| Review Article | ||
Open Vet. J.. 2025; 15(1): 54-68 Open Veterinary Journal, (2025), Vol. 15(1): 54-68 Review Article Bovine spongiform encephalopathy: A review of current knowledge and challengesTita Damayanti Lestari1*, Aswin Rafif Khairullah2, Suzanita Utama1, Sri Mulyati1, Tatik Hernawati1, Ratna Damayanti3, Rimayanti Rimayanti1, Bantari Wisynu Kusuma Wardhani4, Kartika Afrida Fauzia5,6, Ikechukwu Benjamin Moses7, Riza Zainuddin Ahmad2, Syahputra Wibowo8, Ima Fauziah2, Dea Anita Ariani Kurniasih9, Zein Ahmad Baihaqi10, Wasito Wasito2, Muhammad Khaliim Jati Kusala2 and Ertika Fitri Lisnanti111Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 5Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Department of Environmental and Preventive Medicine, Faculty of Medicine, Oita University, Yufu, Japan 7Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 8Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 9Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Research Center for Animal Husbandry, National Research and Innovation Agency (BRIN), Bogor, Indonesia 11Program of Animal Husbandry, Faculty of Agriculture, Universitas Islam Kadiri, Kediri, Indonesia *Corresponding Author: Tita Damayanti Lestari. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: titadlestari [at] fkh.unair.ac.id Submitted: 10/10/2024 Accepted: 31/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
AbstractBovine spongiform encephalopathy (BSE), also referred to as mad cow disease, is a chronic degenerative disease that affects the central nervous system. BSE is caused by a misfolded isoform of the prion protein, a widely expressed glycoprotein. The illness is referred to as Variant Creutzfeldt-Jakob disease (vCJD) in humans. In the United Kingdom (UK), BSE in cattle was first discovered in 1986. Based on epidemiological data, it appears that animal feed containing tainted meat and bone meal (MBM) as a source of meat protein is the common cause of the BSE outbreak in the UK. Clinical indicators in cows include irregular body posture, incoordination, difficulty in standing, weight loss, and temperamental changes, including agitation and hostility. Feeding livestock MBM obtained from BSE-infected livestock contaminated with BSE prions is the only known risk factor for BSE development. Strong evidence linking BSE to human transmission and a variant type of CJD has brought the disease to the attention of many countries. Screening living animals for BSE is challenging. In most cases, suspected animals are usually killed. Typically, the central nervous system is examined for prions to diagnose this illness. There is currently no robust treatment for BSE. The prevention of BSE can be achieved by avoiding the feeding of susceptible animals with ruminant tissues that might carry prions. Keywords: BSE, Cattle, Prion, Public health, vCJD. IntroductionBovine spongiform encephalopathy (BSE), also referred to as mad cow disease, is a chronic degenerative disease that affects the central nervous system (Haley and Richt, 2023). This is caused by a misfolded isoform of prion protein (PrPSc) that deviates from its normal cellular isoform, PrPC. The alternative form of BSE in humans is the variant Creutzfeldt-Jakob Disease (vCJD). BSE is a member of the transmissible spongiform encephalopathies (TSE) disease family (Brown and Abee, 2005; EFSA, 2022). The infectious agent of the disease is believed to be a misfolded protein known as a prion. The disease has a lengthy incubation period, spanning 30 months to 8 years (Poggiolini et al., 2013). The brain and spinal cord develop spongy degeneration as a result of the misfolding and aggregation of additional native prion proteins (PrPs) in the brain caused by these malformed prions (Kumagai et al., 2019). The consumption of tainted meat and bone meal (MBM) in cattle feed results in cattle-to-cattle transmission (Islam et al., 2022). This illness is deadly, and there is currently no known cure. Beginning in the mid-1980s, a cattle outbreak in the United Kingdom (UK) was caused by a novel form of prion disease known as BSE. In 1986, the UK identified the first cases (Lee et al., 2013). Around 200,000 cases of BSE have been confirmed in cattle, nearly all of which occur in the UK (Columbus, 2004). During the UK outbreak in 1992, 37,280 cases were recorded in 1 year (Watson et al., 2021). Native cattle in the majority of European nations, Japan, the US, Canada, and Brazil have been reported to have lower occurrences (EFSA et al., 2017). The BSE epidemic has far-reaching economic repercussions. Trade restrictions on beef commodities were accompanied by a sharp decline in consumer confidence in beef products in countries where BSE cases were prevalent (Jin and Koo, 2003). The traits of BSE-affected cows include altered body posture, decreased milk output, abnormal weight loss, altered temperament (nervous or aggressive), and loss of appetite (Hamir et al., 2011). Typically, symptoms do not appear for 3 to 6 years following the original illness. Typical pathogenic alterations include astrocyte growth, neuronal loss, and gray matter spongy degeneration in the absence of signs of an inflammatory response (Soto and Satani, 2011). Through the use of Western blotting techniques, aberrant prions can be used to diagnose this condition (Olech, 2023). Postmortem identification can be used to diagnose BSE if clinical indications are present. A postmortem examination may show overall neuronal degeneration and microscopic vacuolization of the gray matter of the brain (Rech et al., 2018). While the animal is alive, diagnostic tests are not available. At present, it is recognized that humans can contract BSE by eating contaminated beef or coming into contact with other items made from the diseased cattle’s nerve tissue (Pritzkow et al., 2021). The illness is referred to as vCJD in humans. All age groups are susceptible to this disease, which is extremely difficult to diagnose until almost completely cured (Brandel and Knight, 2018). Early nervous system symptoms, such as depression and loss of coordination, are experienced by those with vCJD. These symptoms eventually lead to dementia (Kelemen et al., 2022). However, in the later phases of the illness, magnetic resonance imaging can identify additional brain abnormalities. Typically, 13 months after the onset of symptoms, vCJD is fatal. This has raised the question of whether and to what extent eating certain foods or other exposures can expose people to prions. The number of cases of BSE disease has increased dramatically in recent decades, ranking among the main health issues. The link between BSE and vCJD in humans has raised awareness of the possible risks associated with BSE. The purpose of this review is to explain the etiology, history, epidemiology, pathogenesis, pathology, clinical symptoms, diagnosis, transmission, host range, risk factors, public health importance, economic impact, and control of BSE. HistoryBefore the identification of BSE, sheep scrapie was the first disease of its kind to be documented, having been present for more than 200 years (Ness et al., 2023). In the UK, BSE in cattle was first discovered in 1986. Although the exact cause of the outbreak is unknown, there is strong evidence linking the use of MBM in cattle feed tainted with an agent similar to scrapie (Nathanson et al., 1997). Regardless of the underlying cause, the epidemic persisted and expanded as a result of the recycling of BSE-contaminated cow material to additional animals starting in the mid-1980s. Since the 1920s, MBM has been used in the UK to manufacture feed supplements for cattle and other livestock (Islam et al., 2022). Modifications made to these processing methods in the 1970s and 1980s may have permitted the infectious agent to endure the manufacturing process. The UK has been the site of most BSE cases. In April 2005, 180,780 BSE cases were confirmed in over 36,000 cattle herds as of April 2005. Almost a thousand probable cases were recorded every week during the height of the pandemic in January 1993 (Alarcon et al., 2023). As of 2005, there have been two documented BSE cases in the US as of 2005. In October 2001, an adult dairy cow was imported from Canada for the first BSE occurrence. In June 2005, a second case was verified (Holman et al., 2010). BSE surveillance found 22 cases in North America as of February 2011: 3 in the US and 19 in Canada (one imported from the UK) (EFSA et al., 2017). Other than BSE, known animal TSEs include transmissible mink encephalopathy, chronic wasting disease (CWD) in deer and elk, and scrapie in sheep and goats (Otero et al., 2021). Feline spongiform encephalopathy (FSE), which has been reported in domestic cats and several wild cat species in zoos (Bencsik et al., 2009), as well as spongiform encephalopathy in several African ruminant species in zoos, is thought to result from infection with the BSE agent (Cunningham et al., 2004). TSEs in humans include Kuru, Gerstmann-Straussler-Scheinker syndrome, Creutzfeld-Jakob disease (CJD), and fatal familial insomnia (Collins et al., 2001). There is compelling evidence that humans can acquire vCJD by consuming by-products from infected cattle, as the same prion strain that causes BSE also causes vCJD in humans (Ritchie et al., 2021). The primary sources of prion-contaminated material from diseased cattle include the brain and spinal cord, as well as some intestines (Hedlin et al., 2012). There are no other animal TSE agents known to cause illness in humans, besides BSE. Between October 1996 and March 2011, 175 cases of vCJD were recorded in the United Kingdom and Northern Ireland (UK), and 49 cases were recorded abroad (Diack et al., 2012). Since 2000, the number of vCJD cases in the UK has decreased following the containment of the BSE pandemic in cattle. Based on the EU TSE annual report, out of 1,021,252 cattle that were tested, 66,121 cattle were tested in eight non-EU reporting countries, with two cases of H-BSE in France and Spain and four cases of L-BSE in France, Germany, and Spain were recorded (EFSA, 2022). Additionally, out of the 311,174 sheep and 118,457 goats that were tested, 551 cases of scrapie, 448 classical scrapie (CS), and 103 atypical scrapie (AS) were reported in sheep. In the other non-EU reporting countries, 55 CS and 1 AS in Iceland, and 8 AS in Norway were reported out of the 27,594 sheep that were tested. In goats, 224 cases of scrapie (219 CS and five AS) were reported (EFSA, 2022). EpidemiologyBased on epidemiological data, it appears that animal feed containing prion-tainted MBM as a source of meat protein was the common cause of the BSE outbreak in the UK (Nathanson et al., 1997; Amin et al., 2023; Betancor et al., 2023). The cause of BSE is the subject of multiple scientific theories. Feeding processed proteins to livestock derived from carcasses of sheep or cattle infected with scrapie with previously undisclosed TSEs may have been the cause of BSE in the UK (Greenlee and Greenlee, 2015; Amin et al., 2023; Betancor et al., 2023; Kaifa et al., 2023). For many years, the use of meat, bone meal, and other similar items was a source of protein in cattle diets. It is possible that modifications to processing procedures in the late 1970s and early 1980s contributed to disease onset (Alarcon et al., 2023). There is no proof that mature cattle that are unrelated to one another or that animals that come into contact with other species transfer BSE horizontally. An infectious agent exposure in 1981–1982 led to an outbreak in the UK, which was correlated with a sharp decline in the usage of organic solvents in the production of MBM (Kumagai et al., 2019). Between 1987 and 1992, the number of BSE cases reported in the UK rose from 446 to 37,280, and after 2010, it decreased to less than 10 (Kumagai et al., 2019). In several European countries, with the exception of the UK, the numbers peaked in 2001–2002 and in Japan in 2006 (Houston and Andréoletti, 2019). The prevalence of BSE was effectively decreased by “feed bans,” which were implemented in nations plagued by BSE to prohibit the recycling of BSE agents (Kao et al., 2003; Amin et al., 2023; Global Times, 2023). The UK resumed surveillance for CJD in 1990 in an effort to track any changes in CJD patterns linked to the BSE outbreak (Will et al., 1996). Concerns regarding the connection between this human disease and BSE were raised when a new vCJD was identified, characterized by a unique neuropathological profile and atypical inclusions at a young age (Ritchie et al., 2021; Amin et al., 2023; Kaifa et al., 2023). A connection between the two diseases is supported by a comparison of Western blot (WB) glycoform patterns and artificial BSE transmission in experimental animals, such as monkeys. A World Health Organization consultation conducted on 2–3 April 1996, determined that although no clear relationship had been identified between BSE and vCJD, exposure to the BSE agent was probably the most likely cause of vCJD. The consultation suggested avoiding animal-to-human BSE transmission and limiting human exposure to BSE. In the UK, France, and other countries, 177, 27, and 25 confirmed instances of definite and probable vCJD occurred from 1995 to 2014, respectively. Two additional cases were reported in 2016 (Chen and Dong, 2016). At necropsy, the tonsils, spleen, and lymph nodes of vCJD patients were shown to have disease-associated PrPSc (Head et al., 2004; Kaifa et al., 2023). Additionally, appendix tissue from vCJD patients was collected 8 months before the commencement of the disease. According to a comprehensive study of appendix samples obtained during surgery in the UK, 16 out of 32,441 samples tested positive for the aberrant PrP, indicating that the prevalence of vCJD in the country is 493 per million (Gill et al., 2020). Before the discovery of the new atypical BSE, prions isolated from BSE cattle were believed to be a strain of TSE prion (Kamali-Jamil et al., 2021). Bovine amyloidotic spongiform encephalopathy (BASE, later also called L-type BSE (L-BSE) and H-type BSE (H-BSE), are two atypical types of BSE that were identified by active surveillance in 2004 in Italy and France (Kumagai et al., 2019). These types of BSE differed from previous cases in terms of neuropathological and molecular features. To differentiate it from atypical BSE, the BSE strain that has been identified as the cause of epidemics in the UK and other countries has since been dubbed classical BSE (C-BSE). The unglycosylated disease-associated isoform (PrPd) from H-BSE and L-BSE animals had a greater molecular mass than that of PrPd produced from C-BSE animals (Hamir et al., 2011). As of November 2018, the Food Safety Commission of Japan reported 135 atypical BSE cases globally, primarily through active surveillance programs for fallen livestock and normal and emergency animal slaughter. Overreaction to external stimuli, unexpected startle response, panic, anxiety, difficulty awakening, and lethargy are examples of atypical clinical symptoms of BSE identified in intraspecies transmission trials. These symptoms are difficult to differentiate from C-BSE (Costassa et al., 2016). EtiologyBSE is caused by a misfolded isoform of the PrP, a widely expressed glycoprotein. PrP is encoded by the PrP gene PRNP and is a typical component of vertebrate cell membranes (Castle and Gill, 2017). An abbreviation for misfolded pathogenic protein isoforms is “prion,” which was coined as a combination of the words “proteinaceous” and “infectious” (Aulić et al., 2013). Conventionally, PrPC is used to indicate the normal cellular isoform of PrP. The cellular form is represented by the superscript C. The prion form, denoted as PrPSc, shares the same amino acid sequence as the normal form (Baldeschi et al., 2022). The superscript in question Sc refers to the classic animal prion disease, scrapie, which afflicts sheep (Imran and Mahmood, 2011). To proliferate, prions attach to the healthy PrPC protein and use it as a template to refold the PrPC molecule into the aberrant PrPSc form (Westergard et al., 2007). PrPC is found in fish, amphibians, birds, mammals, fish, and yeast (Pastore and Zagari, 2007). The protein is highly expressed in the neurological system and is expressed in a wide range of organs in animals, including the spleen, lymph nodes, kidney, pancreas, salivary glands, adrenal glands, liver, thymus, and bone marrow (Tichopad et al., 2003). Nonetheless, the physiological role of PrPC is still unknown, and several mouse strains that have been produced to lack PrPC expression exhibit only minor, non-fatal variations in their physiological and locomotor activities in contrast to wild-type mice (Nico et al., 2005). Some strains of prion disorders, such as scrapie and BSE, have distinct disease characteristics. The protein deposition patterns in the brain and lymphoid tissues, the length of time an animal must incubate following experimental infection, histology, and clinical symptoms are among the differences among the strains. For instance, in the case of scrapie, certain strains are characterized by significant infectivity in lymphoid organs, whereas others preferentially grow in the central nervous system (Scialò et al., 2019). There are three known strains of BSE known to exist. The human vCJD epidemic that accompanied the BSE epidemic that started in the UK and expanded to other nations was caused by a single strain of the prion, known as classical BSE (Ritchie et al., 2021). Atypical strains, referred to as H (high)-BSE and L (low)-BSE, are uncommon and typically found in cattle aged between 8 and 20 (Masujin et al., 2016). They seem to emerge randomly and spontaneously. When it comes to the inactivation of prions, standard sterilizing techniques used to prepare surgical instruments and supplies are infamously ineffective (Sakudo et al., 2022). PrPSc can withstand 70% alcohol treatment, gamma irradiation, UV irradiation at 254 nm, and traditional autoclaving (121°C for 20 minutes). PrPSc can be inactivated by a range of procedures, including harsh autoclaving conditions (134°C for 8–18 minutes) combined with detergents and hydrogen peroxide gas plasma treatment (Sakudo et al., 2020). The infectiousness of prions can be decreased by various processes that alter or hydrolyze proteins. PrPSc has a protease-resistant core and is insoluble in detergents, whereas PrPC is protease-sensitive and soluble in nondenaturing detergents (Yuan et al., 2006). PathogenesisAlthough the pathophysiology of BSE in cattle has been well investigated, there are still many unanswered questions. PrPSc was initially observed in Peyer’s patches in the ileum after calves were orally exposed to contaminated material. It was also found in gut-associated lymphoid tissue (GALT) at the ileocecal junction and jejunum (Stack et al., 2011). Infection occurs in follicular dendritic cells (FDCs) and macrophages. Subsequently, infection can be detected in the enteric nervous system; however, the mechanism by which infection moves from lymphoreticular cells to nervous system cells remains unclear (Natale et al., 2011). Prion infections may occur when they come into contact with thin nerve fibers located just beneath the intestinal mucosa following their passage through the intestinal mucosal barrier (Sigurdson et al., 2019). Once the neurological system is compromised, the infection proceeds to the brain through the sympathetic and parasympathetic nervous systems, which include the vagus and splanchnic nerves, respectively (Ackermann et al., 2021). The involvement of GALT is less in BSE than in scrapie in sheep (Press et al., 2004). It has been proposed that bloodstream infections caused by oral-acquired prion disorders could possibly affect the brain; however, infectious agents were not found in the blood of cattle infected with BSE (Gallardo and Delgado, 2021). In contrast, GALT showed significant infectivity, whereas blood samples were reported to contain prions in experimental BSE in sheep and human vCJD (Mabbott, 2017). It is unknown how PrPSc replication in splenic FDCs contributes to the transmission of agents and may differ throughout animals. According to scrapie research, neuroinvasion can be prevented or delayed by FDC depletion, delayed or prevented by splenic denervation, and promoted by augmenting the spleen’s nerve supply (Mohan et al., 2005). In mice infected with BSE and expressing sheep PrP, splenic PrPSc was detected (Espinosa et al., 2007). Only one of the three cows that were halted at the advanced clinical stage of BSE had splenic PrPSc. Once cells become infected with PrPSc, membrane microparticles harboring PrPSc can disseminate the infection to nearby cells (Fevrier et al., 2004). It has been demonstrated that PrPSc and exosomes can be released from infected cells in vitro, supporting this theory. Exosomes are tiny, membrane-bound vesicles that cells can produce and merge with other cells to form new vesicles (Zhang et al., 2019). Exosome production by lymphoid cells has been demonstrated, but it has not been demonstrated that neurons make exosomes. Another way that PrPSc can spread between neighboring cells is by tunneling nanotubes, which are very thin membrane bridges that can form between cells and facilitate the movement of pathogens, cytoplasmic chemicals, organelles, and parts of the plasma membrane (Gousset and Zurzolo, 2009). Additional suggested routes of propagation inside the nervous system consist: lymph flow around neurons, axonal transport, and successive infection of Schwann cells, which nourish and insulate peripheral nerves (Oliveira et al., 2023). Although some suggestions have been made, the chemical mechanisms responsible for brain injury remain mostly unclear. Given that genetically designed mice with no PrPC at all and mice whose expression of PrPC is turned off in maturity do not exhibit clinical indications of TSE, PrPC depletion does not appear to be the etiology (Lakkaraju et al., 2022). It has been demonstrated that depleting PrPC in mice with prion infection can reverse early spongiform degeneration and prevent disease progression to a clinical stage (Mallucci et al., 2003). These results imply that several PrPC-dependent mechanisms are necessary for PrPSc toxicity. It has been proposed that the conversion of PrPC to PrPSc impairs this neuroprotective effect and promotes neurodegeneration (Mahabadi and Taghibiglou, 2020). An alternative explanation could be that the binding of PrPSc to PrPC initiates a signal transduction cascade that damages nerves (Panes et al., 2021). Based on the in vitro data, further suggestions regarding the pathogenicity of PrPSc include diminished proteasome system degradation, overexpression of genes related to endoplasmic reticulum function, and poor lysosomal breakdown of cellular waste (Goold et al., 2015). PathologyBSE microscopic alterations are pathognomonic and extremely specific. The alterations manifest as bilateral, symmetrical, and degenerative lesions that affect multiple regions of the brain stem’s gray matter (Zerr, 2022). There are two manifestations of neuronal vacuolization. “Sponic encephalopathy” is the term used to describe the presence of 20 μm-sized vacuoles in the neurites of the neuropil. Larger vacuoles (30–40 μm), either single or multiple, in the neuronal pericardium are another presentation. These vacuoles cause the pericardium to expand, resulting in a bloated neuron with only a thin rim of cytoplasm remaining (Kumagai et al., 2019). The primary requirement for a valid histological diagnosis of BSE is the existence of vacuoles in the gray matter neuropil and the neuronal perikaria and cortex (Olech, 2023). Clinical symptomsIn cattleAccording to field data, cattle’s vulnerability to BSE infection peaks at approximately 12 months of age, yet cases of BSE have been reported in cattle that were not fed MBM until they were more than 2 years old (Nathanson et al., 1997). Cattle are thought to take between 30 months and 8 years (on average, 4.5–5.5 years) to fully incubate; nevertheless, the clinical course is brief from the moment clinical indications appear, with most animals dying or necessitating euthanasia within 6 months (EFSA et al., 2017). The age at which clinical indications first appeared was known for 124,000 British cattle, of which 7% were aged 3 years, 31% were aged 4 years, 33% were aged 5 years, and 29% were aged 6 years or older. Clinical indicators in cows include irregular body posture, incoordination, difficulty in standing, weight loss despite continuous eating, and temperamental changes, including agitation and hostility (Saegerman et al., 2004). In humansThere have been > 200 documented incidences of vCJD in people. The age of patients was 17–42. Most patients lived in the UK between 1985 and 1996 (Alarcon et al., 2023). The variant CJD usually manifests 18 months after the onset of symptoms and is always fatal (Maheshwari et al., 2015). Problems with cognition and movement are examples of clinical symptoms and indicators. Patients typically present with psychological or sensory symptoms when the disease first manifests. Psychiatric symptoms that have been reported include anxiety, hostility, recklessness, paranoid delusions, melancholy, apathy, agitation, sleeplessness, poor concentration, and withdrawal (Wall et al., 2005). Approximately one-third of patients experience unpleasant, unusual, and persistent sensory sensations. As the illness worsens, neurological symptoms such as involuntary movements, muscle spasms, and cerebellar ataxia appear. Urinary incontinence, akinetic mutism, and increased immobility are examples of late-onset symptoms (Barnwal et al., 2022). Typically, opportunistic infections result in death. Sporadic CJD is the primary cause of most human cases of prion disease (Tam et al., 2023). In contrast to vCJD, sCJD often affects individuals aged 55–70 years. Unlike vCJD, cerebellar ataxia or progressive dementia is more common in the early stages of sCJD (Cooper et al., 2006). Moreover, vCJD has a unique histopathology. Other than conventional BSE, the only other TSE that has been known to spread orally to humans is the now-extinct illness known as “kuru”, which only affected a limited number of cannibalistic natives in Papua New Guinea (Liberski et al., 2012). DiagnosisEarly, accurate, and rapid diagnosis is vital for the prevention and control of the spread of BSE, especially in the absence of effective treatment strategies and vaccines. Arrays of approaches and methods have been explored and implemented to improve the detection of BSE in cattle; however, each technique has its own drawbacks and strengths. Due to this limitation, several methods and approaches are usually combined to increase the odds of eliminating the disadvantage of using a single method (Olech, 2023). No BSE testing was performed on living animals. The CNS is typically examined for prions to diagnose this illness (Watson et al., 2021). Although the brainstem may occasionally be sampled through the foramen magnum, whole brain sampling is typically performed at the level of the obex (e.g., for surveillance with fast testing) (Olech, 2023). The most precise assays are immunohistochemistry and immunoblotting. There are also some quick diagnostic methods that rely on lateral flow assays, automated immunoblotting WB, and enzyme-linked immunosorbent assays (ELISA). Rapid tests are frequently employed in surveillance and slaughter testing because they enable the screening of large quantities of samples (EFSA et al., 2018). Immunohistochemistry or immunoblotting is the conventional method for confirming positive samples in fast tests (Cooley et al., 2001). However, according to the World Organization for Animal Health (WOAH, 2008), in certain situations, confirming a positive result with a second BSE rapid test may be acceptable. Using electron microscopy, the BSE causative agent can also be identified by observing distinctive prion fibrils known as scrapie-associated fibrils; however, the sensitivity of this test is limited (Simon et al., 2008). While a histological examination of the brain is usually not the only confirmatory test, it can be very helpful in the diagnosis process, as some animals in the early stages of infection exhibit little to no spongy alterations (Olech, 2023). The majority of BSE diagnostic tests are comparatively insensitive and only identify prions in the brain three to 6 months before the manifestation of clinical symptoms. Prion identification can be accelerated by highly sensitive assays, such as real-time quaking-induced conversion (QuIC) and protein misfolding cyclic amplification (PMCA) (Kaelber et al., 2019). The capacity of these methods to transform PrPc, a typical cellular protein, into prions in vitro allows the identification of minute concentrations of prions. Although these methods have not been properly assessed for surveillance programs, they are being investigated for diagnostic purposes. The rodent bioassay, which involves inoculating rats with BSE, is another method for detecting the disease; however, due to the lengthy incubation period, this method is not feasible for routine diagnosis. Because antibodies are not produced against BSE, serology is useless (Corda et al., 2015). The same assays used to diagnose conventional BSE can also be used to detect atypical prions, such as H-BSE and L-BSE. Although these prions can also be identified in obesity, the distribution patterns of H-BSE and L-BSE in the brain differ slightly from those of classical BSE and from one another (Orge et al., 2021). Atypical prions can be identified in immunoblotting tests by their characteristics similar to classical BSE prions. Compared with classical BSE, H-BSE has fragments with larger molecular masses (Moore et al., 2016). Additionally, following proteinase K cleavage, H-BSE interacts with monoclonal antibodies against an N-terminal epitope absent from classical BSE (McCutcheon et al., 2014). The molecular mass of L-BSE is lower than that of the classical BSE prion. It has a unique deposition pattern in the brain, which is marked by amyloid plaques, and its glycosylation pattern is different from that of classic BSE (Fast et al., 2023). It is important to differentiate between BSE in small ruminants and scrapie, a prion disease that affects these animals far more frequently. In most cases, this can be done using a conventional prion test. However, it is difficult to distinguish BSE from other scrapie prions (CH1641) (Gough et al., 2014). The latter two agents can be distinguished from one another using a restricted set of tests, such as PMCA, specific types of specialized immunoblots, PrPSc profiling, and epitope mapping (Elezgarai et al., 2017). The lack of tests with the potential to identify asymptomatic BSE-infected animals that could be employed in screening healthy cattle populations, such as whole herds or specific animals for import or export purposes, represents a major challenge in the effective prevention and control of BSE. In vitro prion techniques, such as PMCA and RT-QuIC, which have the capability to detect minute amounts of prions in various tissues and body fluids, have not been fully evaluated for use in legal BSE surveillance programs, even though they help in the development of systems for antemortem TSE diagnosis (Olech, 2023). TransmissionThere is little evidence that BSE can spread among animals in ways other than feeding tainted with specific tissues from animals that have the disease (Gallardo and Delgado, 2021). This stands in sharp contrast to deer’s chronic wasting disease (CWD) and sheep’s horizontal spread of scrapie (Madsen-Bouterse et al., 2016). Saliva, urine, feces, placentas, and decomposing carcasses are also sources of CWD prions (Saunders et al., 2008). In a previous report, PrPSc was discovered in milk, saliva, placental tissue, nasal secretions, and excrement from sheep with scrapie infections (Vascellari et al., 2007); however, there is no proof that BSE can spread among cattle through this method or through contact with excrement or secretions. However, prions associated with scrapie and CWD persist in the environment, attaching to soil particles or other fomites (EFSA et al., 2019). There is also no proof of vCJD human-to-human vCJD transmission other than through medical procedures such as blood transfusions. It seems that animals can become infected with each other by consuming less than 1 mg of infected brain material. Oral injection of 5 g of infective brain homogenate has been shown to transmit BSE to monkeys; nevertheless, the infective dose of bovine PrPSc for humans remains unknown (Lasmézas et al., 2005). According to current prion theory, the BSE agent, also known as PrPSc, is an abnormally folded isomer of a normal cell surface protein, also known as PrPc. It can cause a conformational change in PrPc, leading to increased production of PrPSc in the central nervous system (CNS) of infected cattle (Thackray et al., 2005). This process culminates in neurological abnormalities typical of BSE after a protracted incubation period. Strong evidence suggests that people who consume offal (particularly the brain and spinal cord) from infected cattle develop vCJD, a neurodegenerative illness that is identical to that of cattle and develops after a protracted incubation period (Houston and Andréoletti, 2019). Consequently, vCJD is the only zoonosis associated with TSEs. Similar to BSE, vCJD is lethal and typically results in months of crippling neurological illness before death (Belay et al., 2005). Dead cattle due to BSE infection, which were processed for MBM and subsequently added to animal feed, were first identified in the UK in 1986 (Kumagai et al., 2019). The transmission pathway of BSE in cattle (Fig. 1) starts with prion infection through contaminated animal feed. The misfolded prions induce the conversion of normal proteins to CNS proteins, leading to neurodegeneration. The sole method of agent transmission between cattle currently known to exist is the ingestion of infectious material in MBM made from animals exposed to BSE (Islam et al., 2022). Human cases of vCJD have also been linked to the consumption of steak tainted with infected cow CNS tissue (Maheshwari et al., 2015). It also becomes clear how contaminated beef products consumed by humans act to cause vCJD (Fig. 1). Four incidences of human-to-human transmission of vCJD through blood or plasma transfusion have been reported in the UK, despite the fact that the majority of cases are caused by the ingestion of tainted beef (McManus et al., 2022). In rare instances, dura mater grafts, growth hormone injections, and corneal transplants have resulted in the transmission of sickle cell disease (sCJD) between individuals (Heath et al., 2006). Because the disease can occur in lifelong vegetarians as well, there is no evidence that sCJD is a TSE of animal origin (Davanipour et al., 2014). Host rangeThrough intracerebral (IC) inoculation, the following species have been experimentally exposed to BSE: rats, sheep, goats, mink, pigs, marmosets, and macaques (Baron, 2002). There have been failed attempts at IC transmission in hamsters. Cattle, sheep, goats, rodents, and mink have all been successfully exposed to BSE through oral transmission (Tamgüney et al., 2009). Pigs have not been proven to be good subjects for oral transmission. In chickens, parenteral and oral transmission has also been attempted; thus, far, no signs of illness have been reported (Moore et al., 2011). In addition to eight captive wild ruminant species, exotic cats (cheetahs, pumas, tigers, and ocelots) and domestic cats have been found to have TSE (Orge et al., 2021). FSE has been reported in approximately 81 domestic cats in the UK and in one domestic cat in each of Norway, Northern Ireland, and Liechtenstein (Iulini et al., 2008). The agent recovered from several of these instances using strain typing in mice was indistinguishable from BSE in cattle, demonstrating that FSE is indeed BSE in exotic and domestic cats. It seems that this also applies to other ruminants. According to epidemiological data, feed tainted with BSE is the primary cause of BSE infection in this species (EFSA et al., 2017).
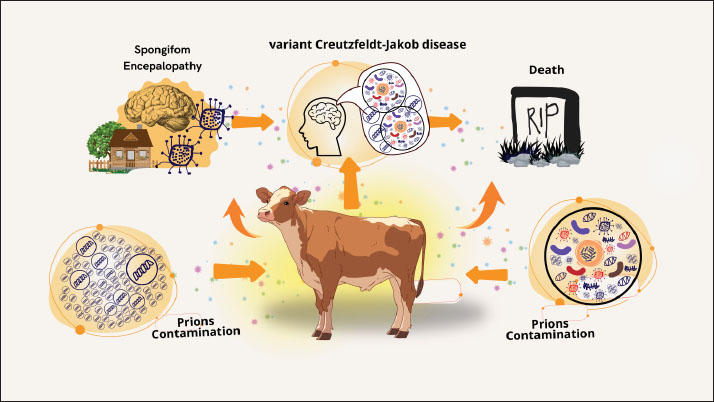
Fig. 1. Understanding BSE transmission: from prion contamination to neurological disease. There have also been reports of spongiform encephalopathy in kudu, eland, nyala, gemsbok, and some exotic cats (Orge et al., 2021). It is believed that tainted feed is also connected to this. It has also been proposed that exposure to BSE prior to the implementation of a ban on certain beef offal (SBO) at slaughterhouses in 1989 may have contributed to 23 cases (up to 31 January 1998) of the variant form of CJD (nvCJD) (human disease) in the (UK Department of Health, 2 March 1998) and 1 case in France (Sanchez-Juan et al., 2007). Finally, a ban on the consumption of brains, spinal cords, and other tissues that could potentially transmit BSE. Risk factorsAnimals cannot contract BSE from one another, and it is not a contagious illness. In addition, milk and other dairy products do not contain BSE. According to research, feeding livestock MBM obtained from BSE-infected livestock contaminated with BSE prions is the only known risk factor for the development of BSE (Islam et al., 2022). Specified risk material refers to specific animal tissues that are likely to contain and consequently spread BSE prions (Shui et al., 2023). The WOAH Terrestrial Animal Health Code lists the brain, spinal cord, eyes, spine, tonsils, skull, and distal ileum as examples of these tissues (WOAH, 2008). BSE prions are not entirely eliminated by processing methods and are resistant to common inactivation techniques like heat and disinfectants (Giles et al., 2008). Public health importanceStrong evidence linking BSE to human transmission and a variant type of CJD has brought the disease to the attention of many countries. British researchers suspected that the cases might reflect the advent of a new form of CJD stemming from human-to-human BSE transmission because of the relatively young age of the victims and their clinicopathological uniformity (Ritchie et al., 2021). Approximately 9 years after BSE was discovered in the UK, the appearance of this variant form of CJD (vCJD) was reported in 1996 (Zou and Gambetti, 2009). The causative link between vCJD and BSE is further supported by the lack of similar instances in other nations with comparable monitoring systems, the fact that it still occurs almost exclusively in the UK, and more laboratory research studies. Human cases of vCJD are increasing as a result of eating meat tainted with central nervous system tissue from diseased cattle (Maheshwari et al., 2015). Four incidences of human-to-human transmission of vCJD through blood or plasma transfusion have been reported in the UK, despite the fact that the majority of cases are caused by the ingestion of prion-tainted beef (McManus et al., 2022). A retrospective study of tonsil and appendix specimens has led to estimates that up to 1 in 4,000 people exposed during the UK outbreak may have been asymptomatic carriers of vCJD, raising concerns about similar transmission (Gill et al., 2020; Ironside et al., 2000). Nonetheless, infectious agents are present in nearly every tissue in the body, including the blood, despite being mostly concentrated in nerve tissue (Brown, 2001). Before regulations on high-risk offal were introduced in 1989, between 460,000 and 482,000 BSE-infected animals had entered the human food chain (Supervie and Costagliola, 2006). In the United Kingdom, the first 10 cases of vCJD were reported in April 1996 (Diack et al., 2014). As of November 1, 2004, there have been 151 recorded instances of vCJD in the UK as of November 1, 2004 (Ritchie et al., 2021). Furthermore, eight cases of vCJD from France and one case from Italy have been detected, along with three cases (one from each of Canada, Ireland, and the United States) among individuals who may have been exposed to BSE in the United Kingdom as a result of their prior residency there. There have been 227 recorded cases of vCJD as of December 2012 (Watson et al., 2021). Most cases (176) occurred in the UK. Economic impactThe global beef sector has been severely damaged by the BSE outbreak. Not only are BSE-affected nations unable to export live ruminants, beef, or beef products, but they also incur financial losses as a result of having to kill livestock that is either afflicted or thought to be infected (Onodera and Kim, 2006). Before the discovery of a potential connection between vCJD and BSE in 1995, the UK exported 77,000 metric tonnes of beef and veal to other countries (Beisel and Morens, 2004). In the month following the 1996 announcement, household consumption of beef had decreased by 26% from the previous year’s level, while domestic sales of beef products in the United Kingdom had declined by 40% (Alarcon et al., 2023). The total economic damage caused by BSE to the UK in the first year of the crisis (1996) was estimated to be between £740 million and £980 million (Nura and Lelisa, 2018). It is anticipated that the UK will export less than 2000 metric tonnes in 2000 because exports will probably stop as more BSE cases are reported (Alarcon et al., 2023). The European Union outlawed the use of substances derived from cows in nonfood goods in 1996. Processed beef by-products, including collagen, elastin, gelatin, and derivatives of beef fat, are used in many of these goods. Historically, byproducts from cows’ hearts, kidneys, spleens, lungs, and brains have been used as food supplements and medications (Latoch et al., 2024). More than 800 medications are on the market that may pose a risk of vCJD (Ponte, 2006). Oral polio vaccination had to be discontinued in the UK in the fall of 2000 due to the discovery that it possibly contained contaminated serum (Sanchez-Juan et al., 2007). The food business has experienced losses as a result of BSE. In Europe, restaurants are cutting back on their beef offerings or completely eliminating them. The demand for European meat distributors, who frequently supply restaurants, has decreased by up to 40%. Almost every aspect of the European food business has been affected by BSE (Meijer et al., 2023). As BSE spread outside Europe to Japan and, in mid-2003, to Canada, USDA enhanced its surveillance efforts and increased funding for BSE-related research. Regulatory efforts to counter the disease were further strengthened when, on December 23, 2003, a dairy cow in Washington State tested positive for BSE. Within days of the Washington state BSE announcement, 53 countries, including major markets, such as Japan, Mexico, South Korea, and Canada, banned imports of U.S. cattle and beef products. The potential impacts of additional BSE measures regarding the ban on animal feed were estimated to have associated costs of $2.16 per head for fed slaughter and $6.77 per head for non-fed slaughter. Additionally, it was estimated that a complete ban on feeding ruminant-derived proteins would cost $14.01 per fed animal and $12.35 per non-fed animal, in addition to adding $4.50 per head to feed costs for a fed animal (Kansas Department of Agriculture, 2005). In 2003, U.S. beef exports totaled $3.95 billion, accounting for 9.6% of U.S. commercial beef production. The import bans caused U.S. beef exports to plummet, and although some important markets, including Mexico and Canada, reopened during 2004, export quantities for the year declined by 82% below 2003’s level (Kansas Department of Agriculture, 2005). A market model that incorporated assumptions about the elasticity of demand for beef and offal to estimate the price impact of additional supplies on the market estimated that the total U.S. beef industry losses arising from the loss of beef and offal exports during 2004 ranged from $3.2 billion to $4.7 billion (Kansas Department of Agriculture, 2005). ControlThere is no treatment for BSE. In most cases, suspected animals are put to death so they can be tested. When a veterinarian comes across or suspects BSE, reports on the illness are usually made in accordance with local or national protocols (Saegerman et al., 2004). Most countries require reports on this condition. In the United States, notification of state or federal veterinary authorities is required immediately (Charatan, 2001). Some nations utilize tests conducted at slaughterhouses to identify BSE cases in cattle and occasionally in sheep and goats. Tests on small ruminants and healthy cattle meant for human consumption are often conducted on animals older than a particular age. Japan tested all cows, regardless of age (Onodera and Kim, 2006). High-risk animals usually have stricter testing criteria (such as cattle that have neurological indications or are unable to move) (EFSA et al., 2018). Only high-risk animals are tested in certain countries. Nations that formerly tested most or all cattle have lowered their testing criteria as the prevalence of BSE has decreased (Olech, 2023). Following the identification of diseased animals, the afflicted herd is typically placed under quarantine while the infection’s cause is investigated (Simon et al., 2008). Groups of animals that are infected (such as those that were born or raised in the same herd during their first year of life) are frequently tested and put to death because they are most likely to have shared feed during the peak of their vulnerability (Kumagai et al., 2019). These animals may also be euthanized because the progeny of affected cattle have an elevated risk of BSE. Preventing BSE can be achieved by avoiding feeding vulnerable species with ruminant tissues that might carry prions (EFSA et al., 2017). Because prions cannot be completely inactivated by cooking or processing, complete avoidance is usually required (Lee, 2023). Nowadays, the use of ruminant or mammalian proteins in animal feed is prohibited in many nations, with the exception of specific foods like milk and blood (Ferreira et al., 2019). The specific types of prohibited and banned sources of protein differ among nations. In several nations, fertilizer and other types of animal feed are also prohibited. By taking these last measures, it may be possible to avoid cross-contamination and unintentional exposure of cattle to BSE prions (Kumagai et al., 2019). Feed restrictions have the potential to curb the spread of the BSE epidemic, but because the illness has a protracted incubation period, there may be a delay in the number of cases. Moreover, the importation of live cattle and specific ruminant proteins from impacted nations may be prohibited under trade agreements (Onodera and Kim, 2006). Countries that meet specific criteria (e.g., feed bans, laboratory support, and BSE awareness programs for people working with livestock) and have surveillance and traceability programmes that comply with WOAH standards are recognized as being at “negligible risk” or “managed risk” for BSE (WOAH, 2008). Countries classified as controlled risk have had more recent cases of BSE, whereas countries under negligible risk have not reported any cases of classical BSE in local animals or contaminated animals born more than 11 years ago (EFSA et al., 2017). “Undetermined risk” refers to nations that do not fit the criteria for insignificant or controlled risk. Most countries with cases of BSE are implementing certain stringent measures, such as bans on live animals (especially cattle), meat products, and some animal feedstuffs, disease surveillance programmes, restrictions on feeding some suspected prion-infected animal tissues to ruminant animals, and restrictions on blood donations from individuals who previously resided in BSE-affected countries, in a way to drastically curtail/eliminate the spread of BSE and its risk materials from the food chain (Boujon et al., 2016; Amin et al., 2023). For example, Brazil, one of the world’s top exporters of beef, especially to China, has already announced a reduction in beef shipment to China due to concerns arising from the spread of BSE (Global Times, 2023). ConclusionA transmissible neurological disease known as BSE affects cattle and is caused by misfolded proteins. The main route of BSE transmission among cattle is through the ingestion of infectious material found in MBM made from diseased animals. This illness has an influence on the economy and public health. The main route of contracting the virus is through eating beef contaminated with tissue from diseased cattle’s central nervous system. There is no known cure for BSE. Reared cattle tissues that may contain prions should not be fed to vulnerable species to prevent BSE. AcknowledgmentsThe authors would like to acknowledge the Kementerian Pendidikan, Kebudayaan, Riset, and Teknologi who funded this research. Conflict of interestThe authors declare no conflict of interest. FundingThe authors thank Universitas Airlangga for their managerial support. Author’s contributionsTDL, ARK, BWKW, and SW drafted the manuscript. ZAB, DAAK, WW, and IBM revised and edited the manuscript. KAF, RZA, EFL, TH, and RD participated in the preparation and critical checking of the manuscript. IF, SU, SM, RR, and MKJK edited the references. All authors have read and approved the final manuscript. Data availabilityAll references are open-access, so data can be obtained from the online literature. ReferencesAckermann, I., Ulrich, R., Tauscher, K., Fatola, O.I., Keller, M., Shawulu, J.C., Arnold, M., Czub, S., Groschup, M.H. and Balkema-Buschmann, A. 2021. Prion infectivity and PrPBSE in the peripheral and central nervous system of cattle 8 months post oral BSE Challenge. Int. J. Mol. Sci. 22(21), 11310. Alarcon, P., Wall, B., Barnes, K., Arnold, M., Rajanayagam, B. and Guitian, J. 2023. Classical BSE in Great Britain: RA review of its epidemic, risk factors, policy, and impact. Food Control. 146(1), 109490. Amin, R., Darwin, R., Chakraborty, S., Chandran, D., Chopra, H. and Dhama, K. 2023. Bovine spongiform encephalopathy, “Mad cow’s disease” and variant Creutzfeldt-Jakob disease in humans: a critical update. Arch. Med. Res. 54(5), 102854. Aulić, S., Bolognesi, M.L. and Legname, G. 2013. Small-molecule theranostic probes: a promising future in neurodegenerative diseases. Int. J. Cell. Biol. 2013(1), 150952. Baldeschi, A.C., Zattoni, M., Vanni, S., Nikolic, L., Ferracin, C., La Sala, G., Summa, M., Bertorelli, R., Bertozzi, S.M., Giachin, G., Carloni, P., Bolognesi, M.L., De Vivo, M. and Legname, G. 2022. Innovative non-PrP-targeted drug strategy designed to enhance prion clearance. J. Med. Chem. 65(1), 8998–9010. Barnwal, S., Jha, G., Sola, S.C., Anand, P. and Shariff, S.Y. 2022. Creutzfeldt-Jakob disease: a case report and literature review for understanding the big picture. Cureus 14(11), e31303. Baron, T. 2002. Identification of inter-species transmission of prion strains. J. Neuropathol. Exp. Neurol. 61(5), 377–383. Beisel, C.E. and Morens, D.M., 2004. Variant Creutzfeldt-Jakob disease and acquired and transmissible spongiform encephalopathies. Clin. Infect. Dis. 38(5), 697–704. Belay, E.D., Sejvar, J.J., Shieh, W.J., Wiersma, S.T., Zou, W.Q., Gambetti, P., Hunter, S., Maddox, R.A., Crockett, L., Zaki, S.R. and Schonberger, L.B. 2005. Variant Creutzfeldt-Jakob disease death, United States. Emerg. Infect. Dis. 11(9), 1351–1354. Bencsik, A., Debeer, S., Petit, T. and Baron, T. 2009. A case of maternal transmission of feline spongiform encephalopathy in a captive cheetah. PLoS One 4(9), e6929. Betancor, M., Marín, B., Otero, A., Hedman, C., Romero, A., Barrio, T., Sevilla, E., Douet J, Huor, A., Badiola, J., Andreoletti, O. and Bolea, R. 2023. Detection of classical BSE prions in asymptomatic cows after inoculation with atypical/Nor98 scrapie. Vet. Res. 54, 89. Boujon, C., Serra, F. and Seuberlich, T. 2016. Atypical variants of bovine spongiform encephalopathy: rare diseases with consequences for BSE surveillance and control. Switzerland Arch. Tierheilkd. 158, 171–177. Brandel, J.P. and Knight, R. 2018. Variant Creutzfeldt-Jakob disease. Handb. Clin. Neurol. 153(1), 191–205. Brown, P. 2001. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease. BMJ 322(7290), 841–844. Brown, P. and Abee, C.R. 2005. Working with transmissible spongiform encephalopathy agents. ILAR J. 46(1), 44–52. Castle, A.R. and Gill, A.C. 2017. Physiological functions of the cellular prion protein. Front. Mol. Biosci. 4(1), 19. Charatan, F. 2001. The United States employs precautions against BSE. West J. Med. 174(4), 235–237. Chen, C. and Dong, X.P. 2016. Epidemiological characteristics of human prion diseases. Infect. Dis. Poverty 5(1), 47. Collins, S., McLean, C.A. and Masters, C.L. 2001. Gerstmann-Sträussler-Scheinker syndrome, fatal familial insomnia, and kuru: a review of these less common human transmissible spongiform encephalopathies. J. Clin. Neurosci. 8(5), 387–397. Columbus, C. 2004. Mad cow and other diseases: updates on emerging infectious diseases. Proc. Bayl. Univ. the Med. Cent. 17(4), 411–417. Cooley, W.A., Clark, J.K., Ryder, S.J., Davis, L.A., Farrelly, S.S. and Stack, M.J. 2001. Evaluation of a rapid western immunoblotting procedure for the diagnosis of bovine spongiform encephalopathy (BSE) in the UK. J. Comp. Pathol. 125(1), 64–70. Cooper, S.A., Murray, K.L., Heath, C.A., Will, R.G. and Knight, R.S. 2006. Sporadic Creutzfeldt-Jakob disease with cerebellar ataxia at onset in the UK. J. Neurol. Neurosurg. Psychiatry 77(11), 1273–1275. Corda, E., Thorne, L., Beck, K.E., Lockey, R., Green, R.B., Vickery, C.M., Holder, T.M., Terry, L.A., Simmons, M.M. and Spiropoulos, J. 2015. Ability of the wild-type mouse bioassay to detect bovine spongiform encephalopathy (BSE) in the presence of excess scrapie. Acta Neuropathol. Commun. 3(1), 21. Costassa, E.V., Iulini, B., Mazza, M., Acutis, P., Maurella, C., Meloni, D., Pautasso, A., Capucci, L., Bozzetta, E., Simmons, M.M., Zanusso, G., Pocchiari, M., Corona, C. and Casalone, C. 2016. Pathogenesis and transmission of classical and atypical BSE in cattle. Food Saf. 4(4), 130–134. Cunningham, A.A., Kirkwood, J.K., Dawson, M., Spencer, Y.I., Green, R.B. and Wells, G.A. 2004. Bovine spongiform encephalopathy infectivity in greater kudu (Tragelaphus strepsiceros). Emerg. Infect. Dis. 10(6), 1044–1049. Davanipour, Z., Sobel, E., Ziogas, A., Smoak, C., Bohr, T., Doram, K. and Liwnicz, B. 2014. Dietary risk factors for sporadic Creutzfeldt-Jakob disease: a confirmatory case–control study. Br. J. Med. Med. Res. 4(12), 2388–2417. Diack, A.B., Head, M.W., McCutcheon, S., Boyle, A., Knight, R., Ironside, J.W., Manson, J.C. and Will, R.G. 2014. Variant CJD. Eighteen years of research and surveillance. Prion 8(4), 286–295. Diack, A.B., Ritchie, D., Bishop, M., Pinion, V., Brandel, J.P., Haik, S., Tagliavini, F., Van Duijn, C., Belay, E.D., Gambetti, P., Schonberger, L.B., Piccardo, P., Will, R.G. and Manson, J.C. 2012. Constant transmission properties of variant Creutzfeldt-Jakob disease in 5 countries. Emerg. Infect. Dis. 18(10), 1574–1579. EFSA (European Food Safety Authority) 2022. Annual report of the scientific network on BSE-TSE 2022. EFSA supporting publication 2022: EN-7656. Parma: European Food Safety Authority, pp: 11. EFSA Panel on Biological Hazards (BIOHAZ), Koutsoumanis, K., Allende, A., Alvarez-Ordoňez, A., Bolton, D., Bover-Cid, S., Chemaly, M., Davies, R., De Cesare, A., Herman, L., Hilbert, F., Lindqvist, R., Nauta, M., Peixe, L., Ru, G., Skandamis, P., Suffredini, E., Andreoletti, O., Benestad, S.L., Comoy, E., Nonno, R., da Silva Felicio, T., Ortiz-Pelaez, A. and Simmons, M.M. 2019. Update on chronic wasting disease (CWD) III. EFSA J. 17(11), e05863. EFSA Panel on Biological Hazards (BIOHAZ), Ricci, A., Allende, A., Bolton, D., Chemaly, M., Davies, R., Escámez, P.S.F., Gironés, R., Herman, L., Koutsoumanis, K., Lindqvist, R., Nørrung, B., Robertson, L., Ru, G., Sanaa, M., Skandamis, P., Snary, E., Speybroeck, N., Kuile, B.T., Threlfall, J., Wahlström, H., Adkin, A., Greiner, M., Marchis, D., Prado, M., Da Silva Felicio, T., Ortiz-Pelaez, A. and Simmons, M. 2018. Updated quantitative risk assessment (QRA) of the risk of BSE associated with processed animal protein (PAP). EFSA J. 16(7), e05314. EFSA Panel on Biological Hazards (BIOHAZ), Ricci, A., Allende, A., Bolton, D., Chemaly, M., Davies, R., Escámez, P.S.F., Gironés, R., Herman, L., Koutsoumanis, K., Lindqvist, R., Nørrung, B., Robertson, L., Sanaa, M., Simmons, M., Skandamis, P., Snary, E., Speybroeck, N., Kuile, B.T., Threlfall, J., Wahlström, H., Adkin, A., De Koeijer, A., Ducrot, C., Griffin, J., Pelaez, A.O., Latronico, F. and Ru, G. 2017. Bovine spongiform encephalopathy (BSE) cases born after the total feed ban. EFSA J. 15(7), e04885. Elezgarai, S.R., Fernández-Borges, N., Eraña, H., Sevillano, A.M., Charco, J.M., Harrathi, C., Saá, P., Gil, D., Kong, Q., Requena, J.R., Andréoletti, O. and Castilla, J. 2017. Generation of a new infectious recombinant prion: a model to understand Gerstmann-Sträussler-Scheinker syndrome. Sci. Rep. 7(1), 9584. Espinosa, J.C., Andréoletti, O., Castilla, J., Herva, M.E., Morales, M., Alamillo, E., San-Segundo, F.D., Lacroux, C., Lugan, S., Salguero, F.J., Langeveld, J. and Torres, J.M. 2007. Sheep-passaged bovine spongiform encephalopathy agent exhibits altered pathobiological properties in bovine PRP transgenic mice. J. Virol. 81(2), 835–843. Fast, C., Graham, C., Kaatz, M., Santiago-Mateo, K., Kaatz, T., MacPherson, K., Balkema-Buschmann, A., Ziegler, U., Groschup, M.H. and Czub, S. 2023. Discrimination between classical and atypical BSE by a distinct immunohistochemical PrPSc profile. Pathogens 12(2), 353. Ferreira, R.S. Jr., da Silva, D.A.F., Biscola, N.P., Sartori, M.M.P., Denadai, J.C., Jorge, A.M., Dos Santos, L.D. and Barraviera, B. 2019. Traceability of animal protein byproducts in ruminants by multivariate analysis of isotope ratio mass spectrometry to prevent the transmission of prion diseases. J. Venom Anim. Toxins Incl. Trop. Dis. 25(1), e148718. Fevrier, B., Vilette, D., Archer, F., Loew, D., Faigle, W., Vidal, M., Laude, H. and Raposo, G. 2004. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U S A 101(26), 9683–9688. Gallardo, M.J. and Delgado, F.O. 2021. Animal prion diseases: a review of intraspecies transmission. Open Vet. J. 11(4), 707–723. Giles, K., Glidden, D.V., Beckwith, R., Seoanes, R., Peretz, D., DeArmond, S.J. and Prusiner, S.B. 2008. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathog. 4(11), e1000206. Gill, O.N., Spencer, Y., Richard-Loendt, A., Kelly, C., Brown, D., Sinka, K., Andrews, N., Dabaghian, R., Simmons, M., Edwards, P., Bellerby, P., Everest, D.J., McCall, M., McCardle, L.M., Linehan, J., Mead, S., Hilton, D.A., Ironside, J.W. and Brandner, S. 2020. Prevalence of abnormal prion protein in human appendices before and after exposure to the cattle BSE epizootic. Acta Neuropathol. 139(6), 965–976. Global Times (2023). Brazilian beef exports to China suspended after mad cow disease. Australian supply may be boosted. Available via https://www.globaltimes.cn/page/202302/1286114.shtml. (Accessed 4 December 2024). Goold, R., McKinnon, C. and Tabrizi, S.J. 2015. Prion degradation pathways: potential for therapeutic intervention. Mol. Cell. Neurosci. 66(Pt A), 12–20. Gough, K.C., Bishop, K. and Maddison, B.C. 2014. Highly sensitive detection of small ruminant bovine spongiform encephalopathy within transmissible spongiform encephalopathy mixes by serial protein misfolding cyclic amplification. J. Clin. Microbiol. 52(11), 3863–3868. Gousset, K. and Zurzolo, C. 2009. Tunneling nanotubes: a highway for particle spreading? Prion 3(2), 94–98. Greenlee, J.J. and Greenlee, M.H. 2015. The transmissible spongiform encephalopathies of livestock. ILAR J. 56(1), 7–25. Haley, N.J. and Richt, J.A. 2023. Classical bovine spongiform encephalopathy and chronic wasting disease: two sides of the prion coin. Anim. Dis. 3(1), 24. Hamir, A.N., Kehrli, M.E. Jr., Kunkle, R.A., Greenlee, J.J., Nicholson, E.M., Richt, J.A., Miller, J.M. and Cutlip, R.C. 2011. Experimental interspecies transmission studies of transmissible spongiform encephalopathies to cattle: comparison to bovine spongiform encephalopathy in cattle. J. Vet. Diagn. Invest. 23(3), 407–420. Head, M.W., Ritchie, D., Smith, N., McLoughlin, V., Nailon, W., Samad, S., Masson, S., Bishop, M., McCardle, L. and Ironside, J.W. 2004. Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: an immunohistochemical, quantitative, and biochemical study. Am. J. Pathol. 164(1), 143–153. Heath, C.A., Barker, R.A., Esmonde, T.F., Harvey, P., Roberts, R., Trend, P., Head, M.W., Smith, C., Bell, J.E., Ironside, J.W., Will, R.G. and Knight, R.S. 2006. Dura mater-associated Creutzfeldt-Jakob disease: experience from surveillance in the UK. J. Neurol. Neurosurg. Psychiatry 77(7), 880–882. Hedlin, P., Taschuk, R., Potter, A., Griebel, P. and Napper, S. 2012. Detection and control of prion diseases in food animals. ISRN Vet. Sci. 2012(1), 254739. Holman, R.C., Belay, E.D., Christensen, K.Y., Maddox, R.A., Minino, A.M., Folkema, A.M., Haberling, D.L., Hammett, T.A., Kochanek, K.D., Sejvar, J.J. and Schonberger, L.B. 2010. Human prion diseases in the United States. PLoS One 5(1), e8521. Houston, F. and Andréoletti, O. 2019. Animal prion diseases: risks to human health. Brain Pathol. 29(2), 248–262. Imran, M. and Mahmood, S. 2011. An overview of animal prion diseases. Virol. J. 8(1), 493. Ironside, J.W., Hilton, D.A., Ghani, A., Johnston, N.J., Conyers, L., McCardle, L.M. and Best, D. 2000. Retrospective study of prion-protein accumulation in tonsil and appendix tissue. Lancet 355(9216), 1693–1694. Islam, M.N., Siddiqui, M.S.I., Islam, M.T., Islam, M.R. and Chowdhury, E.H. 2022. Usage of meat and bone meal in animal, poultry, and fish feeds: a survey and risk analysis for the occurrence of bovine spongiform encephalopathy in Bangladesh. Vet. Med. Sci. 8(1), 377–386. Iulini, B., Cantile, C., Mandara, M.T., Maurella, C., Loria, G.R., Castagnaro, M., Salvadori, C., Porcario, C., Corona, C., Perazzini, A.Z., Maroni, A., Caramelli, M. and Casalone, C. 2008. Neuropathology of Italian cats with feline spongiform encephalopathy. Vet. Pathol. 45(5), 626–633. Jin, H.J. and Koo, W.W. 2003. The effect of the BSE outbreak in Japan on consumers’ preferences. Eur. Rev. Agric. Econ. 30(2), 173–192. Kaelber, North, Bett, C., Asher, D.M. and Gregori, L., 2019. Quaking-induced conversion of prion protein on a thermal mixer accelerates detection in brains infected with transmissible spongiform encephalopathy agents. PLoS One 14(12), e0225904. Kaifa, F.H., Bhattacharya, P. and Islam, M.A. 2023. Possible BSE case in Brazil: beef export halted to China. New Microbes New Infect. 53, 101121. Kamali-Jamil, R., Vázquez-Fernández, E., Tancowny, B., Rathod, V., Amidian, S., Wang, X., Tang, X., Fang, A., Senatore, A., Hornemann, S., Dudas, S., Aguzzi, A., Young, H.S. and Wille, H. 2021. The ultrastructure of infectious L-type bovine spongiform encephalopathy prions constrains molecular models. PLoS Pathog. 17(6), e1009628. Kansas Department of Agriculture 2005. The economic impact of BSE on the U.S. Beef Industry: product value losses, regulatory costs, and consumer reactions. Available via www.agmanager.info/livestock/marketing/bulletins_2/industry (Accessed 6 December 2024). Kao, R.R., Houston, F., Baylis, M., Chihota, C.M., Goldmann, W., Gravenor, M.B., Hunter, N. and McLean, A.R. 2003. Epidemiological implications of the susceptibility to BSEinf putatively resistant sheep. J. Gen Virol. 84(Pt 12), 3503–3512. Kelemen, K., Kövecsi, A., Banias, L., Klára, I., Mihály, I., Forró, C., Szász, J.A. and Szatmári, S. 2022. A rare case of histopathologically confirmed Creutzfeldt-Jakob disease from Romania, long route to diagnosis-case report, and an overview of the Romanian CJD situation. J. Clin. Med. 11(16), 4803 Kumagai, S., Daikai, T. and Onodera, T., 2019. Bovine spongiform encephalopathy: a review from the perspective of food safety. Food Saf. 7(2), 21–47. Lakkaraju, A.K.K., Sorce, S., Senatore, A., Nuvolone, M., Guo, J., Schwarz, P., Moos, R., Pelczar, P. and Aguzzi, A. 2022. Glial activation in prion diseases is selectively triggered by neuronal PrPSc. Brain Pathol. 32(5), e13056. Lasmézas, C.I., Comoy, E., Hawkins, S., Herzog, C., Mouthon, F., Konold, T., Auvré, F., Correia, E., Lescoutra-Etchegaray, N., Salès, N., Wells, G., Brown, P. and Deslys, J.P. 2005. Risk of oral bovine spongiform encephalopathy agent in primates. Lancet 365(9461), 781–783. Latoch, A., Stasiak, D.M. and Siczek, P. 2024. Edible offal as a valuable source of nutrients in the diet-a review. Nutrients 16(11), 1609. Lee, J., Kim, S.Y., Hwang, K.J., Ju, Y.R. and Woo, H.J. 2013. Prion diseases are transmissible zoonotic diseases. Osong. P.H. Res. Perspect. 4(1), 57–66. Lee, Y.C.J. 2023. Prions: a threat to health security that requires effective medical countermeasures. Glob. Health J. 7(1), 43–48. Liberski, P.P., Sikorska, B., Lindenbaum, S., Goldfarb, L.G., McLean, C., Hainfellner, J.A. and Brown, P. 2012. Kuru: genes, cannibals, and neuropathology. J. Neuropathol. Exp. Neurol. 71(2), 92–103. Mabbott, N.A. 2017. How do PrPSc Prions spread between and within host species? Pathogens 6(4), 60. Madsen-Bouterse, S.A., Schneider, D.A., Zhuang, D., Dassanayake, R.P., Balachandran, A., Mitchell, G.B. and O’Rourke, K.I. 2016. Primary transmission of chronic wasting disease versus scrapie prions from small ruminants to transgenic mice expressing ovine or cervid prion protein. J. Gen Virol. 97(9), 2451–2460. Mahabadi, H.M. and Taghibiglou, C., 2020. Cellular prion protein (PrPc): putative interacting partners and consequences of the interaction. Int. J. Mol. Sci. 21(19), 7058. Maheshwari, A., Fischer, M., Gambetti, P., Parker, A., Ram, A., Soto, C., Concha-Marambio, L., Cohen, Y., Belay, E.D., Maddox, R.A., Mead, S., Goodman, C., Kass, J.S., Schonberger, L.B. and Hussein, H.M. 2015. Recent US case of variant Creutzfeldt-Jakob disease: global implications. Emerg. Infect. Dis. 21(5), 750–759. Mallucci, G., Dickinson, A., Linehan, J., Klöhn, P.C., Brandner, S. and Collinge, J. 2003. Depleting neuronal PrP during prion infection prevents disease and reverses spongiosis. Science 302(5646), 871–874. Masujin, K., Orrú, C.D., Miyazawa, K., Groveman, B.R., Raymond, L.D., Hughson, A.G. and Caughey, B. 2016. Detection of atypical H-type bovine spongiform encephalopathy and discrimination of bovine prion strains by real-time shaking-induced conversion. J. Clin. Microbiol. 54(3), 676–686. McCutcheon, S., Langeveld, J.P., Tan, B.C., Gill, A.C., de Wolf, C., Martin, S., Gonzalez, L., Alibhai, J., Blanco, A.R., Campbell, L., Hunter, N. and Houston, E.F. 2014. Prion protein-specific antibodies that detect multiple TSE agents with high sensitivity. PLoS One 9(3), e91143. McManus, H., Seed, C.R., Hoad, V.C., Kiely, P., Kaldor, J.M., Styles, C.E., Yang, H., Law, M. and Gosbell, I.B. 2022. Risk of variant Creutzfeldt-Jakob disease transmission by blood transfusion in Australia. Vox. Sang. 117(8), 1016–1026. Meijer, N., Van Raamsdonk, L.W.D., Gerrits, E.W.J. and Appel, M.J. 2023. Use of animal by-products in circular bioeconomy: time for a TSE road map 3? Heliyon 9(3), e14021. Mohan, J., Bruce, M.E. and Mabbott, N.A. 2005. Follicular dendritic cell dedifferentiation reduces scrapie susceptibility following inoculation via the skin. Immunology 114(2), 225–234. Moore, J., Hawkins, S.A., Austin, A.R., Konold, T., Green, R.B., Blamire, I.W., Dexter, I., Stack, M.J., Chaplin, M.J., Langeveld, J.P., Simmons, M.M., Spencer, Y.I., Webb, P.R., Dawson, M. and Wells, G.A. 2011. Studies on the transmissibility of bovine spongiform encephalopathy agents to domestic chickens. BMC Res. 4(1), 501. Moore, S.J., Greenlee, M.H.W., Smith, J.D., Vrentas, C.E., Nicholson, E.M. and Greenlee, J.J. 2016. A comparison of classical and H-type bovine spongiform encephalopathy associated with E211K prion protein polymorphism in wild-type and EK211 cattle following intracranial inoculation. Front. Vet. Sci. 3(1), 78. Natale, G., Pasquali, L., Paparelli, A. and Fornai, F. 2011. Parallel manifestations of neuropathologies in the enteric and central nervous systems. Neurogastroenterol. Motil. 23(12), 1056–1065. Nathanson, N., Wilesmith, J. and Griot, C. 1997. Bovine spongiform encephalopathy (BSE): causes and consequences of a common source epidemic. Am. J. Epidemiol. 145(11), 959–969. Ness, A., Aiken, J. and McKenzie, D. 2023. Sheep scrapie and deer rabies were found in England prior to 1800. Prion 17(1), 7–15. Nico, P.B., de-Paris, F., Vinadé, E.R., Amaral, O.B., Rockenbach, I., Soares, B.L., Guarnieri, R., Wichert-Ana, L., Calvo, F., Walz, R., Izquierdo, I., Sakamoto, A.C., Brentani, R., Martins, V.R. and Bianchin, M.M. 2005. Altered behavioral response to acute stress in mice lacking cellular prion protein. Behav. Brain Res. 162(2), 173–181. Nura, D. and Lelisa, K. 2018. Review of bovine spongiform encephalopathy. Am. Eurasian. J. Agric. Environ. Sci. 13(4), 85–92. Olech, M. 2023. Conventional and state-of-the-art detection methods of bovine spongiform encephalopathy (BSE). Int. J. Mol. Sci. 24(8), 7135–7147. Oliveira, J.J., Yanick, C.T., Wein, N. and Limia, C.E.G. 2023. Neuron-Schwann cell interactions in peripheral nervous system homeostasis, disease, and preclinical treatment. Front. Cell. Neurosci. 17(1), 1248922. Onodera, T. and Kim, C.K. 2006. BSE situation and establishment of the Food Safety Commission in Japan. J. Vet. Sci. 7(1), 1–11. Orge, L., Lima, C., Machado, C., Tavares, P., Mendonça, P., Carvalho, P., Silva, J., Pinto, M.L., Bastos, E., Pereira, J.C., Gonçalves-Anjo, N., Gama, A., Esteves, A., Alves, A., Matos, A.C., Seixas, F., Silva, F., Pires, I., Figueira, L., Vieira-Pinto, M., Sargo, R. and Pires, M.D.A. 2021. Neuropathology of animal prion diseases. Biomolecules 11(3), 466. Otero, A., Velásquez, C.D., Aiken, J. and McKenzie, D. 2021. Chronic wasting disease: a cervid prion infection looming to spillover. Vet. Res. 52(1), 115. Panes, J.D., Saavedra, P., Pineda, B., Escobar, K., Cuevas, M.E., Moraga-Cid, G., Fuentealba, J., Rivas, C.I., Rezaei, H. and Muñoz-Montesino, C. 2021. PrPC as a transducer of physiological and pathological signals. Front. Mol. Neurosci. 14(1), 762918. Pastore, A. and Zagari, A. 2007. A structural overview of the vertebrate prion proteins. Prion 1(3), 185–197. Poggiolini, I., Saverioni, D. and Parchi, P. 2013. Prion protein misfolding, strains, and neurotoxicity: an update from studies on Mammalian prions. Int. J. Cell. Biol. 2013(1), 910314. Ponte, M.L. 2006. Insights into the management of emerging infections: regulating variant Creutzfeldt-Jakob disease transfusion risk in the UK and the US. PLoS Med. 3(10), e342. Press, C.M., Heggebø, R. and Espenes A. 2004. Involvement of ruminants’ gut-associated lymphoid tissue in the spread of transmissible spongiform encephalopathies. Adv. Drug Deliv. Rev. 56(6), 885–899. Pritzkow, S., Gorski, D., Ramirez, F. and Soto C. 2021. Prion dissemination through the environment and medical practices: facts and risks for human health. Clin. Microbiol. Rev. 34(4), e0005919. Rech, R.R., Giaretta, P.R., Brown, C. and Barros, C.S.L. 2018. Gross and histopathological pitfalls found in the examination of 3,338 cattle brains submitted to the BSE surveillance program in Brazil. Bras. 38(11), 2099–2108. Ritchie, D.D.L., Peden, A.H. and Barria, M.A. 2021. Variant CJD: reflections on a quarter of a century on. Pathogens 10(11), 1413. Saegerman, C., Speybroeck, N., Roels, S., Vanopdenbosch, E., Thiry, E. and Berkvens, D. 2004. Decision support tools for clinical diagnosis of disease in cows with suspected bovine spongiform encephalopathy. J. Clin. Microbiol. 42(1), 172–178. Sakudo, A., Anraku, D. and Itarashiki, T. 2020. Inactivation of prions by low-temperature sterilization technology using vaporized gas derived from a hydrogen peroxide-peracetic acid mixture. Pathogens 10(1), 24. Sakudo, A., Yamashiro, R. and Onodera, T. 2022. Recent advances in prion inactivation by plasma sterilizer. Int. J. Mol. Sci. 23(18), 10241–10252. Sanchez-Juan, P., Cousens, S.N., Will, R.G. and van Duijn, C.M. 2007. Source of variant Creutzfeldt-Jakob disease outside the United Kingdom. Emerg. Infect. Dis. 13(8), 1166–1169. Saunders, S.S.E., Bartelt-Hunt, S.L. and Bartz, J.C. 2008. Prions in the environment: occurrence, fate, and mitigation. Prion 2(4), 162–169. Scialò, C., De Cecco, E., Manganotti, P. and Legname, G. 2019. Prion- and prion-like protein strains: deciphering the molecular basis of heterogeneity in neurodegeneration. Viruses 11(3), 261. Shui, T., Li, A., Chae, M., Xu, C.C. and Bressler, D.C. 2023. Valorization strategies for hazardous proteinaceous waste from rendering production: recent advances in specified risk materials (SRMs) conversion. J. Hazard Mater. 453(1), 131339. Sigurdson, C.J., Bartz, J.C. and Glatzel, M. 2019. Cellular and molecular mechanisms of prion disease. Annu. Rev Pathol. 14(1), 497–516. Simon, S., Nugier, J., Morel, N., Boutal, H., Créminon, C., Benestad, S.L., Andréoletti, O., Lantier, F., Bilheude, J.M., Feyssaguet, M., Biacabe, A.G., Baron, T. and Grassi, J. 2008. Rapid typing of transmissible spongiform encephalopathy strains with differential ELISA. Emerg. Infect. Dis. 14(4), 608–616. Simon, S.L., Lamoureux, L., Plews, M., Stobart, M., LeMaistre, J., Ziegler, U., Graham, C., Czub, S., Groschup, M. and Knox, J.D. 2008. Identification of disease-induced biomarkers in the urine of BSE infected cattle. Proteome Sci. 6(1), 23. Soto, C. and Satani, N. 2011. The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol. Med. 17(1), 14–24. Stack, M.J., Moore, S.J., Vidal-Diez, A., Arnold, M.E., Jones, E.M., Spencer, Y.I., Webb, P., Spiropoulos, J., Powell, L., Bellerby, P., Thurston, L., Cooper, J., Chaplin, M.J., Davis, L.A., Everitt, S., Focosi-Snyman, R., Hawkins, S.A., Simmons, M.M. and Wells, G.A. 2011. Experimental bovine spongiform encephalopathy: detection of PrP(Sc) in the small intestine relative to exposure dose and age. J. Comp. Pathol. 145(2–3), 289–301. Supervie, V. and Costagliola, D. 2006. How was the French BSE epidemic underestimated? C. R. Biol. 329(2), 106–116. Tam, J., Centola, J., Kurudzhu, H., Watson, N., MacKenzie, J., Leitch, M., Hughes, T., Green, A., Summers, D., Barria, M., Smith, C. and Pal, S. 2023. Sporadic Creutzfeldt-Jakob disease in the young (50 and below): 10-year review of United Kingdom surveillance. J. Neurol. 270(2), 1036–1046. Tamgüney, G., Miller, M.W., Giles, K., Lemus, A., Glidden, D.V., DeArmond, S.J. and Prusiner, S.B. 2009. Transmission of scrapie and sheep-passaged bovine spongiform encephalopathy prions to transgenic mice expressing elk prion protein. J. Gen Virol. 90(Pt 4), 1035–1047. Thackray, A.M., Ryder, S.J. and Bujdoso, R. 2005. Modification of blood cell PrP epitope exposure during prion disease. Biochem. J. 390(Pt 2), 563–571. Tichopad, A., Pfaffl, M.W. and Didier, A. 2003. Tissue-specific expression pattern of the bovine prion gene: quantification using real-time RT-PCR. Mol. Cell. Probes. 17(1), 5–10. Vascellari, M., Nonno, R., Mutinelli, F., Bigolaro, M., Di Bari, M.A., Melchiotti, E., Marcon, S., D’Agostino, C., Vaccari, G., Conte, M., De Grossi, L., Rosone, F., Giordani, F. and Agrimi, U. 2007. PrPSc in the salivary glands of scrapie-affected sheep. J. Virol. 81(9), 4872–4876. Wall, C.A., Rummans, T.A., Aksamit, A.J., Krahn, L.E. and Pankratz, V.S. 2005. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J. Neuropsychiatry Clin. Neurosci. 17(4), 489–495. Watson, N., Brandel, J.P., Green, A., Hermann, P., Ladogana, A., Lindsay, T., Mackenzie, J., Pocchiari, M., Smith, C., Zerr, I. and Pal, S. 2021. The importance of ongoing international surveillance for Creutzfeldt-Jakob disease. Nat. Rev. Neurol. 17(6), 362–379. Westergard, L., Christensen, H.M. and Harris, D.A. 2007. The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim. Biophys. Acta 1772(6), 629–644. Will, R.G., Ironside, J.W., Zeidler, M., Cousens, S.N., Estibeiro, K., Alperovitch, A., Poser, S., Pocchiari, M., Hofman, A. and Smith, P.G. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347(9006), 921–925. WOAH. 2008. Chapter 2.4.6.—Bovine spongiform encephalopathy. Terrestrial Manual. Paris, France: WOAH. pp: 671–682. Yuan, J., Xiao, X., McGeehan, J., Dong, Z., Cali, I., Fujioka, H., Kong, Q., Kneale, G., Gambetti, P. and Zou, W.Q. 2006. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J. Biol. Chem. 281(46), 34848–34858. Zerr, I. 2022. Prion 2022 Conference abstracts: pushing the boundaries. Prion 16(1), 95–253. Zhang, Y., Liu, Y., Liu, H. and Tang, W.H. 2019. Exosomes: biogenesis, biologic function, and clinical potential. Cell. Biosci. 9(1), 19. Zou, W.Q. and Gambetti, P. 2009. Variant Creutzfeldt-Jakob disease: French versus British. Ann. Neurol. 65(3), 233–235. | ||
| How to Cite this Article |
| Pubmed Style Lestari TD, Khairullah AR, Utama S, Mulyati S, Hernawati T, Damayanti R, Rimayanti R, Wardhani BWK, Fauzia KA, Moses IB, Ahmad RZ, Wibowo S, Fauziah I, Kurniasih DAA, Baihaqi ZA, Wasito W, Kusala MKJ, Lisnanti EF. Bovine spongiform encephalopathy: A review of current knowledge and challenges. Open Vet. J.. 2025; 15(1): 54-68. doi:10.5455/OVJ.2025.v15.i1.5 Web Style Lestari TD, Khairullah AR, Utama S, Mulyati S, Hernawati T, Damayanti R, Rimayanti R, Wardhani BWK, Fauzia KA, Moses IB, Ahmad RZ, Wibowo S, Fauziah I, Kurniasih DAA, Baihaqi ZA, Wasito W, Kusala MKJ, Lisnanti EF. Bovine spongiform encephalopathy: A review of current knowledge and challenges. https://www.openveterinaryjournal.com/?mno=224174 [Access: October 19, 2025]. doi:10.5455/OVJ.2025.v15.i1.5 AMA (American Medical Association) Style Lestari TD, Khairullah AR, Utama S, Mulyati S, Hernawati T, Damayanti R, Rimayanti R, Wardhani BWK, Fauzia KA, Moses IB, Ahmad RZ, Wibowo S, Fauziah I, Kurniasih DAA, Baihaqi ZA, Wasito W, Kusala MKJ, Lisnanti EF. Bovine spongiform encephalopathy: A review of current knowledge and challenges. Open Vet. J.. 2025; 15(1): 54-68. doi:10.5455/OVJ.2025.v15.i1.5 Vancouver/ICMJE Style Lestari TD, Khairullah AR, Utama S, Mulyati S, Hernawati T, Damayanti R, Rimayanti R, Wardhani BWK, Fauzia KA, Moses IB, Ahmad RZ, Wibowo S, Fauziah I, Kurniasih DAA, Baihaqi ZA, Wasito W, Kusala MKJ, Lisnanti EF. Bovine spongiform encephalopathy: A review of current knowledge and challenges. Open Vet. J.. (2025), [cited October 19, 2025]; 15(1): 54-68. doi:10.5455/OVJ.2025.v15.i1.5 Harvard Style Lestari, T. D., Khairullah, . A. R., Utama, . S., Mulyati, . S., Hernawati, . T., Damayanti, . R., Rimayanti, . R., Wardhani, . B. W. K., Fauzia, . K. A., Moses, . I. B., Ahmad, . R. Z., Wibowo, . S., Fauziah, . I., Kurniasih, . D. A. A., Baihaqi, . Z. A., Wasito, . W., Kusala, . M. K. J. & Lisnanti, . E. F. (2025) Bovine spongiform encephalopathy: A review of current knowledge and challenges. Open Vet. J., 15 (1), 54-68. doi:10.5455/OVJ.2025.v15.i1.5 Turabian Style Lestari, Tita Damayanti, Aswin Rafif Khairullah, Suzanita Utama, Sri Mulyati, Tatik Hernawati, Ratna Damayanti, Rimayanti Rimayanti, Bantari Wisynu Kusuma Wardhani, Kartika Afrida Fauzia, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Syahputra Wibowo, Ima Fauziah, Dea Anita Ariani Kurniasih, Zein Ahmad Baihaqi, Wasito Wasito, Muhammad Khaliim Jati Kusala, and Ertika Fitri Lisnanti. 2025. Bovine spongiform encephalopathy: A review of current knowledge and challenges. Open Veterinary Journal, 15 (1), 54-68. doi:10.5455/OVJ.2025.v15.i1.5 Chicago Style Lestari, Tita Damayanti, Aswin Rafif Khairullah, Suzanita Utama, Sri Mulyati, Tatik Hernawati, Ratna Damayanti, Rimayanti Rimayanti, Bantari Wisynu Kusuma Wardhani, Kartika Afrida Fauzia, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Syahputra Wibowo, Ima Fauziah, Dea Anita Ariani Kurniasih, Zein Ahmad Baihaqi, Wasito Wasito, Muhammad Khaliim Jati Kusala, and Ertika Fitri Lisnanti. "Bovine spongiform encephalopathy: A review of current knowledge and challenges." Open Veterinary Journal 15 (2025), 54-68. doi:10.5455/OVJ.2025.v15.i1.5 MLA (The Modern Language Association) Style Lestari, Tita Damayanti, Aswin Rafif Khairullah, Suzanita Utama, Sri Mulyati, Tatik Hernawati, Ratna Damayanti, Rimayanti Rimayanti, Bantari Wisynu Kusuma Wardhani, Kartika Afrida Fauzia, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Syahputra Wibowo, Ima Fauziah, Dea Anita Ariani Kurniasih, Zein Ahmad Baihaqi, Wasito Wasito, Muhammad Khaliim Jati Kusala, and Ertika Fitri Lisnanti. "Bovine spongiform encephalopathy: A review of current knowledge and challenges." Open Veterinary Journal 15.1 (2025), 54-68. Print. doi:10.5455/OVJ.2025.v15.i1.5 APA (American Psychological Association) Style Lestari, T. D., Khairullah, . A. R., Utama, . S., Mulyati, . S., Hernawati, . T., Damayanti, . R., Rimayanti, . R., Wardhani, . B. W. K., Fauzia, . K. A., Moses, . I. B., Ahmad, . R. Z., Wibowo, . S., Fauziah, . I., Kurniasih, . D. A. A., Baihaqi, . Z. A., Wasito, . W., Kusala, . M. K. J. & Lisnanti, . E. F. (2025) Bovine spongiform encephalopathy: A review of current knowledge and challenges. Open Veterinary Journal, 15 (1), 54-68. doi:10.5455/OVJ.2025.v15.i1.5 |