 (2025), 314-324. doi:10.5455/OVJ.2025.v15.i1.30 MLA (The Modern Language Association) Style Rashed, Hadeer Abd El-hak, Layla Omran Elmajdoub, Eman Fayad, and Ali Hussein Abu Almaaty. "Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt." Open Veterinary Journal 15.1 (2025), 314-324. Print. doi:10.5455/OVJ.2025.v15.i1.30 APA (American Psychological Association) Style Rashed, H. A. E., Elmajdoub, . L. O., Fayad, . E. & Almaaty, . A. H. A. (2025) Morphometric and richness analysis of free-living and parasite taxa-invading street wetlands in Port Said, Egypt. Open Veterinary Journal, 15 (1), 314-324. doi:10.5455/OVJ.2025.v15.i1.30 |