| Research Article | ||
Open Vet. J.. 2025; 15(1): 307-313 Open Veterinary Journal, (2025), Vol. 15(1): 307-313 Research Article Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot studyFrancesca Perondi1, Ilaria Lippi1, Natascia Bruni2, Nicolò Lonigro3 and Elisa Martello4*1Department of Veterinary Science, University of Pisa, Pisa, Italy 2Candioli Pharma S.r.l., Beinasco, Turin, Italy 3Department of Drug Science and Technology, University of Turin, Turin, Italy 4Division of Epidemiology and Public Health, School of Medicine, University of Nottingham, Nottingham, UK *Corresponding Author: Elisa Martello. Division of Epidemiology and Public Health, School of Medicine, University of Nottingham, Nottingham, UK. Email: martello.elisa [at] gmail.com Submitted: 16/10/2024 Accepted: 27/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
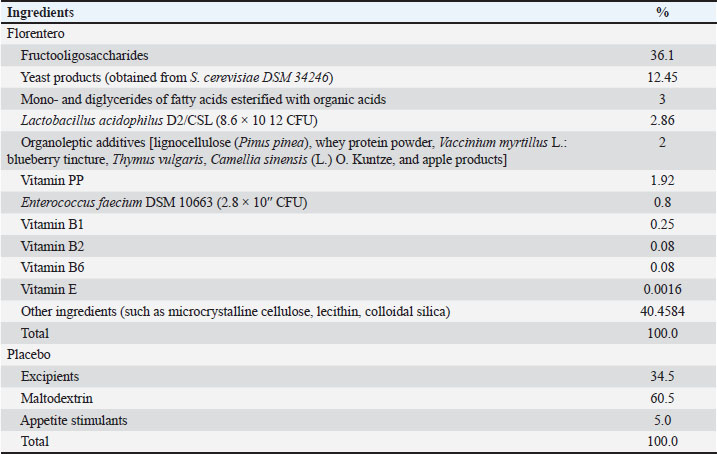
AbstractBackground: Chronic kidney disease (CKD) in dogs is often accompanied by dysbiosis and abnormal gut microbiota composition, leading to gastrointestinal issues. Probiotics, including Lactobacillus acidophilus, Enterococcus faecium, and Saccharomyces cerevisiae, have shown promise in maintaining a healthy gut microbiota in both healthy and CKD animals. Aim: This pilot double-blinded, randomized controlled study aimed to assess the effects of a feed supplement containing these probiotics on clinical parameters and fecal consistency in dogs with CKD and intestinal disorders. Methods: Eight dogs with CKD were randomly assigned to receive the supplement (TRT, n=4) or placebo (CTR, n=4) alongside a renal commercial complete dietetic feed and another supplement specific for CKD for 50 days. Clinical parameters, such as body condition score and fecal score, were regularly monitored. Results: The dogs in the TRT group exhibited improvements in fecal consistency and clinical parameters compared with the CTR group. Conclusion: The feed supplement under investigation seems beneficial for improving clinical conditions, gut health, and stool quality in dogs with CKD. Future studies with larger sample sizes and microbiota analyses are needed to gain a better understanding of the potential benefits of this supplement in managing CKD in dogs. Keywords: Renal disease, Beneficial bacteria, Gut, Stool texture, Canine, CKD. IntroductionChronic kidney disease (CKD) is characterized by persistent abnormalities in kidney structure or function lasting for >3 months. This condition commonly affects dogs (Polzin, 2011; Bartges, 2012). Dysbiosis and abnormal composition of the intestinal microbiota have been reported in both humans with CKD and veterinary patients (Ephraim and Jewell, 2020; Meineri et al., 2021; Bartochowski et al., 2022). Several gastrointestinal issues have been reported in patients with CKD, including reduced gut motility, increased intestinal permeability, bacterial translocation, and intestinal inflammation (Bartochowski et al., 2022). The gut–kidney axis has gained increasing attention in recent years, with studies conducted on both in vivo and animal models of CKD, aiming to understand the changes occurring in the gut microbiota of these patients (Bartochowski et al., 2022). The gastrointestinal tract appears to play a role in the pathophysiology of uremic syndrome and contributes to clinical symptoms, such as anorexia, nausea, diarrhea, malnutrition, and protein-energy wasting (Lippi et al., 2017; Bartochowski et al., 2022). Conversely, in CKD, uremic metabolites can damage the intestinal epithelial tight junctions and disrupt barrier function, leading to alterations in the gut microbiota (Ephraim and Jewell, 2020). The use of probiotics has been documented in both healthy and CKD animals as a means to maintain a healthy balance of the gastrointestinal microbiota (Bruni et al., 2020, Marelli et al., 2020; Meineri et al., 2021). Probiotics have shown the ability to modulate the intestinal microbiota, leading to improvements in fecal parameters, such as the fecal score (FS) (Bruni et al., 2020). Additionally, there have been reports of probiotics being effective in controlling the progression of CKD in dogs (Lippi et al., 2017; Meineri et al., 2021). Among the probiotics Lactobacilli, Lactobacillus acidophilus is one of the most commonly studied strains, and it has demonstrated beneficial effects in maintaining the nutritional status and improving kidney function in dogs with CKD (Lippi et al., 2017; Meineri et al., 2021). Lactobacillus acidophilus stabilizes the gut microbiota and strengthens the gastrointestinal barrier by limiting the adhesion of pathogens (Meineri et al., 2021). It has also been found to suppress mucosal inflammation and restore cytokine balance, thereby promoting a healthier gut environment [8]. Enterococcus faecium is another probiotic used in dogs that has shown an improvement and positive effect on fecal quality (Gagné et al., 2013; Nybroe et al., 2023). While probiotics, including L. acidophilus and E. faecium, have demonstrated positive effects on the gut microbiota in healthy dogs (Tanprasertsuk et al., 2021), there is currently no evidence regarding their impact on gastrointestinal health, specifically in dogs with kidney failure. However, recent studies have shown that live yeast products derived from Saccharomyces cerevisiae have beneficial effects on intestinal functionality in dogs, with positive effects on microbial function (Bastos et al., 2023). Saccharomyces cerevisiae supplementation appears to modulate the gut microbiota, promoting a balanced microbial community and improving gut homeostasis while reducing the presence of potential pathogens (Bastos et al., 2023). The aim of our study was to evaluate the synergistic effect of probiotics (L. acidophilus D2/CSL, E. faecium) and yeast products (S. cerevisiae) administered as a food supplement on FS and clinical parameters in dogs with CKD. Materials and MethodsA double-blind randomized controlled study on azotemic dogs with a confirmed diagnosis of CKD was conducted at the Veterinary Teaching Hospital of the University of Pisa, Italy. A total of eight dogs were enrolled in the trial, and they were randomly divided into two groups: a treatment group (TRT) consisting of four dogs and a control group (CTR) also consisting of four dogs. The assignment of the dogs to the respective groups was unknown to both the investigator and the owners at the end of the study. Prior to the study, the owners were informed about the study protocol and provided informed consent. The experimental protocol was designed in accordance with the guidelines outlined in the current European and Italian laws concerning the protection of animals used for scientific purposes, specifically directive 2010/63/EU. These laws were enforced in Italy under regulation D.L. 2014/26. All eight animals in the study were fed a commercial complete dietetic dog feed formulated to support renal function in cases of chronic or temporary renal insufficiency (Monge Vetsolution Renal and Oxalate Canine). In addition to the commercial complete dietetic feed, the dogs in both groups received a treatment (composed of sodium bicarbonate (HCO3), chitosan, and calcium carbonate) for addressing the progression of CKD (Polzin, 2011; Bartges, 2012; IRIS Guidelines, 2023). Furthermore, in the CTR group, a placebo was added to the food along with renal therapy. On the other hand, the TRT group received a new supplement called Florentero [Candioli Pharma S.r.l., 1 tablet 5–10 kg or 2 tablets 10–20 kg body weight (BW)/day]. This supplement is rich in probiotics and bacteriocins. The compositions of the supplement and placebo groups are presented in Table 1. Each dog participated in the trial for 50 days. A complete clinical examination by the same veterinarian and the blood and urinary analysis were performed at the beginning of the study (T0) and then every 10 days [days 10 (T1), 20 (T2), 30 (T3), 40 (T4), and 50 (T5)]. Dogs were included if they were affected by CKD (from stage 2 to 4) following the IRIS guidelines (IRIS Guidelines, 2023), whereas they were excluded if they were affected by pancreatitis and/or primary chronic enteropathy before the diagnosis of renal disease. Primary chronic enteropathy is defined as chronic gastrointestinal disease (including weight loss, vomiting, diarrhea, and decreased appetite) persisting for at least 3 weeks. In addition, abdominal ultrasound at the beginning of the study was used to exclude patients with extraintestinal or nonprimary inflammatory intestinal diseases (e.g., intussusception, foreign bodies, or intestinal tumors). The diagnosis of pancreatitis was based on two or more of the following clinical signs: (1) abdominal pain, diarrhea, vomiting, or anorexia/hyporexia; (2) the presence of an abdominal ultrasound performed by a radiologist (Xario XG, Toshiba) without other identifiable extra-pancreatic diseases; and (3) an abnormal IDEXX SNAP cPL test result. The following examinations were performed by the same trained veterinarian, and the data were collected at each time point (T0 to T5) during the trial. Clinical examinationClinical examination: BW; body condition score (BCS) (9-point scale) (Laflamme, 1997); and muscular condition score (MCS) (3-point scale) (WSAVA, 2013). Systemic blood pressure (SBP) (mmHg) (IRIS Guidelines, 2023). Canine inflammatory bowel activity index (CIBDAI): The CIBDAI scoring system assesses six clinical data of disease severity, including evaluation of attitude and activity [from 0 (normal) to 3 (severely decreased)], appetite [from 0 (normal) to 3 (severely decreased)), vomiting [from 0 (<1 event/week) to 3 (>3 events/week)], fecal consistency [from 0 (normal) to 3 (watery diarrhea)], fecal frequency [from 0 (normal; <2 events/day) to 3 (severely increased; >5 events/day)], and weight loss [from 0 (none) to 3 (> 10%)]. Based on the CIBDAI value, the severity of enteropathy is assessed as a clinically insignificant-mild disease (CIBDAI 0-5) and moderate-to-very severe disease (CIBDAI≥ 6) (Jergens et al., 2003). FS (7-point scale) (Greco, 2011). Table 1. Composition of the feed supplement (Florentero, Candioli Pharma S.r.l.) and the placebo used during the study.
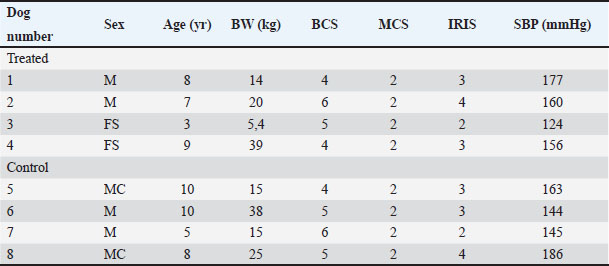
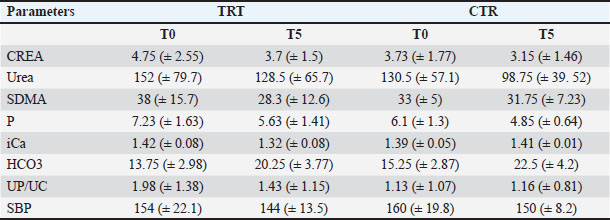
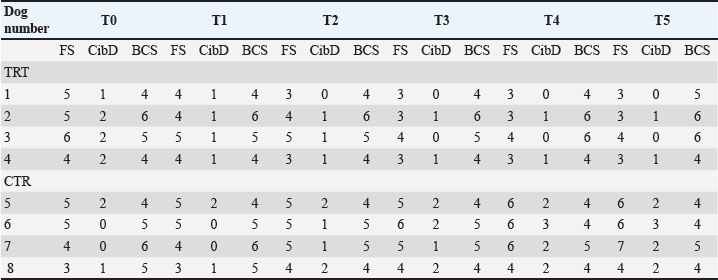
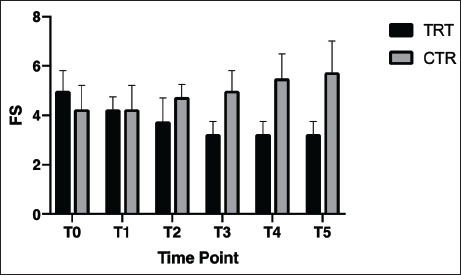
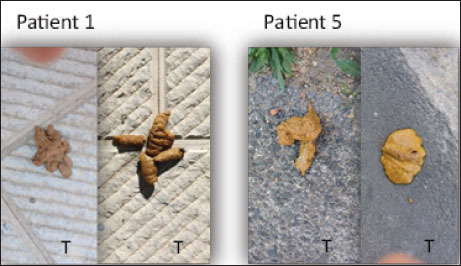
Blood, urine, and fecal samplesComplete blood count and serum biochemistry [creatinine-CREA, urea, phosphorous (P), ionic calcium (iCa), (HCO3)], Symmetric dimethylarginine (SDMA) (Hall et al., 2016), Complete urinalysis Urinary protein/Urinary creatinine (UP/UC: total protein-to-creatinine ratio) with sediment fecal examination. Statistical analysisDescriptive statistics were used to summarize the data for blood parameters (mean and standard deviation). Median values were used for the FS comparison between the two groups. A two-way ANOVA (Geisser-Greenhouse correction) was performed using GraphPad Prism 8. ResultsIn the present study, a total of 10 dogs were initially included. However, two dogs were excluded from the study after a few days. One dog was excluded due to worsening symptoms, while the other dog died. Out of the remaining eight dogs, there were two sterilized females, two sterilized males, and four intact males. The age of the dogs in the study is presented in Table 2. Additionally, Table 2 provides information about the clinical examination, including BW, BCS, and MCS. The IRIS classification with the corresponding upstaging parameters (SBP and UP/UC values) of all dogs at the time of inclusion is also presented in Table 2. Most of the dogs were in the advanced stages of kidney disease, with four dogs in IRIS stage 3 (including two in the TRT group), two dogs in IRIS stage 4 (including one in the TRT group), and only two dogs in IRIS stage 2 (including one in the TRT group). In addition, three dogs were hypertensive (including one in the TRT group), and four dogs had significant proteinuria (including two in the TRT group) (Table 3). In Table 3, we reported the mean values of different hematological and urinary parameters of the two groups (TRT and CTR) at the baseline (T0) and the end of the study (T5). All the animals involved in the study belonging to both groups showed improvement in these parameters after 50 days of trial. During the trial, progressive improvements in FS and BCS were observed over time in all four dogs that received the treatment (Table 4 and Fig. 1). A slight decrease in the CIBIDAI score (CibD) was observed in the TRT group but not in the CTR (Table 4). Notably, improvement in FS was noted relatively early in the trial in the TRT group (T2). In Figure 2, a visual comparison was conducted between the fecal images provided by the owners for dog number 1 (belonging to the TRT group) and dog number 5 (belonging to the CTR group) at both the beginning and end of the trial (T0 vs. T5). Table 2. Patient characteristics at baseline [sex, age, BW, BCS, MCS, IRIS stage, and systolic blood pressure (SBP)]
Table 3. Mean values for blood parameters [creatinine (CREA), urea, SDMA, phosphorous (P), iCa HCO3, total protein-to-creatinine ratio UP/UC and urinary parameters, and SBP at the baseline (T0) and the end of the study (T5) for each group].
DiscussionIn the present randomized controlled study, a feed supplement containing a combination of probiotics and yeasts was tested in dogs affected by CKD to determine its effect on nutritional status and fecal consistency. Improvements in blood and urinary parameters, usually used to monitor kidney disease, were observed in both groups between T0 and T5 (Table 4). This result was expected because both groups received standard renal therapy during the entire trial to manage their renal condition (Polziin, 2011; Bartges, 2012; IRIS Guidelines, 2023). The use of probiotics as supplements in animal diets has recently increased, both in healthy pets (Bruni et al., 2020; Marelli et al., 2020; Tanprasertsuk et al., 2021) and pets with different disorders, such as kidney disease (Lippi et al., 2017; Meineri et al., 2021; Thakur et al., 2021). In particular, one study reported the improvement of gut health in dogs with CKD, which suppressed the growth of harmful bacteria and decreased the production of uremic toxins (Thakur et al., 2021). In other recent studies, the use of probiotics containing L. acidophilus has been demonstrated to maintain the proper nutritional status and improve blood parameters relevant to monitor renal disease progression (Meineri et al., 2021) and to reduce the deterioration of the glomerular filtration rate in dogs with CKD (Lippi et al., 2017). The main reason for adding probiotics to the diet when a dog is affected by CKD and also has intestinal disorders is their ability to remove uremic toxins from the intestine (Lippi et al., 2017). In particular, they reduce blood concentrations of uremic toxins and slow down the progression of renal impairment in both human and animal studies (Koppe et al., 2015; Lippi et al., 2017; Thakur et al., 2021). Table 4. Fecal (FS) and clinical parameters (BCS and CIBIDAI score – CibD) for each dog at each time point.
Fig. 1. Median values of the (FS) (range 1 to 7) for the two groups (treated : TRT and control: –CTR) at different time points (T0–T5). To our knowledge, no study has investigated the effect of L. acidophilus D2/CSL on fecal parameters in dogs affected by CKD. In our study, the dogs that received the feed supplement containing probiotics showed improved clinical parameters, such as BCS and FS, compared with the CTR group during the trial (Table 3 and Fig. 1). Previous studies have demonstrated the safety and efficacy of L. acidophilus D2/CSL, showing improvements in fecal parameters and clinical conditions, such as BCS, in healthy dogs (Bruni et al., 2020; Marelli et al., 2020). Furthermore, it has been reported that this particular probiotic is capable of modifying the intestinal microbiota and reducing gut inflammation in dogs with CKD (Mutsaers et al., 2013). Animal models of CKD have also reported motility dysfunction and altered intestinal barrier function, resulting in leaky gut syndrome and bacterial translocation (Bartochowski et al., 2022). In these cases, dysbiosis and alterations in the gut microbiota can contribute to the excretion of softer, watery, or diarrheal stools (Marelli et al., 2020). As an illustrative example, Figure 2 shows images depicting the improvement in fecal consistency at different time points (T0 to T5) in a dog treated with a supplemented diet compared with another dog that received a placebo. These findings are consistent with a previous study in which FS and fecal moisture were improved in healthy dogs following the addition of L. acidophilus D2/CSL (Bruni et al., 2020). Furthermore, the addition of L. acidophilus D2/CSL to the diet also resulted in improved nutritional status, as assessed by the BCS and CIBIDAI scores in this study. A similar study on healthy dogs showed improvement in BCS and fecal parameters (Marelli et al., 2020). Both studies suggest that the inclusion of L. acidophilus D2/CSL in the diet can have positive effects on the nutritional status of dogs, regardless of their health condition.
Fig. 2. Images of fecal samples at different time points (T0 to T5) in a dog treated with a supplemented diet (Patient 1) compared to another dog that received the placebo (Patient 5). Indeed, it is possible that the observed effect on stool consistency in the current study could be attributed to other ingredients present in the supplement diet, such as E. faecium and S. cerevisiae. Enterococcus faecium has been reported to have a positive impact on the microbiome and improved fecal quality in dogs (Nybroe et al., 2023). A study conducted on healthy dogs fed a commercial diet containing E. faecium demonstrated that the frequency of soft stools was reduced, and there was an increase in fecal microbial diversity. In addition, the probiotic diet led to a decrease in serum cholesterol levels in these dogs (Nybroe et al., 2023). Regarding S. cerevisiae, the mechanisms by which it influences intestinal functionality are not yet fully understood. However, yeast products derived from S. cerevisiae have been shown to positively influence microbial function, leading to improvements in digestive conditions (Bastos et al., 2023). It has been reported that dietary supplementation with S. cerevisiae in dogs results in reduced Escherichia coli counts in feces and modulation of immunity and intestinal microbiota (Bastos et al., 2023). These effects may be attributed to the production of metabolites, including short-chain fatty acids, antioxidants, and B vitamins, which interact with other microorganisms (Bastos et al., 2023). The present pilot study has some limitations that should be considered. First, the sample size of dogs included in the study was small, which prevented a statistical analysis between the two groups. This limitation affects the strength and generalizability of the findings. Second, a comprehensive analysis of the microbiota, which would provide valuable insights into the actual effects of the supplement on gut health, was not conducted in this study. The absence of microbiota analysis limits our understanding of specific changes occurring in gut microbial composition. ConclusionIn conclusion, feed supplementation containing L. acidophilus D2/CSL, E. faecium, and S. cerevisiae seems to have beneficial effects on the clinical condition, gut health, and stool quality of dogs with CKD. These promising results can help plan further studies to enhance our knowledge of the potential benefits of gastroenteric complications in dogs with CKD. AcknowledgmentsWe would like to thank Monge & C. S.p.A for providing the commercial complete dietetic feed. Conflicts of interestTwo authors are consultants, and one is an employee of Candioli Pharma S.r.l which supported the study and provided the tested supplements. FundingThis research was supported by Candioli Pharma S.r.l. Institutional review board statementThe experimental protocol was designed in accordance with the guidelines outlined in the current European and Italian laws concerning the protection of animals used for scientific purposes, specifically directive 2010/63/EU. These laws were implemented in Italy under the regulation D.L. 2014/26. Informed consent statementInformed consent was obtained from all owners of the dogs involved in the study. Author contributionsConceptualization, FP, EM, NB.; methodology, FP, EM; software, FP; validation, FP; formal analysis, FP; investigation, FP, EM; resources, NB; data curation, FP, EM; writing—original draft preparation, FP, EM; writing—review and editing, FP, EM, IL, NL, NB; visualization, FP, EM; supervision, NB; project administration, NB, NL; funding acquisition, NB. All authors have read and agreed to the published version of the manuscript. Data availabilityData are available upon request from the corresponding author. ReferencesBartges, J.W. 2012. Chronic kidney disease in dogs and cats. Vet. Clin. North Am. Small. Anim. Pract. 42, 669–692. Bartochowski, P., Gayrard, N., Bornes, S., Druart, C., Argilés, A., Cordaillat-Simmons, M. and Duranton, F. 2022 Gut–kidney axis investigations in animal models of chronic kidney disease. Toxins 14, 626. Bastos, T.S., Menezes Souza, C.M., Legendre, H., Richard, N., Pilla, R., Suchodolski, J.S., de Oliveira, S.G., Lesaux, A.A. and Portella Félix, A. 2023. Effect of yeast Saccharomyces cerevisiae as a probiotic on diet digestibility, fermentative metabolites, and composition and functional potential of the fecal microbiota of dogs submitted to an abrupt dietary change. Microorganisms 11, 506. Bruni, N., Martello, E., Fusi, E., Meineri, G. and Giardini, A. 2020. Study of faecal parameters and body condition in dogs with a diet supplemented with Lactobacillus acidophilus D2/CSL (CECT 4529). Ital. J. Anim. Sci. 19(1) 704–711. Ephraim, E. and Jewell, D.E. 2020. Effects of added dietary betaine and soluble fiber on metabolites and fecal microbiome in dogs with early renal disease. Metabolism 10, 0370. Gagné, J.W., Wakshlag, J.J., Simpson, K.W., Dowd, S.E., Latchman, S., Brown, D.A., Brown, K., Swanson, K.S. and Fahey, G.C. 2013. Effects of a synbiotic on fecal quality, short-chain fatty acid concentrations, and the microbiome of healthy sled dogs. BMC Vet. Res. 9, 246. Greco, D. 2011. Diagnosis and dietary management of gastrointestinal disease. Pur. Vet. Diets. VET 2229A-0808. Hall, J.A., Yerramilli, M., Obare, E., Yerramilli, M., Almes, K. and Jewell., D.E. 2016. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J. Vet. Intern. Med. 30, 794–802. IRIS Guidelines. 2023. Available via http://www.iris-kidney.com/guidelines/ (Accessed July 2024). Jergens, A.E., Schreiner, C.A., Frank, D.E., Niyo, Y., Ahrens, F.E., Eckersall, P.D., Benson, T.J. and Evans, R. 2003. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 17, 291–297. Koppe, L., Mafra, D. and Fouque, D. 2015. Probiotics and chronic kidney disease. Kid. Intern. 88, 958–966. Laflamme, D.R.P.C. 1997. Development and validation of a body condition score system for dogs. Can. Prac. 22, 10–15. Lippi, I., Perondi, F., Ceccherini, G., Marchetti, V. and Guidi, G. 2017. Effects of probiotic VSL#3 on glomerular filtration rate in dogs with chronic kidney disease: a pilot study. Can. Vet. J. 58, 1301–1305. Marelli, S.P., Fusi, E., Giardini, A., Martino, P.A., Polli, M., Bruni, N. and Rizzi, R. 2020. Effects of probiotic Lactobacillus acidophilus D2/CSL (CECT 4529) on the nutritional and health status of boxer dogs. Vet. Rec. 187(4), e28. Meineri, G., Saettone, V., Radice, E., Bruni, N., Martello, E. and Bergero, D. 2021. The synergistic effect of prebiotics, probiotics and antioxidants on dogs with chronic kidney disease. Ital. J. Anim. Sci. 20(1), 1079–1084. Mutsaers, H.A.M., Engelke, U.F.H., Wilmer, M.J.G., Wetzels, J.F.M., Wevers, R.A., van den Heuvel, L.P., Hoenderop, J.G. and Masereeuw, R. 2013. Optimized metabolomic approach to identify uremic solutes in plasma of stage 3–4 chronic kidney disease patients. PLoS One 8(8), e71199. Nybroe, S., Horsman, P.B., Krag, K., Hosbjerg, T.G., Stenberg, K., Khakimov, B., Baymler, J., Bjørnvad C.R. and Kieler, I.N. 2023. Alterations in healthy adult canine faecal microbiome and selected metabolites as a result of feeding a commercial complete synbiotic diet with Enterococcus faecium NCIMB 10415. Animals 13, 144. Polzin, D.J. 2011. Chronic kidney disease. In Nephrology and urology of small animals, 1st ed. Eds., Bartges, J., Polzin, D.J. Chicester, UK: Blackwell Publishing Ltd., pp. 433–471. Tanprasertsuk, J., Jha, A.R., Shmalberg, J., Jones, R.B., Perry, L.M., Maughan, H. and Honaker, R.W. 2021. The microbiota of healthy dogs demonstrates individualized responses to synbiotic supplementation in a randomized controlled trial. Anim. Microb. 3, 36. Thakur, K., Dhoot, V.M., Bhojne, G.R., Upadhye, S.V. and Somkuwar, A.P. 2021. Effects of probiotics on hemato-biochemical alterations in dogs with chronic kidney disease. Pharm. Innov. J. 10(6), 507–513. WSAVA. 2013. World small animal veterinary association Global Nurition Committee (Body Condition Score). Available online: https://www.edu-veterinar.ro/files/download/prezentari/ghiduri-practice/WSAVA-Animal-Welfare-Guidelines-(2018).pdf (accessed on 15 November 2024). | ||
| How to Cite this Article |
| Pubmed Style Perondi F, Lippi I, Bruni N, Lonigro N, Martello E. Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study. Open Vet. J.. 2025; 15(1): 307-313. doi:10.5455/OVJ.2025.v15.i1.29 Web Style Perondi F, Lippi I, Bruni N, Lonigro N, Martello E. Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study. https://www.openveterinaryjournal.com/?mno=224880 [Access: September 04, 2025]. doi:10.5455/OVJ.2025.v15.i1.29 AMA (American Medical Association) Style Perondi F, Lippi I, Bruni N, Lonigro N, Martello E. Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study. Open Vet. J.. 2025; 15(1): 307-313. doi:10.5455/OVJ.2025.v15.i1.29 Vancouver/ICMJE Style Perondi F, Lippi I, Bruni N, Lonigro N, Martello E. Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study. Open Vet. J.. (2025), [cited September 04, 2025]; 15(1): 307-313. doi:10.5455/OVJ.2025.v15.i1.29 Harvard Style Perondi, F., Lippi, . I., Bruni, . N., Lonigro, . N. & Martello, . E. (2025) Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study. Open Vet. J., 15 (1), 307-313. doi:10.5455/OVJ.2025.v15.i1.29 Turabian Style Perondi, Francesca, Ilaria Lippi, Natascia Bruni, Nicolò Lonigro, and Elisa Martello. 2025. Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study. Open Veterinary Journal, 15 (1), 307-313. doi:10.5455/OVJ.2025.v15.i1.29 Chicago Style Perondi, Francesca, Ilaria Lippi, Natascia Bruni, Nicolò Lonigro, and Elisa Martello. "Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study." Open Veterinary Journal 15 (2025), 307-313. doi:10.5455/OVJ.2025.v15.i1.29 MLA (The Modern Language Association) Style Perondi, Francesca, Ilaria Lippi, Natascia Bruni, Nicolò Lonigro, and Elisa Martello. "Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study." Open Veterinary Journal 15.1 (2025), 307-313. Print. doi:10.5455/OVJ.2025.v15.i1.29 APA (American Psychological Association) Style Perondi, F., Lippi, . I., Bruni, . N., Lonigro, . N. & Martello, . E. (2025) Effect of a feed supplement containing probiotics on fecal score and clinical parameters in dogs with chronic kidney disease and intestinal disorders: A pilot study. Open Veterinary Journal, 15 (1), 307-313. doi:10.5455/OVJ.2025.v15.i1.29 |