| Research Article | ||
Open Vet. J.. 2025; 15(1): 270-276 Open Veterinary Journal, (2025), Vol. 15(1): 270-276 Research Article First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and HerzegovinaAmela Livnjak1*, Nejra Hadžimusić1, Hans Peter Fuehrer2, Bita Shahi-Barogh2, Jovana Šupić1 and Lejla Pašić31Department for Clinical Sciences, University of Sarajevo-Veterinary Faculty, Sarajevo, Bosnia and Herzegovina 2Department of Pathobiology, Institute of Parasitology, University of Veterinary Medicine, Vienna, Austria 3Sarajevo Medical School, Sarajevo School of Science and Technology, Sarajevo, Bosnia and Herzegovina *Corresponding Author: Amela Livnjak. Department for Clinical Sciences, University of Sarajevo-Veterinary Faculty, Sarajevo, Bosnia and Herzegovina. Email: amela.livnjak [at] vfs.unsa.ba Submitted: 26/09/2024 Accepted: 13/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
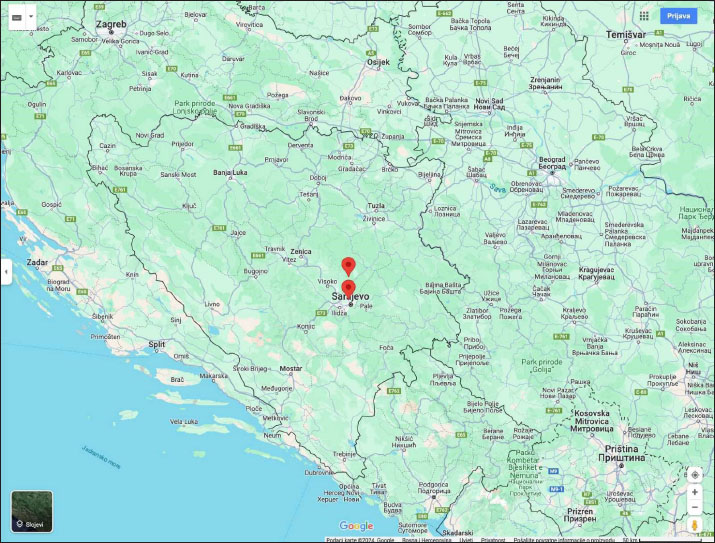
AbstractBackground: Hemoplasmas represent the type of bacteria that infect red blood cells, potentially leading to various health impacts, including changes in blood parameters. The close interaction between hemoplasma and red blood cells results in cell damage through immune-related and other unspecified mechanisms. Even with a strong immune response and antibiotic treatment, affected animals are likely to remain chronic carriers once clinical symptoms have subsided. These microorganisms were previously documented in sheep and other small ruminants worldwide. Aim: Since there is a lack of research on the link between Mycoplasma infection and blood parameters, our aim was to investigate how Mycoplasma infection affects these blood parameters. In addition, the study conducted in Bosnia and Herzegovina represents the first documented research of hemoplasma infection in goats within this region. Methods: In this research, 20 Alpine goats were sampled to investigate the presence of hemoplasma using polymerase chain reaction (PCR) analysis. Sequences of the 16S rRNA gene fragments were identified subsequently. The effect of Mycoplasma ovis (M. ovis) infection was observed on the following hematological parameters: Red blood cell count (RBC), hematocrit (HCT), hemoglobin (HGB), mean cell volume (MCV), mean cell hemoglobin, mean cell hemoglobin concentration, Reticulocyte count, and white blood cell (WBC). Effect on white blood cell differentiation, absolute white blood cell counts, platelet count, and mean platelet volume were also investigated. Results: PCR analysis confirmed the presence of Mycoplasma spp. in 7 out of the 20 blood samples. Sequencing of the 16S rRNA gene fragments revealed that all positive samples were identified as M. ovis. The research findings highlighted potential effects on blood parameters in infected goats. Goats infected with M. ovis exhibited higher mean levels of HGB and HCT compared to uninfected goats. However, there were no statistically significant differences in RBC counts between infected and uninfected groups. The study also noted significantly higher WBC counts in goats without M. ovis infection. Conclusion: 35% of animals tested positive for M. ovis. Our study’s findings showed notable differences in hematological parameters between goats infected with M. ovis and those that were not infected. Keywords: Bosnia and Herzegovina, Goats, Hematological parameters, Hemoplasma infection, Mycoplasma ovis. IntroductionGoats play a crucial role in mixed farming systems due to their adaptability, resistance to pathogens, and efficient feed conversion. Despite the favorable conditions for raising and breeding goats in every part of Bosnia and Herzegovina, the current goat population in the country is approximately 73,000, according to data from the Statistical Agency of Bosnia and Herzegovina (Livnjak and Hadžimusić, 2022). The herds in Bosnia and Herzegovina consist mainly of Alpine goats that are primarily used for milk production. Available data suggests that herds usually range between 50 and 200 goats, although there is a rare instance of a single herd comprising over 700 animals. The most commonly found hemotropic mycoplasmas (hemoplasmas) in goats and various other small ruminants are Mycoplasma ovis (M. ovis) and Candidatus Mycoplasma haemovis. These non-cultivatable small bacteria are pleomorphic, wall-less, and reside in the erythrocytes infecting various vertebrate hosts. Previously, they were classified under the Anaplasmataceae family as Haemobartonella and Eperythrozoon, but they have since been reclassified as Mycoplasma due to their phylogenetic placement based on the 16S rRNA gene sequences (Galon et al., 2019). Hemoplasmosis is typically transmitted naturally via arthropods including ticks, mosquitoes, horseflies, and certain other biting flies. However, hemoplasmas can also be transmitted mechanically. Iatrogenic transmission may be facilitated through fomites, i.e., hypodermic needles and equipment used for tail-docking, castrating, or disbudding animals (Shi et al., 2023). The severity of hemoplasma infection varies depending on the susceptibility of the host and the pathogenicity of the species. Mycoplasma ovis and Candidatus Mycoplasma haemovis are recognized as causes of hemolytic anemia and decreased exercise tolerance in sheep suffering from acute infections (Byamukama et al., 2020). However, clinical signs are rarely seen in goats (Stuen, 2016). Infected goats, on the other hand, often show mild clinical signs and may become persistent carriers due to low levels of bacteremia (Machado et al., 2017). Hemoplasma infections have a considerable effect on small ruminant production, primarily due to the mortality and productivity losses observed in chronically infected animals. (Shi et al., 2019). To the best of the authors’ knowledge, there are no documented cases of hemotropic Mycoplasma in goats from Bosnia and Herzegovina. Additionally, information on the effects of these infections on the blood parameters of naturally infected goats is scarce. This study aims to (1) identify the presence of hemotropic Mycoplasma in goats from both suburban and rural regions of Bosnia and Herzegovina using polymerase chain reaction (PCR), and (2) assess any potential correlation between hemoplasmosis and the hematological parameters of the infected goats. Materials and MethodsBlood samplingBlood samples were gathered from goats situated on farms in Bosnia and Herzegovina, specifically from farm A (43°53’47.4”N 18°23’28.7”E) in a suburban area (n=10) and farm B (44°03’33.2”N 18°27’09.0”E) in a rural location (n=10) (Fig. 1). Blood samples were transported in portable refrigerators at a temperature of 4°C to the University of Sarajevo—Veterinary Faculty, where they underwent additional processing. The study was conducted during the winter of 2019. Throughout the examination period, temperatures in both areas fluctuated between −10°C and −17°C, while the annual average precipitation was 1,000 mm for the year 2019. These particular areas were chosen due to the presence of pastoralist farmers who co-graze their livestock with wildlife, increasing the likelihood of pathogen transmission between species. The age of sampled animals ranged from 6 months to 7 years. Hematology and serum biochemistry analysisFor hematological analyses and molecular diagnostics, blood samples were gathered in vacutainers that contained the anticoagulant ethylenediaminetetraacetic acid (EDTA). Hematological parameters, including red blood cell count (RBC), hematocrit (HCT), hemoglobin (HGB), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), Reticulocyte count (RETIC), white blood cell (WBC), as well as cell differentiation of WBC and determination of absolute values of Neutrophils, Lymphocytes, Monocytes, Eosinophils, Basophils; platelet count (PLT), and mean platelet volume (MPV) was determined using automatic analyzer (ProCyte Dx, IDEXX, USA). Blood vacutainers without anticoagulant were allowed to coagulate, centrifuged at 3,000 rpm for 10 minutes, and measured using standard procedures with the Catalyst OneTM Chemistry Analyzer (IDEXX Laboratories, Netherlands). The concentration of glucose (GLU), albumin (ALB), globulin (GLOB), albumin globulin ratio, total protein (TP), creatinine (CREA), urea (UREA), ratio blood urea nitrogen and creatinine (BUN/CREA), total bilirubin (TB), cholesterol (CHOL), calcium (Ca), phosphorus (PHOS), as well as the activity of alanine aminotransferase (ALT), alkaline phosphatase (ALKP), gamma-glutamyl transferase (GGT), amylase (AMYL), and lipase (LIPA) had been determined. DNA isolationTotal genomic DNA from 100 µl of anticoagulated whole blood was isolated using DNeasy® Blood and Tissue kit (Qiagen, Germany) following the manufacturer’s guidelines and was eluted from the column using 100 µl of Tris-EDTA buffer. DNA concentration was estimated using Qubit® fluorimeter (ThermoFisher Scientific, USA) according to the manufacturer’s instructions. DNA was stored at −20°C until PCR testing. Confirmation of Mycoplasma spp. by polymerase chain reactionWe amplified 600 bp fragments of 16S rRNA of Mycoplasma spp. using the following primers: HBT-F: 5’-ATA CGG CCC ATA TTC CTA CG-3’ and HBT-R: 5’-TGC TCC ACC ACT TGT T CA-3’ (Criado-Fornelio et al., 2003). The composition of each 25 µl reaction mixture was as follows: 12.675 µl of sterile distilled water, 0.125 µl of GoTaq® DNA polymerase (Promega, USA), 5 µl of Green Reaction Buffer (Promega, USA), 0.2 µl of dNTPs, 1 µl of each primer, and 5 µl of template DNA. These reactions were prepared in batches of 10. The conditions of the PCR reaction were as follows: initial denaturation at 94°C for 2 minutes, followed by 40 cycles of 95°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute, with a final extension step at 72°C for 7 minutes. All samples were tested three times. Positive and negative controls were utilized to confirm the results. PCR products were examined through electrophoresis on 2% agarose gels stained with Midori Green Advance DNA stain (Nippon Genetics Europe, Germany). Positive samples were forwarded to a commercial laboratory (LGC Genomics GmbH, Germany) for sequencing using amplification primers. The representative sequence of seven identical clones was deposited to GenBank under the number PP359532.
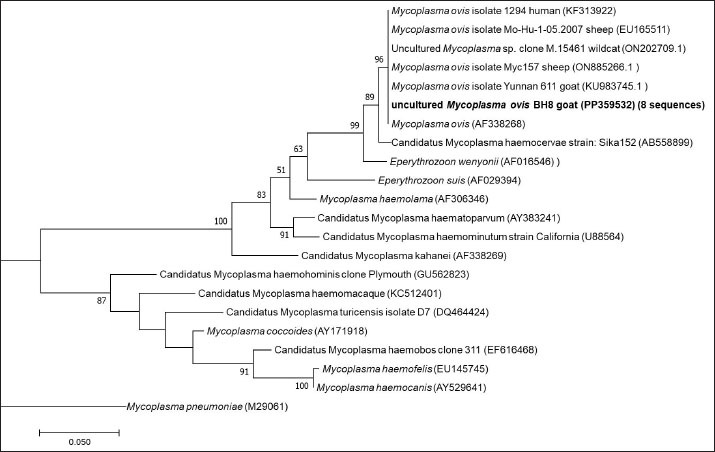
Fig. 1. Map of Bosnia and Herzegovina showing the location of the two sampling locations (Farm A and Farm B). The figure was generated and modified using https://www.google.com/maps. Phylogenetic analysis of Mycoplasma sequencesObtained sequences and relevant phylogenetic data from GenBank were aligned using MUSCLE (Edgar, 2004). A phylogenetic tree demonstrating the relationship of sequences obtained in this study and known Mycoplasma sequences was created by the Maximum likelihood method based on the General Time Reversible model (Nei and Kumar, 2000) and considering among-site variation by employing a four-category discrete approximation of a Γ distribution with a fraction of invariable sites using MEGA11 (Tamura et al., 2021). Bootstrap support values were assessed by 1,000 bootstrap replications. Statistical analysisAll results are presented as means ± standard deviation (SD). A p-value of < 0.05 was deemed statistically significant. Comparisons between groups were made using the unpaired-sample Student’s t-test. Statistical analyses were performed using the Mini Tab software (Version 19/2020). ResultsUsing a PCR assay 35% (7 of 20) animals tested positive for Mycoplasma infection. Sequence comparisons revealed that the 16S rRNA fragments were clonal as they were 100% identical. Comparisons with the nucleotide collection database available from GenBank showed that the sequences were 100% identical to M. ovis (AF338268) as well as to several other M. ovis sequences including sequences originating from goats (KU983745), sheep (EU16551, ON885266), wildcat (ON202709), and human (KF313922). On a phylogenetic tree (Fig. 2.), these sequences formed a separate cluster with M. ovis. The results of our study revealed significant hematological differences for some parameters between goats infected with M. ovis and those without the infection. Although the obtained values for the examined parameters within the reference values, indicated that none of the goats became anemic, statistical differences were still observed among the groups of animals studied.
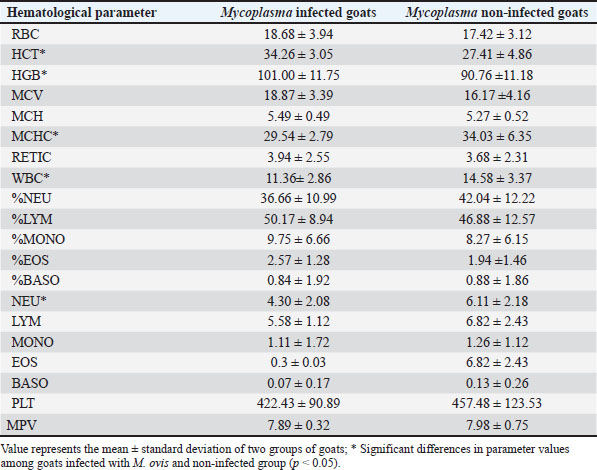
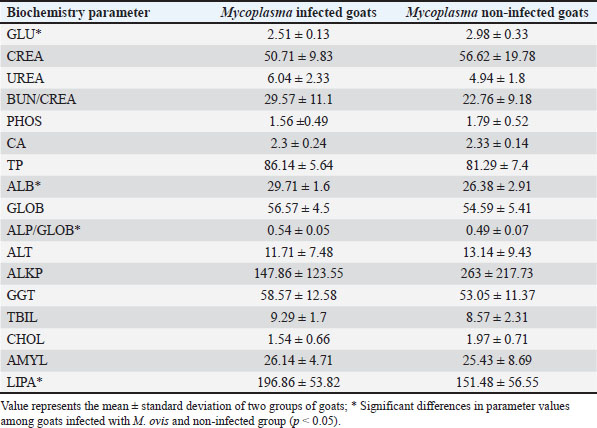
Fig. 2. ML phylogenetic tree showing the phylogenetic position of sequences obtained in this study and other mycoplasmas. Only bootstrap values of >75% are shown. The M. ovis sequences detected in this study are shown in bold. DiscussionThis research examines the occurrence of hemotropic M. ovis in randomly chosen goat herds in Bosnia and Herzegovina. Since M. ovis is an obligate epicellular bacterium that does not remain stable under laboratory conditions and is easily damaged by drying or disinfectants, it cannot be grown in cell-free media (Windsdor, 2022). Before the development of PCR assays, estimating parasitemia in haemoplasma infection relied on subjective blood smear examination, demanding a high level of expertise (Paul et al., 2020). The effectiveness of blood film assessment in terms of sensitivity and specificity is also limited (Wangai et al., 2011). Additionally, studies have noted that the degree of bacteremia observed in goats via microscopy is lower compared to that in sheep; this observation has been confirmed through molecular techniques (Johnson et al., 2016). The use of more advanced and accurate molecular techniques, such as conventional PCR assays and quantitative PCR assays, has greatly improved the ability to diagnose hemoplasma infections in various host species. There are few reports on the hematological and biochemical parameters of small ruminants with haemotropic mycoplasmosis. Some authors report neutrophilic left shift, monocytosis, and lymphocytosis (Souza et al., 2019). Besides, there are reports on M. ovis infection accompanied by significant biochemical alterations, such as hyponatremia, hypocalcemia, hypoalbuminemia, reduced protein levels, and a simultaneous rise in serum creatinine, indirect bilirubin, GGT, AST, ALP, and BUN (Souza et al., 2019). It is possible that an untested concurrent infection could have contributed to the observed lower values in HCT and hemoglobin levels in goats that tested negative for M. ovis. Another study found that 73% of the animals in their cohort had coinfections (Paul, 2020). In addition to this synergistic relationship, hemotropic mycoplasmas, acting independently, have been shown to induce severe anemia (Adduci et al., 2022). Goats negative for M. ovis may be experiencing different types of immune responses or activation of alternate immune mechanisms, which could lead to higher baseline WBC counts. Additionally, goats testing positive for M. ovis may be experiencing immune suppression or a subdued immune response. This can occur due to the pathogen’s capacity to evade or suppress the host’s immune system, particularly the inflammatory responses (Askar et al., 2021). It is essential to highlight that individual variation and environmental factors significantly influence immune responses. The observed significant increase in neutrophil counts in goats that tested negative for M. ovis (Table 1), coupled with lower levels in goats that tested positive for the pathogen aligns with the previously discussed variations in white blood cell counts, suggesting a complex interaction between M. ovis infection and the host’s immune response. A study indicates that in a goat, co-infection with Ehrlichia ewingii and hemotropic Mycoplasma resulted in macrocytic hypochromic anemia characterized by anisocytosis, macrocytosis, basophilic stippling, elevated levels of AST, CK, and GGT, and reduced ALP activity (Meichner et al., 2015). Likewise, reports have indicated co-infections of erythrocytes with hemotropic Mycoplasma and Anaplasma species in goats (Ait Lbacha et al., 2017). Concurrent infection with other tick-borne hemopathogens heightens the vulnerability of animals to hemotropic mycoplasmas. (Varanat et al., 2011). This occurs because the presence of multiple co-infecting pathogens induces complex and varied responses, facilitating synergy and more effective colonization within the host (Baneth, 2014). The hypoglycemia found in the blood samples (Table 2) is believed to have a dual potential cause: (1) the utilization of circulating glucose by M. ovis within the host, resulting in genuine hypoglycemia; and (2) the consumption of glucose in vitro by M. ovis present within the collected blood sample may cause artifactually decreased blood glucose levels (Burkhard and Garry, 2004; Almy et al., 2006). Studies on M. suis suggest that the severity of hypoglycemia appears to correspond to the level of haemoplasma bacteremia (Tasker et al., 2009). Furthermore, two studies specifically highlight significant hypoglycemia in the absence of any related clinical signs (Burkhard and Garry, 2004, Humann-Ziehank and Ganter, 2012). Changes in serum biochemical parameters with goats positive for M. ovis showed values lower than the reference range for total protein concentration in both groups of animals, as well as lower concentrations of creatinine and cholesterol, higher concentration of TBIL, and lower activity of ALT and GGT (Table 2). Mycoplasma infections are among significant goat diseases and cause notable losses in many European countries, the United States, as well as Australia and Africa (DaMassa et al., 1992). The actual distribution of M. ovis remains uncertain as there are limited studies documented in the literature. Previous investigations suggest that the infection is widespread (Paul at al., 2020). In Europe, M. ovis has been detected in several countries including Cyprus, France, Germany, Hungary, Ireland, Norway, and the UK (Stuen, 2016). This research is, to the best of the authors’ knowledge, the first molecular detection of M. ovis in goats in Bosnia and Herzegovina as well as the first report in this geographical region. This research represents the inaugural confirmed identification of hemoplasma infection in goats from Bosnia and Herzegovina, achieved through a PCR-based assay. The findings from this study aim to enhance awareness among farmers and veterinarians, leading to improved health management practices and overall goat herd well-being. Including more comprehensive immune response markers in future studies could elucidate the reasons behind the observed discrepancies in WBC counts among different groups. Table 1. Values of hematological parameters in goats infected with M. ovis and non infected goats.
Table 2. Values of biochemistry parameters in goats infected with M. ovis and non infected goats.
AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Authors’ contributionsN.H., L.P., J.Š.: Conceptualization, Methodology, Software; L.P., H.P.F., B.S.B: Project administration; L.P.: Data curation, Supervision, Validation; A.L, N.H, L.P: Writing- Original draft preparation; A.L., N.H., L.P., J.Š.: Visualization, Investigation. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAdduci, I., Sajovitz, F., Hinney, B., Lichtmannsperger, K., Joachim, A., Wittek, T. and Yan, S. 2022. Haemonchosis in sheep and goats, control strategies and development of vaccines against Haemonchus contortus. Animals. 12, 2339. Ait Lbacha, H., Alali, S., Zouagui, Z., El Mamoun, L., Rhalem, A., Petit, E. and Maillard, R. 2017. High prevalence of Anaplasma spp. in small ruminants in Morocco. Transbound. Emerg. Dis. 64, 250–263. Almy, F.S., Ladd, S.M., Sponenberg, D.P., Crisman, M.V. and Messick, J.B. 2006. Mycoplasma haemolamae infection in a 4-day-old cria: support for in utero transmission by use of a polymerase chain reaction assay. Can. Vet. J. 47, 229. Askar, H., Chen, S., Hao, H., Yan, X., Ma, L., Liu, Y. and Chu, Y. 2021. Immune evasion of Mycoplasma bovis. Pathogens. 10, 297. Baneth, G. 2014. Tick-borne infections of animals and humans: a common ground. Int. J. Parasitol. 44, 591–596. Burkhard, M.J. and Garry, F. 2004. Artifactual hypoglycemia associated with hemotrophic mycoplasma infection in a lamb. Vet. Clin. Pathol. 33, 244–248. Byamukama, B., Tumwebaze, M.A., Tayebwa, D.S., Byaruhanga, J., Angwe, M.K., Li, J. and Xuan, X. 2020. First molecular detection and characterization of hemotropic mycoplasma species in cattle and goats from uganda. Animals. 10, 1624. Criado-Fornelio, A., Martinez-Marcos, A., Buling-Saraña, A. and Barba-Carretero, J.C. 2003. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet. Microbiol. 93, 307–317. DaMassa, A.J., Wakenell, P.S. and Brooks, D.L. 1992. Mycoplasmas of goats and sheep. J. Vet. Diagn. Invest. 4, 101–113. Edgar, R.C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic. Acids Res. 32, 1792–1797. Galon, E.M.S., Moumouni, P.F.A., Ybanez, R.H.D., Macalanda, A.M.C., Liu, M., Efstratiou, A. and Xuan, X. 2019. Molecular evidence of hemotropic mycoplasmas in goats from Cebu, Philippines. J. Vet. Med. Sci. 81, 869–873. Humann-Ziehank, E. and Ganter, M. 2012. Pre-analytical factors affecting the results of laboratory blood analyses in farm animal veterinary diagnostics. Animal. 6, 1115–1123. Johnson, K.A., do Nascimento, N.C., Bauer, A.E., Weng, H.Y., Hammac, G.K. and Messick, J.B. 2016. Detection of hemoplasma infection of goats by use of a quantitative polymerase chain reaction assay and risk factor analysis for infection. Am. J. Vet. Res. 77, 882–889. Livnjak, A. and Hadžimusić, N. 2022. Hematological parameters of Alpine goats in Bosnia and Herzegovina. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Food Sci. Technol. 79, 42–46. Machado, C.A., Vidotto, O., Conrado, F.O., Santos, N.J., Valente, J.D., Barbosa, I.C. and Vieira, R.F. 2017. Mycoplasma ovis infection in goat farms from northeastern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 55, 1–5. Meichner, K., Qurollo, B.A., Anderson, K.L., Grindem, C.B., Savage, M. and Breitschwerdt, E.B. 2015. Naturally occurring Ehrlichia ewingii and Mycoplasma sp. Co-Infection in a Goat. J. Vet. Intern. Med. 29, 1735. Nei, M. and Kumar, S. 2000. Molecular evolution and phylogenetics. Oxford, UK: Oxford University Press. Paul, B.T., Jesse, F.F.A., Chung, E.L.T., Che-Amat, A., Mohd Lila, M.A., Hashi, H.A. and Norsidin, M.J. 2020. Review of clinical aspects, epidemiology and diagnosis of haemotropic Mycoplasma ovis in small ruminants: current status and future perspectives in tropics focusing on Malaysia. Trop. Anim. Health Prod. 52, 2829–2844. Shi, H., Hu, Y., Leng, C., Shi, H., Jiao, Z., Chen, X. and Yao, L. 2019. Molecular investigation of “Candidatus Mycoplasma haemobos” in goats and sheep in central China. Transbound. Emerg. Dis. 66, 22–27. Shi, H., Hui, R., Zhou, M., Wang, L., Li, G., Bai, Y. and Yao, L. 2023. Abortion outbreak in pregnant goats and cows with coinfection of ‘Candidatus Mycoplasma haemobos’ and HoBi-like pestivirus. Vet. Microbiol. 279, 109690. Souza, U.A., Oberrather, K., Fagundes-Moreira, R., Almeida, B.A.D., Valle, S.D.F., Girotto-Soares, A. and Soares, J.F. 2019. First molecular detection of Mycoplasma ovis (Hemotropic mycoplasmas) from Sheep in Brazil. Rev. Bras. Parasitol. Vet. 28, 360–366. Stuen, S.J.S.R.R. 2016. Haemoparasites in small ruminants in European countries: challenges and clinical relevance. Small. Rumin. Res. 142, 22–27. Tamura, K., Stecher, G. and Kumar, S. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027. Tasker, S., Peters, I.R., Papasouliotis, K., Cue, S.M., Willi, B., Hofmann-Lehmann, R. and Helps, C.R. 2009. Description of outcomes of experimental infection with feline haemoplasmas: copy numbers, haematology, Coombs’ testing and blood glucose concentrations. Vet. Microbiol. 139, 323–332. Varanat, M., Maggi, R.G., Linder, K.E. and Breitschwerdt, E.B. 2011. Molecular prevalence of B artonella, B abesia, and Hemotropic Mycoplasma sp. in dogs with splenic disease. J. Vet. Intern. Med. 25, 1284–1291. Wangai, L.N., Karau, M.G., Njiruh, P.N., Sabah, O., Kimani, F.T., Magoma, G. and Kiambo, N. 2011. Sensitivity of microscopy compared to molecular diagnosis of P. falciparum: implications on malaria treatment in epidemic areas in Kenya. Afr. J. Infect. Dis. 5, 1–6. Windsdor, P.A. 2022. Anaemia in lambs caused by Mycoplasma ovis: global and Australian perspectives. Animals. 12, 1372. | ||
| How to Cite this Article |
| Pubmed Style Livnjak A, Hadžimusić N, Fuehrer HP, Shahi-barogh B, Šupić J, Pašić L. First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina. Open Vet. J.. 2025; 15(1): 270-276. doi:10.5455/OVJ.2025.v15.i1.25 Web Style Livnjak A, Hadžimusić N, Fuehrer HP, Shahi-barogh B, Šupić J, Pašić L. First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina. https://www.openveterinaryjournal.com/?mno=222095 [Access: October 31, 2025]. doi:10.5455/OVJ.2025.v15.i1.25 AMA (American Medical Association) Style Livnjak A, Hadžimusić N, Fuehrer HP, Shahi-barogh B, Šupić J, Pašić L. First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina. Open Vet. J.. 2025; 15(1): 270-276. doi:10.5455/OVJ.2025.v15.i1.25 Vancouver/ICMJE Style Livnjak A, Hadžimusić N, Fuehrer HP, Shahi-barogh B, Šupić J, Pašić L. First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina. Open Vet. J.. (2025), [cited October 31, 2025]; 15(1): 270-276. doi:10.5455/OVJ.2025.v15.i1.25 Harvard Style Livnjak, A., Hadžimusić, . N., Fuehrer, . H. P., Shahi-barogh, . B., Šupić, . J. & Pašić, . L. (2025) First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina. Open Vet. J., 15 (1), 270-276. doi:10.5455/OVJ.2025.v15.i1.25 Turabian Style Livnjak, Amela, Nejra Hadžimusić, Hans Peter Fuehrer, Bita Shahi-barogh, Jovana Šupić, and Lejla Pašić. 2025. First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina. Open Veterinary Journal, 15 (1), 270-276. doi:10.5455/OVJ.2025.v15.i1.25 Chicago Style Livnjak, Amela, Nejra Hadžimusić, Hans Peter Fuehrer, Bita Shahi-barogh, Jovana Šupić, and Lejla Pašić. "First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina." Open Veterinary Journal 15 (2025), 270-276. doi:10.5455/OVJ.2025.v15.i1.25 MLA (The Modern Language Association) Style Livnjak, Amela, Nejra Hadžimusić, Hans Peter Fuehrer, Bita Shahi-barogh, Jovana Šupić, and Lejla Pašić. "First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina." Open Veterinary Journal 15.1 (2025), 270-276. Print. doi:10.5455/OVJ.2025.v15.i1.25 APA (American Psychological Association) Style Livnjak, A., Hadžimusić, . N., Fuehrer, . H. P., Shahi-barogh, . B., Šupić, . J. & Pašić, . L. (2025) First molecular evidence of hemotropic mycoplasmas in goats from Bosnia and Herzegovina. Open Veterinary Journal, 15 (1), 270-276. doi:10.5455/OVJ.2025.v15.i1.25 |