| Research Article | ||
Open Vet. J.. 2025; 15(1): 118-125 Open Veterinary Journal, (2025), Vol. 15(1): 118-125 Research Article Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotionAsadig Emhemmed Alghoull1,2, Salma Rifqi Firdausi3, Yuyun Ika Christina4,5,6, Sri Widyarti4, Muhaimin Rifa’i4,6 and Muhammad Sasmito Djati4,5,6*1Doctoral Program, Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, East Java, Indonesia 2Department of Zoology, Faculty of Science, University of Zawia, Zawiya, Libya 3Master Program, Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, East Java, Indonesia 4Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, East Java, Indonesia 5Research Center of Complementary Medicine and Functional Food, Brawijaya University, Malang, East Java, Indonesia 6Dewan Jamu Indonesia East Java Region, Malang, East Java, Indonesia *Corresponding Author: Muhammad Sasmito Djati. Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, Indonesia. Email: msdjati [at] ub.ac.id Submitted: 17/09/2024 Accepted: 13/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
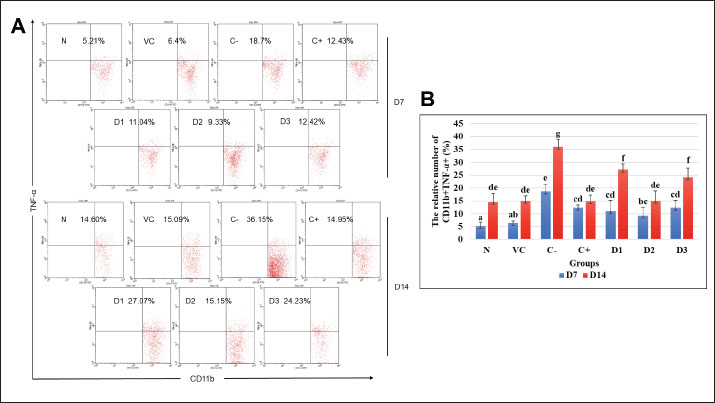
AbstractBackground: Pulmonary fibrosis represents the most prevalent form of idiopathic interstitial pneumonia. The pathogenesis of pulmonary fibrosis using a bleomycin-induced mice model has indicated an imbalanced immune response such as an early massive inflammatory response, followed by fibrosis development. Therapy focused on restraining inflammation is one of the ways to inhibit fibrosis development. Elephantopus scaber ethanolic extract (ESEE) is known to have many beneficial compounds that were proven to possess anti-inflammatory activities, but its prospect in inhibiting pulmonary fibrosis development needs to be investigated. Aim: This study aimed to evaluate the potency of ESEE treatment in inhibiting fibrosis development in the bleomycin-induced pulmonary fibrosis mice model. Methods: Healthy male BALB/c mice were divided into seven experimental groups (n=8): healthy mice (N), vehicle mice (VC), pulmonary fibrosis mice (C-), pulmonary fibrosis received dexamethasone (C+), and pulmonary fibrosis mice received ESEE at a 0.0504 mg/kg body weight (BW) (D1), 0.1008 mg/kg BW (D2), and 0.2016 mg/kg BW (D3). Mice were given ESEE orally and intraperitoneal bleomycin injection daily for 14 days. Mice were then sacrificed on days 7 and 14 and spleens were isolated to determine the production of IL-10, TNF-α, and IFN-γ using flow cytometry. Results: The results revealed that a remarkable increase of TNF-α was found in the macrophage of pulmonary fibrosis mice model from day 7 to 14. An increase in IFN-γ production was also observed on day 7 and then decreased on day 14. The production of IL-10 was reduced in the fibrosis group at day 7 and continued to increase at day 14. Interestingly, ESEE treatment for 14 days could effectively reduce TNF-α and increase IFN-γ production. ESEE treatment could also maintain a stable production of IL-10 at each time point. ESEE at 0.1004 mg/kg BW (D2) showed the most effective activity in reducing pro-fibrotic cytokine than the dexamethasone group. Conclusion: Ethanolic extract of ESEE has demonstrated its beneficial prospect in regulating pro-inflammatory and pro-fibrotic cytokine to inhibit fibrosis development. Keywords: Elephantopus scaber, IFN-γ, IL-10, Pulmonary fibrosis, TNF-α. IntroductionPulmonary fibrosis is a progressive and chronic lung disease that is characterized by the histopathological pattern of interstitial pneumonia (Patil et al ., 2016; Sgalla et al., 2018; Gul et al., 2023). Pulmonary fibrosis is not a single disease, but a family of diseases that includes more than 200 different lung diseases that are very similar. As its main causes are unknown, the most effective treatment and medication to cure it also need more investigation. Research in pulmonary fibrosis treatment has been increasing since the outbreak of COVID-19, as it was found to be progressing in many surviving patients (Megha et al., 2021; Aliyu et al., 2022). This study used bleomycin injection to model pulmonary fibrosis in mice, based on several previous studies by Patil et al. (2016), Tashiro et al. (2017), and Gul et al. (2023). The lung injury by bleomycin induction comprises interstitial edema with an influx of inflammatory and immune cells, prolonged tissue inflammation, and massive activation of fibroblastic cells, which later lead to the enhanced production and deposition of collagen and other matrix components in the tissue, forming fibrosis (Kato et al., 2018; Liang et al., 2020; Wen et al., 2020). Consequently, researchers have been interested in observing beneficial compounds in well-known traditional plants that may have significant benefits in improving pulmonary fibrosis. Plants are one of the most important sources of medicine. Plant-derived compounds, or phytochemicals, have been attracting much interest as natural alternatives to synthetic compounds (Shaw et al., 2010). One of the potential medicinal plants is Tapak liman (Elephantopus scaber L.), a traditional herb that has been gaining attention for its promising therapeutic effect. In Indonesia, E. scaber is used as a treatment for anemia and blood booster in Indonesia (Aldi et al., 2019). It is a common wild weed that grows at 1,200 m above sea level. This plant belongs to the Asteraceae family (Djati and Christina, 2019; Qi et al., 2020) which is widely spread in many tropical countries including South and West Asia, Africa, India, and Australia (Firdausi et al., 2024). Elephantopus scaber has been listed in the Chinese Pharmacopeia for its efficacy in treating fever, sore throat, and other related inflammation disorders (Qi et al., 2020). Then, several researchers from Japan, America, and Asia started to explore more biological activities of this plant as an anticancer, wound healing, anti-diabetic, antibacterial, antioxidant, analgesic, and anti-asthmatic (Hiradeve and Rangari, 2014; Djati and Christina, 2019; Qi et al., 2020). Previous studies also suggested that this plant can reduce the levels of low-density lipoprotein in the experimental animals (Wang et al., 2014; Hiradeve and Rangari, 2014; Pandey et al., 2020; Asfi et al., 2022). Many research has been done to investigate the efficacy of E. scaber in various diseases. However, the effect of E. scaber in fibrosis mice model remains unclear. Therefore, this study focused on investigating the potential of E. scaber ethanolic extract (ESEE) in bleomycin-induced pulmonary fibrosis in mice, by observing CD11b+TNF-α+, CD11b+IFN-γ+, and CD4+CD25+IL-10+ cell populations. The anti-fibrotic potential of E. scaber presents a promising opportunity with applications started from drug development into modern treatment. Materials and MethodsPlant materials and extractionThe fresh leaves of E. scaber L. were purchased from the UPT Laboratorium Herbal Materia Medica, Batu, Indonesia, and identified by a qualified botanist from this institution (sample number: 067/656/102.20/2023). Subsequently, the leaves of E. scaber were dried and pulverized to a fine powder. A total of 100 g of the powdered samples were then macerated with 1,000 ml of 96% ethanol (1:10, w/v) for 24 hours at room temperature with several stirrings. The sample was then filtered through Whatman No. 1 filter paper and concentrated using a vacuum pump evaporator at 70°C until a paste consistency was obtained. ESEE were stored in a refrigerator at a temperature of 3°C before use. Experimental research designThe current study utilized male BALB/c mice, aged 6–7 weeks, sourced from the Faculty of Pharmacy, Airlangga University, Surabaya. The healthy mice were selected based on the following criteria: average body weight (BW) between 25 and 30 g, active, and no physical defects. The research employed an experimental design with a completely randomized design. The mice were acclimated for 14 days and then randomly divided into seven groups (n=8): healthy mice (N), vehicle mice (VC), pulmonary fibrosis mice (C-), pulmonary fibrosis received dexamethasone (C+), pulmonary fibrosis mice received ESEE at a 0.0504 mg/kg BW (D1), 0.1008 mg/kg BW (D2), and 0.2016 mg/kg BW (D3). Mice were given ESEE orally and intraperitoneal bleomycin injection daily for 14 days. A total of 10 g bleomycin (Med Chem Express LLC, USA) was dissolved in 1 mL phosphate buffer saline (PBS) and divided into five propylene tubes of 200 µl each. Subsequently, 7.8 ml of PBS was added to each propylene tube. Each mouse was intraperitoneally administered with bleomycin at a dose of 2 mg/kg BW daily for 14 days, under the protocol outlined by van Gijsel-Bonnello et al. (2022). Dexamethasone as a control drug (C+) which given at 3 mg/kg BW. Each dose of ESEE was dissolved in corn oil and given to the mice in D1, D2, and D3 groups orally. Mice were sacrificed on days 7 and 14 after treatment by cervical dislocation. Lymphocyte isolation, antibody staining, and flow cytometry analysisThe mice were sacrificed for spleen isolation. The spleens were washed three times with PBS and then crushed clockwise with the bottom of the syringe in a petri dish containing 1 ml PBS. Four milliliters of PBS were added to the petri dish and transferred to a 15 ml polypropylene tube. Subsequently, the homogenate was centrifuged at 2,500 rpm for 5 minutes at 10°C. The supernatant was discarded, and the pellet was resuspended in 1 ml PBS. Then, 50 μl of the cell suspension was transferred to a 1.5 ml microtube and subjected to antibody staining. Antibody staining was performed by extracellular and intracellular (IC) staining. For extracellular staining, 50 µl of cell suspension was added to 50 µl of specific extracellular antibody solution [Fluorescein isothiocyanate (FITC) conjugate anti-mouse CD4, FITC conjugate anti-mouse CD11b, and phycoerythrin (PE) conjugate anti-mouse CD25 (Biolegend, California, USA)] and incubated at 4°C for 20 minutes in a dark room in an ice box. For IC staining, 50 µl of IC fixation buffer (eBioscienceTM, Thermo Fisher Scientific, USA) was added to the cell suspension and incubated at 4°C for 20 minutes in a dark room on an ice box. The cell suspension was mixed with 400 µl permeabilization buffer (eBioscienceTM, Thermo Fisher Scientific, USA) (10× dilution) and homogenized. Samples were centrifuged at 2,500 rpm, 10°C, for 5 minutes. Pellets were added with 50 µl of specific IC antibody solution PE/Cy5 conjugated anti-mouse IFN-γ, PE/Cy5 conjugated anti-mouse TNF-α and PE/Cy5 conjugated anti-mouse IL-10 (Biolegend, California, USA) and incubated again at 4°C for 20 minutes in the dark on an ice box. After incubation, 400 μl of PBS was added to the sample, transferred to the cuvette, and subjected for flow cytometric analysis using flow cytometer (BD FACS Calibur™, San Jose, CA). Data were then analyzed using BD CellQuest Pro™ software. Data analysisThe relative number of cell population were tabulated and presented as mean percentage (%). All data was subjected to normality and homogeneity tests using the IBM SPSS Statistics 26 for Windows. Parametric analysis was conducted using two-way ANOVA followed by Duncan’s post hoc test with p < 0.05 considered statistically different. Ethical approvalThis research has been granted ethical approval by the Brawijaya University Research Ethics Commission (No. 182-KEP-UB-2023). ResultsEffects of ESEE on CD11b+TNF-α+ and CD11b+IFN-γ+cell populationsThis study revealed a significant increase (p < 0.05) in the CD11b+TNF-α+ cell population in mice exposed to bleomycin compared to the healthy mice on day 7 (Fig. 1A and B). The fibrosis mice model (C-) reached the highest CD11b+TNF-α+ cell population, up to 18.7%, after bleomycin injection for 7 days. After 7 days of treatment, dexamethasone (C+) and different doses of ESEE (D1, D2, and D3) showed a significant increase in the CD11b+TNF-α+ cell population compared to the C- group. Although there was no significant difference in cell population between the ESEE groups and the dexamethasone group, the D2 group showed a lower cell population among all. At day 14, the fibrosis model group (C+) showed a significant increase in the CD11b+TNF-α+ cell population compared to day 7, indicating continuous inflammation. ESEE treatment groups, D1 and D3 also showed significant increase, indicating weak suppression activity. Meanwhile, treatment with dexamethasone (C+) and ESEE at 0.1008 mg/kg (D2) showed a lower CD11b+TNF-α+ cell population after 14 days of treatment compared to the other ESEE group, indicating the effective dose for regulating CD11b+TNF-α+ cell population, thereby suppressing inflammation and inhibit fibrosis development.
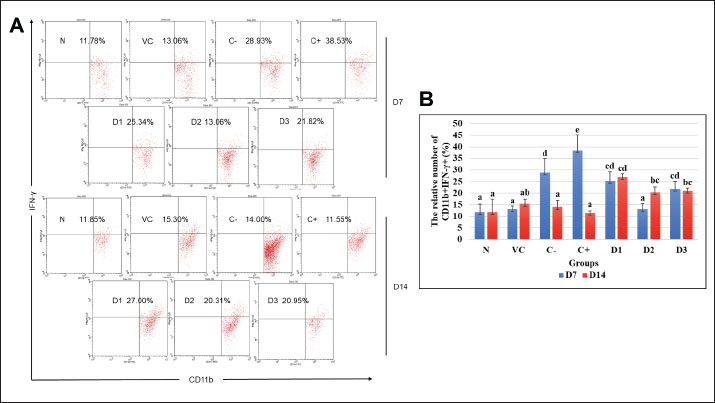
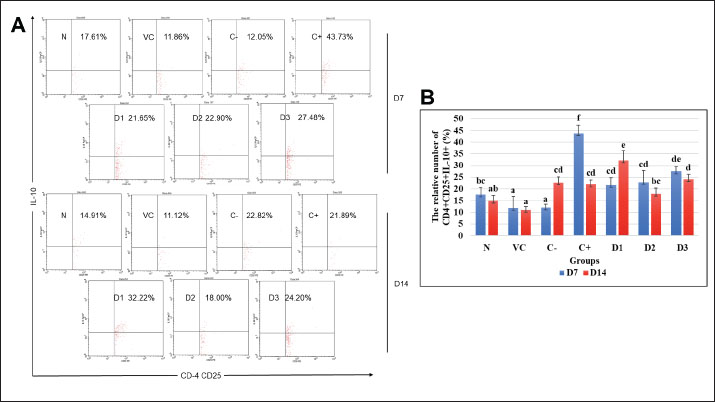
Fig. 1. Flow cytometry result of CD11b+TNF-α+ cell population at days 7 and 14 for each treatment group. (A) Dot plots demonstrated the CD11b+TNF-α+ cell population. (B) The representative bar showed the mean relative percentage of the CD11b+TNF-α+ cell population. Data were shown as mean ± standard deviation (SD). Lowercase marks showed significant differences based on Duncan’s HSD post hoc test (p < 0.05). N: healthy mice group; VC: vehicle control group; F: fibrosis model group with bleomycin; D: positive control group with dexamethasone; D1: ESEE treatment at a dose of 0.0504 mg/kg; D2: ESEE treatment at a dose of 0.1008 mg/kg; E3: ESEE treatment at a dose of 0.2016 mg/kg. The result also showed a higher increase in CD11b+IFN-γ+ cell populations after 7 days of bleomycin injection in the fibrosis mice model compared to healthy mice (38.53% vs. 11.78%) (Fig. 2A and B). Treatment with ESEE at 0.1008 mg/kg BW for 7 days showed no significant difference compared to healthy mice, indicating beneficial activities of ESEE in decreasing IFN-γ production. However, on day 14, the fibrosis mice model group (C-) and dexamethasone treatment group (C+) showed decreasing CD11b+IFN-γ+ cell populations. D1 and D3 groups showed no significant changes in the production of IFN-γ both days 7 and 14. Meanwhile, the D2 group showed a significant increase in CD11b+IFN-γ+ cell populations at day 14. All groups receiving ESEE showed no significant differences between groups at day 14 observations (Fig. 2B). Effects of ESEE on IL-10 production (CD4+CD25+IL-10+)The result found a lower IL-10 production in the fibrosis mice (C-) compared to healthy mice (N) at day 7 and then continued to increase at day 14 (Fig. 3A). The dexamethasone treatment group showed the highest IL-10 production compared to another group. All ESEE groups showed a significant increase in CD4+CD25+IL-10+ cell populations compared to the fibrosis group (C-), but no significant difference between the ESEE group. At day 14, there were no significant differences between the treatment groups and fibrosis group, except for the D1 group, which showed significantly highest CD4+CD25+IL-10+ cell populations. DiscussionBleomycin, a chemotherapeutic agent and antibiotic, has been demonstrated to induce pulmonary fibrosis in both animal models and humans (Patil et al., 2016). When administered to mice, bleomycin causes lung injury, which is characterized by inflammation, fibroblast proliferation, collagen deposition, and ultimately fibrosis (Cowley et al., 2019). Bleomycin attacks lung epithelial cells, causing programmed cell death due to DNA damage. Injured cells in the tissue signaled immune cells to come and clean up the damage, initiating inflammation. This inflammation can last too long due to repetitive bleomycin damage, causing irregular tissue repair. Massive activation of fibroblast cells was also the key to tissue fibrosis initiation (Thannickal et al., 2004; Pahwa et al., 2023). This current study used bleomycin as a model inducer of pulmonary fibrosis in mice to investigate the beneficial prospect of ESEE treatment in pulmonary fibrosis.
Fig. 2. Flow cytometry result of CD11b+IFN-γ+ cell population at days 7 and 14 for each treatment group. (A) Dot plots demonstrated the CD11b+IFN-γ+ cell population. (B) The representative bar showed the mean relative percentage of the CD11b+IFN-γ+ cell population. Data were shown as mean ± standard deviation (SD). Lowercase marks showed significant differences based on Duncan’s HSD post hoc test (p < 0.05). N: healthy mice group; VC: vehicle control group; F: fibrosis model group with bleomycin; D: positive control group with dexamethasone; D1: ESEE treatment at a dose of 0.0504 mg/kg; D2: ESEE treatment at a dose of 0.1008 mg/kg; E3: ESEE treatment at a dose of 0.2016 mg/kg. The results of this study demonstrated the increase of TNF-α and IFN-γ production in macrophage cells after 7 days of bleomycin exposure. In the fibrosis model group (C-), the production of TNF-α (CD11b+TNF-α+) was found to increase compared to healthy mice (N). CD11b+TNF-α+ cells are activated macrophage cells in response to damaged cells. Activated macrophage cells then produced more inflammatory cytokines and mediators to signal another immune cell to come to the site. This cytokine can be produced through the NF-κB pathway in macrophage cells (Hirano, 2021). TNF-α is a pro-inflammatory cytokine produced mainly by macrophages and other immune cells (Hou et al., 2018). It is involved in the initiation and propagation of the inflammatory response. During bleomycin injury, TNF-α contributes to the recruitment and activation of inflammatory cells in the lungs, which exacerbates inflammation and tissue damage (Thomson et al., 2012; Redente et al., 2014). It also promotes the activation of fibroblasts, the cells responsible for producing collagen and other extracellular matrix components, leading to excessive fibrosis. This study demonstrated that treatment with ESEE could reduce the activation of macrophage cells to its inflammatory M1 type, marked by a significantly lower CD11b+TNF-α+ cell population in all groups receiving it (D1, D2, and D3). ESEE treatment successfully prevents the continuous inflammation to day 14. ESEE contains its unique beneficial bio-compounds, such as deoxyelephantopin and scabertopin, which are known to act as an anti-inflammatory agent by inhibiting NF-κB pathway activation (Hiradeve and Rangari, 2014; Djati et al., 2016; Han et al., 2020; Pandey et al., 2020; Asfi et al., 2022). This pathway is the main producer of inflammatory mediators, inhibition of it could significantly reduce inflammation.
Fig. 3. Flow cytometry result of CD4+CD25+IL-10+ cell population at days 7 and 14 for each treatment group. (A) Dot plots demonstrated the CD4+CD25+IL-10+ cell population. (B) The representative bar showed the mean relative percentage of the CD4+CD25+IL-10+ population. Data were shown as mean ± standard deviation (SD). Lowercase marks showed significant differences based on Duncan’s HSD post hoc test (p < 0.05). N: healthy mice group; VC: vehicle control group; F: fibrosis model group with bleomycin; D: positive control group with dexamethasone; D1: ESEE treatment at a dose of 0.0504 mg/kg; D2: ESEE treatment at a dose of 0.1008 mg/kg; E3: ESEE treatment at a dose of 0.2016 mg/kg. Activation of macrophage cells, antigen-presenting cells, will later also initiate the activation of T helper and T regulatory cells. T helper type 1 cell is activated by IL-12 cytokine produced by activated macrophage and is the main producer of IFN-γ (Sivangala and Sumanlatha, 2015; Giunta et al., 2020). But it also could influence macrophage and act as a positive feedback loop. At day 7 observation, there was also a significant increase of this cytokine in the fibrosis model group, indicating inflammation. ESEE treatment for 7 days in dose 2 (D2) showed a significant decrease of this cytokine, due to inhibition of macrophage activation. However, on day 14, the fibrosis model group showed a significant decrease of CD11b+IFN-γ+ compared to day 7. This result indicates a highly pro-fibrotic environment in the lung, suppressing IFN-γ cytokine as it will block and inhibit fibroblast activation. Treatment with ESEE, on the contrary, showed a higher CD11b+IFN-γ+ cell population at day 14, showing its role in promoting IFN-γ production and domination, to inhibit fibrosis development. IFN-γ exerts its anti-fibrotic role as it could directly block fibroblast activation and promote matrix metalloproteinase activation to degrade excessive extracellular matrix (Zhou et al., 2008; Vu et al., 2019). As the beneficial compounds of ESEE successfully suppressed inflammation, the immune environment was then dominated by anti-inflammatory activity. Regulatory T cells (Tregs) play a critical role in maintaining immune homeostasis and modulating immune responses (Birjandi et al., 2016). The production of IL-10 by regulatory T cell (Treg) was found reduced in the fibrosis model group (C-). At day 7 observation, the CD4+CD25+IL-10+ cell population was found highest in the dexamethasone group (C+), suggesting its strong anti-inflammatory activity. All ESEE groups also showed a significant increase in IL-10-producing Treg cells. IL-10 helps inhibit the activation of macrophages and other inflammatory cells, reducing overall inflammation. There were no significant changes in the IL-10 production at day 14, except for dose 1 ESEE treatment (D1), which showed a significantly higher cell population. IL-10 could directly exert its anti-fibrotic role by inhibiting more fibroblast activation, but when it is over-accumulated, it could also over-stimulate TGF-β cytokine, which is a key mediator of fibrosis (Shi et al., 2014; Maranatha et al., 2022). Some studies have indicated that IL-10 may exert anti-fibrotic effects by suppressing pro-inflammatory cytokines and modulating immune cell responses (Moore and Hogaboam, 2008). Some studies have indicated that IL-10 may also directly affect the fibrotic process by inhibiting the activation and proliferation of fibroblasts, the progenitor cells responsible for collagen deposition and scar formation in pulmonary fibrosis (Zhou et al., 2008). The precise mechanisms underlying the elevation of IL-10 expression in bleomycin-treated mice and its impact on the progression of fibrosis remain to be elucidated. However, in this study, persistent CD4+CD25+IL-10+ cell populations were more beneficial as they could still suppress inflammation but also did not overstimulate pro-fibrotic cytokine. ConclusionESEE has indicated its beneficial properties in inhibiting pulmonary fibrosis development by reducing inflammation, which is demonstrated with lower CD11b+TNF-α+ and CD11b+IFN-γ+ cell populations and higher CD4+CD25+IL-10+ cell populations in all ESEE treatment groups. Dose 2 of ESEE at 0.1008 mg/kg BW (D3) showed the most efficient activity in reducing CD11b+TNF-α+ and CD11b+IFN-γ+ cell populations at day 14. Future studies are required to investigate another immune marker in fibrosis mice models after ESEE treatment. AcknowledgmentThe authors would like to express their gratitude to all members of the Animal Physiology, Structure, and Development Laboratory for their invaluable technical support and constructive discussions. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research was supported by the Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, Indonesia, through a professor research grant (Grant no: 02172.8/UN10.F0901/B/KS/2024). Author contributionsAEA, SRF, YIC, and MSD contributed to the study design and conceptualization. SRF and YIC contributed to laboratory work and data collection. AEA analyzed the data and wrote the original draft. YIC conducted the project administration and validated the data. SW interpreted and visualized the results. MR performed a critical revision of the article. MSD performed funding acquisition. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data supporting the findings of this study are mentioned within the manuscript. ReferencesAldi, Y., Dillasamola, D. and Rifa, N. 2019. Effect of ethanol from extract of Tapak Liman leaves (Elephantopus scaber Linn.) on hematopoiesis of anemia mice. Int. J. Pharm. Res. Allied Sci. 8, 157–167. Aliyu, M., Zohora, F.T., Anka, A.U., Ali, K., Maleknia, S., Saffarioun, M. and Azizi, G. 2022. Interleukin-6 cytokine: an overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 111, 1–14. Asfi, N., Christina, Y.I., Dwijayanti, D.R., Rifa’i, M. and Djati, M.S. 2022. Anti-inflammatory activity of Elephantopus scaber and Sauropus androgynus combination in pregnant mice infected with Escherichia coli. J. Exp. Life Sci. 12, 117–123. Birjandi, S.Z., Palchevskiy, V., Xue, Y.Y., Nunez, S., Kern, R., Weigt, S.S., Lynch, J.P., Chatila, T.A. and Belperio, J.A. 2016. CD4+CD25hiFoxp3+ cells exacerbate bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 186, 2008–2020. Cowley, P.M., Roberts, C.R. and Baker, A.J. 2019. Monitoring the health status of mice with bleomycin-induced lung injury by using body condition scoring. Comp. Med. 69, 95–102. Djati, M.S. and Christina, Y.I. 2019. Traditional Indonesian Rempah-rempah as a Modern Functional Food and Herbal Medicine. Funct. Foods Health Dis. 9, 241–264. Djati, M.S., Dwijayanti, D.R. and Rifa’I, M. 2016. Herbal supplement formula of Elephantopus scaber and Sauropus androgynus promotes IL-2 cytokine production of CD4+T cells in pregnant mice with typhoid fever. Open Life Sci. 11, 211–219. Firdausi, S.R., Nur’aini, R.A.R., Izzah, F.N., Nabilah, S.N., Christina, Y.I., Dwijayanti, D.R., Rahayu, S., Rifa’i, M. and Djati, M.S. 2024. Elephantopus scaber ethanol extract suppresses inflammation via regulation of the NF-κB pathway expression in pulmonary fibrosis. Trop. J. Nat. Prod. Res. 8, 8554–8560. Giunta, E.F., Barra, G., De Falco, V., Argenziano, G., Napolitano, S., Vitale, P., Zanaletti, N., Terminiello, M., Martinelli, E., Morgillo, F., Ciardiello, D., De Palma, R., Ciardiello, F. and Troiani, T. 2020. Baseline IFN-γ and IL-10 expression in PBMCs could predict response to PD-1 checkpoint inhibitors in advanced melanoma patients. Sci. Rep. 10, 1–11. Gul, A., Yang, F., Xie, C., Du, W., Mohammadtursun, N., Wang, B., Le, J. and Dong, J. 2023. Pulmonary fibrosis model of mice induced by different administration methods of bleomycin. BMC Pulm. Med. 23, 1–16. Han, Y., Li, X., Zhang, X., Gao, Y., Qi, R., Cai, R. and Qi, Y. 2020. Isodeoxyelephantopin, a sesquiterpene lactone from Elephantopus scaber Linn., inhibits pro-inflammatory mediators’ production through both NF-κB and AP-1 pathways in LPS-activated macrophages. Int. Immunopharmacol. 84, 1–9. Hiradeve, S.M. and Rangari, V.D. 2014. Elephantopus scaber Linn.: a review on its ethnomedical, phytochemical and pharmacological profile. J. Appl. Biomed. 12, 49–61. Hirano, T. 2021. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 33, 127–148. Hou, J., Ma, T., Cao, H., Chen, Y., Wang, C., Chen, X., Xiang, Z. and Han, X. 2018. TNF-α-induced NF-κB activation promotes myofibroblast differentiation of LR-MSCs and exacerbates bleomycin-induced pulmonary fibrosis. J. Cell. Physiol. 233, 2409–2419. Kato, S., Inui, N., Hakamata, A., Suzuki, Y., Enomoto, N., Fujisawa, T., Nakamura, Y., Watanabe, H. and Suda, T. 2018. Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis. Respir. Res. 19, 1–12. Liang, Q., Lin, Q., Li, Y., Luo, W., Huang, X., Jiang, Y., Qin, C., Nong, J., Chen, X., Sooranna, S.R. and Pinhu, L. 2020. Effect of SiS3 on extracellular matrix remodeling and repair in a lipopolysaccharide-induced ARDS rat model. J. Immunol. Res. 2020, 1–13. Maranatha, D., Hasan, H., Bakhtiar, A., Widyoningroem, A. and Aryati 2022. Association of TNF-α, TGF-β1, amphiregulin, IL-2, and EGFR WITH pulmonary fibrosis in COVID-19. J. Infect. Public Health 15, 1072–1075. Megha, K.B., Joseph, X., Akhil, V. and Mohanan, P. V. 2021. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 91, 1–17. Moore, B.B. and Hogaboam, C.M. 2008. Murine models of pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, 152–160. Pahwa, R., Goyal, A. and Jialal, I. 2023. Chronic inflammation. [Updated 2023 Aug 7]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024 Jan-. Available via https://www.ncbi.nlm.nih.gov/books/NBK493173/ Pandey, V., Tripathi, A., Rani, A. and Dubey, P.K. 2020. Deoxyelephantopin, a novel naturally occurring phytochemical impairs growth, induces G2/M arrest, ROS-mediated apoptosis and modulates lncRNA expression against uterine leiomyoma. Biomed. Pharmacother. 131, 1–13. Patil, N., Paulose, R.M., Udupa, K.S., Ramakrishna, N. and Ahmed, T. 2016. Pulmonary toxicity of bleomycin - a case series from a tertiary care Center in Southern India. J. Clin. Diagn. Res. 10, FR01–FR03. Qi, R., Li., X., Zhang, X., Huang, Y., Fei, Q., Han, Y., Cai, R., Gao., Y. and Qi, Y. 2020. Ethanol extract of Elephantopus scaber Linn. Attenuates inflammatory response via the inhibition of NF-κB signaling by dampening p65-DNA binding activity in lipopolysaccharide-activated macrophages. J. Ethnopharmacol. 250, 1–10. Redente, E.F., Keith, R.C., Janssen, W., Henson, P.M., Ortiz, L.A., Downey, G.P., Bratton, D.L. and Riches, D.W.H. 2014. Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am. J. Respir. Cell Mol. Biol. 50, 825–837. Sgalla, G., Iovene, B., Calvello, M., Ori, M., Varone, F. and Richeldi, L. 2018. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir. Res. 19, 1–18. Shaw, J.E., Sicree, R.A. and Zimmet, P.Z. 2010. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14. Shi, K., Jiang, J., Ma, T., Xie, J., Duan, L., Chen, R., Song, P., Yu, Z., Liu, C., Zhu, Q. and Zheng, J. 2014. Pathogenesis pathways of idiopathic pulmonary fibrosis in bleomycin-induced lung injury model in mice. Respir. Physiol. Neurobiol. 190, 113–117. Sivangala, R. and Sumanlatha, G. 2015. Cytokines that mediate and regulate immune responses. Innov. Immunol. 4, 1–26. Tashiro, J., Rubio, G.A., Limper, A.H., Williams, K., Elliot, S.J., Ninou, I., Aidinis, V., Tzouvelekis, A. and Glassberg, M.K. 2017. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front. Med. 4, 1–11. Thannickal, V.J., Toews, G.B., White, E.S., Lynch, J.P. and Martinez, F.J. 2004. Mechanisms of pulmonary fibrosis. Annu. Rev. Med.,55, 395–417. Thomson, E.M., Williams, A., Yauk, C.L. and Vincent, R. 2012. Overexpression of tumor necrosis factor-α in the lungs alters immune response, matrix remodeling, and repair and maintenance pathways. Am. J. Pathol. 180, 1413–1430. van Gijsel-Bonnello, M., Darling, N.J., Tanaka, T., Di Carmine, S., Marchesi, F., Thomson, S., Clark, K., Kurowska-Stolarska, M., McSorley, H.J., Cohen, P. and Arthur, J.S.C. 2022. Salt-inducible kinase 2 regulates fibrosis during bleomycin-induced lung injury. J. Biol. Chem. 298, 102644. Vu, T.N., Chen, X., Foda, H.D., Smaldone, G.C. and Hasaneen, N.A. 2019. Interferon-γenhances the antifibrotic effects of pirfenidone by attenuating IPF lung fibroblast activation and differentiation. Respir. Res. 20, 1–14. Wang, J., Li, P., Li, B., Guo, Z., Kennelly, E.J. and Long, C. 2014. Bioactivities of compounds from Elephantopus scaber, an ethnomedicinal plant from Southwest China. Evidence-based Complement. Altern. Med. 2014, 1–7. Wen, G., Li, T., He, H., Zhou, X. and Zhu, J. 2020. Ganoderic acid A inhibits bleomycin-induced lung fibrosis in mice. Pharmacology 105, 568–575. Zhou, L., Wang, H., Zhong, X., Jin, Y., Mi, Q.S., Sharma, A., McIndoe, R.A., Garge, N., Podolsky, R. and She, J.X. 2008. The IL-10 and IFN-γ pathways are essential to the potent immunosuppressive activity of cultured CD8+ NKT-like cells. Genome Biol. 9, R119.1–R119.18 | ||
| How to Cite this Article |
| Pubmed Style Alghoull AE, Firdausi SR, Christina YI, Widyarti S, Rifa'i M, Djati MS. Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion. Open Vet. J.. 2025; 15(1): 118-125. doi:10.5455/OVJ.2025.v15.i1.11 Web Style Alghoull AE, Firdausi SR, Christina YI, Widyarti S, Rifa'i M, Djati MS. Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion. https://www.openveterinaryjournal.com/?mno=220904 [Access: January 08, 2026]. doi:10.5455/OVJ.2025.v15.i1.11 AMA (American Medical Association) Style Alghoull AE, Firdausi SR, Christina YI, Widyarti S, Rifa'i M, Djati MS. Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion. Open Vet. J.. 2025; 15(1): 118-125. doi:10.5455/OVJ.2025.v15.i1.11 Vancouver/ICMJE Style Alghoull AE, Firdausi SR, Christina YI, Widyarti S, Rifa'i M, Djati MS. Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion. Open Vet. J.. (2025), [cited January 08, 2026]; 15(1): 118-125. doi:10.5455/OVJ.2025.v15.i1.11 Harvard Style Alghoull, A. E., Firdausi, . S. R., Christina, . Y. I., Widyarti, . S., Rifa'i, . M. & Djati, . M. S. (2025) Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion. Open Vet. J., 15 (1), 118-125. doi:10.5455/OVJ.2025.v15.i1.11 Turabian Style Alghoull, Asadig Emhemmed, Salma Rifqi Firdausi, Yuyun Ika Christina, Sri Widyarti, Muhaimin Rifa'i, and Muhammad Sasmito Djati. 2025. Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion. Open Veterinary Journal, 15 (1), 118-125. doi:10.5455/OVJ.2025.v15.i1.11 Chicago Style Alghoull, Asadig Emhemmed, Salma Rifqi Firdausi, Yuyun Ika Christina, Sri Widyarti, Muhaimin Rifa'i, and Muhammad Sasmito Djati. "Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion." Open Veterinary Journal 15 (2025), 118-125. doi:10.5455/OVJ.2025.v15.i1.11 MLA (The Modern Language Association) Style Alghoull, Asadig Emhemmed, Salma Rifqi Firdausi, Yuyun Ika Christina, Sri Widyarti, Muhaimin Rifa'i, and Muhammad Sasmito Djati. "Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion." Open Veterinary Journal 15.1 (2025), 118-125. Print. doi:10.5455/OVJ.2025.v15.i1.11 APA (American Psychological Association) Style Alghoull, A. E., Firdausi, . S. R., Christina, . Y. I., Widyarti, . S., Rifa'i, . M. & Djati, . M. S. (2025) Evaluating the efficacy of ethanolic extract of Tapak Liman (Elephantopus scaber L.) leaf in inhibiting pulmonary fibrosis: Mechanisms through anti-fibrotic cytokine promotion. Open Veterinary Journal, 15 (1), 118-125. doi:10.5455/OVJ.2025.v15.i1.11 |