| Research Article | ||
Open Vet. J.. 2025; 15(1): 222-243 Open Veterinary Journal, (2025), Vol. 15(1): 222-243 Research Article Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus)Yi Yuan1, Amira A. Omar2, Wasseem Emam3 and Radi A. Mohamed1*1Department of Aquaculture, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Kafr El-Sheikh, Egypt 2Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt 3Ethical Seafood Research, Glasgow, UK *Corresponding Author: Radi A. Mohamed. Department of Aquaculture, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Kafr El-Sheikh, Egypt. Email: r.mohamed.vet [at] gmail.com Submitted: 03/09/2024 Accepted: 03/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
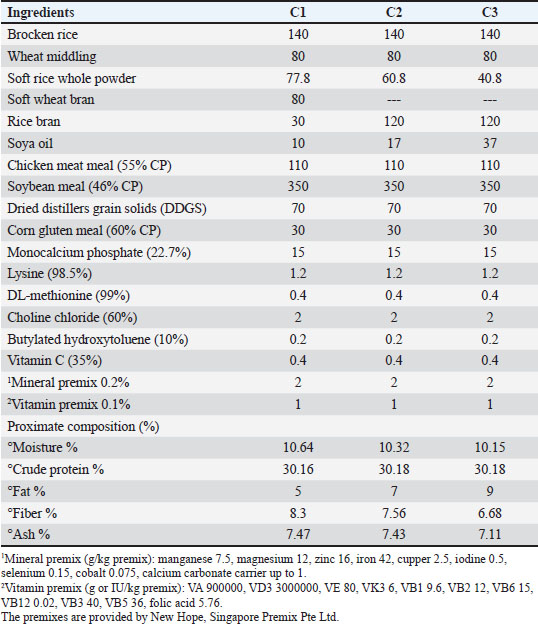
AbstractBackground: Bile acids (BAs) are made from cholesterol in the liver and are then coupled with taurine or glycine before being expelled by the hepatocyte. BAs are very important for the emulsification of dietary fat for easy nutrient absorption processes. Aim: The aim of this study is to investigate the effects of dietary BA supplementation and dietary fat percent on the growth performance, morphology of the intestine, immune-physiological responses, and transcriptomic responses of Nile tilapia (Oreochromis niloticus). Methods: Using diets containing three different inclusion levels of fat (5%, 7%, and 9%) with or without BA supplementation (0.4 g/kg), fish were fed for 90 days. Results: BA supplementation significantly (p < 0.05) improved growth performance and feed utilization, with fish-fed BA-supplemented diets exhibiting higher final weight (FW), weight gain (WG), and feed conversion ratio. Dietary fat levels also significantly affected growth performance, with higher fat levels leading to higher FW, WG, and specific growth rate. BA supplementation also positively (p < 0.05) affected intestinal morphology, immune response, and antioxidant capacity. Fish-fed BA-supplemented diets had higher intestinal villus height, lysozyme activity, superoxide dismutase (SOD) and catalase (CAT) activities, and lower malonaldehyde concentration. Gene expression analysis revealed that BA supplementation upregulated (p < 0.05) the expression of antioxidant-related genes (SOD, glutathione peroxidase, and CAT, growth-related genes (GHr1 and insulin growth factor 1), and intestinal mucin gene (MUC2) while downregulating (p < 0.05) the expression of fatty acid synthase and pro-inflammatory genes (interleukin 1β and tumor necrosis factor alpha). Conclusion: BA dietary supplementation accompanied with 7% fat can be a valuable tool for improving Nile tilapia’s growth performance, feed utilization, intestinal health, immune function, and antioxidant capacity. Keywords: Bile acid, Fat, Growth, Immune-oxidative response, Oreochromis niloticus. IntroductionThe State of World Fisheries and Aquaculture reported that there was an increase in total aquaculture production by 4.4% in 2022 compared to 2020. Furthermore, the 2022 production reached up to 223.2 million tonnes from which 185.4 million tonnes were finfish and shellfish and the rest was algae (FAO Report, 2024). The rapid growth of aquaculture has led to an increased demand for protein sources in feed production. In addition to providing energy, lipids and carbohydrates also have a “protein-sparing” impact (Arenas et al., 2021). However, consuming excessive amounts of fats and carbohydrates in the diet can result in the buildup of fat in the liver, impair the body’s antioxidant and immune functions, and impact the growth and quality of meat in farmed fish (Ahmedou et al., 2025). Dietary lipids are crucial in fish feed due to their importance as the main energy source and provider of critical fatty acids (Ding et al., 2020). Several studies have shown a correlation between high-lipid diets and enhanced fish growth (Boujard et al., 2004; Martins et al., 2007). Recently, there has been an increased use of high-lipid diets in different aquaculture systems and advanced fish farming techniques for cost-efficiency, indicating potential benefits for improving protein utilization and maximizing nitrogen retention in fish (Boujard et al., 2004). This may be attributed to bile acid (BA), which can promote the synthesis of healthy lipids and reduce abnormal lipid accumulation (Borges et al., 2009; Li and Apte, 2015). On the other hand, Lee et al. (2002) and Tibbetts et al. (2005) stated that providing farmed fish with a high-fat diet for a long time can disturb their lipid metabolism, leading to unfavorable effects on their growth and physiological well-being. The liver is where BAs are first produced; they are physiological deodorants. As molecular signals, they can control energy metabolism and state of health in addition to accelerating the absorption and transportation of lipid and lipid-soluble nutrients (Wang et al., 2023a,b). Cholic acid and cyprinol are the most common BAs and alcohols, respectively. Bile consists of amphipathic molecules produced in the liver from cholesterol and kept in the gallbladder. Bile contains smaller quantities of fats (0.4%–0.5%), including phospholipids, cholesterol, and fatty acids. Biliverdin and bilirubin are responsible for the yellowish-to-green color of bile (Hofmann et al., 2010). Bile is discharged into the beginning of the intestine and works as a surfactant by breaking down lipids into micelles. By increasing the number of locations where lipase can break down lipid molecules, digestion is enhanced (Liu et al., 2024). Furthermore, the activation of “bile-salt activated lipase”, which exhibits a broad substrate specificity, is dependent on the presence of bile (Romano et al., 2020). Bile also improves the uptake of other fat-soluble nutrients such as carotenoids, astaxanthin, and vitamins A, D, E, and K (Sallam et al., 2017). In addition, bile assists in removing harmful substances such as cholesterol and other metabolites that can be dangerous if they accumulate, especially bilirubin, a byproduct of hemoglobin (Hb) breakdown in old red blood cells (RBCs) (Hofmann et al., 2010). BAs are synthesized from cholesterol in the liver and then conjugated with taurine or glycine before being excreted by the hepatocyte (Yeo et al., 2023). One of the most important tasks carried out by BAs is the movement of cholesterol in bile from the hepatocyte to the intestinal lumen for elimination (Vourakis et al., 2021). BAs play important roles in manipulating the gut microbiota, as well as in the absorption of lipids and fat-soluble vitamins, and nutrient digestion (Zhao et al., 2024). Therefore, in the 1980s, BA was used as a powerful emulsifying agent and approved as a common feed additive in 2014 in the field of aquaculture for its strong antioxidant, immunostimulant, growth-promoting, and physiological modulator of various body functions (Romano et al., 2020). Because of its prompt growth and strong ability to survive, Nile tilapia has become a significant freshwater species in semi-intensive aquaculture systems globally (Ponzoni et al., 2011). Lowering production costs and increasing profitability for this fish has been achieved by creating cost-effective feed using alternative ingredients such as soybean meal, cottonseed meal, rapeseed meal, or unconventional protein sources such as meat and bone meal, blood meal, and feather meal, along with plant seed oil (El-Naggar et al., 2021). Therefore, a crucial obstacle for the feed sector is to enhance the growth performance and health of Nile tilapia feed (Jiang et al., 2018; Elkaradawy et al., 2022). Hence, the objective of this research was to assess the effects of BA and fat percentage dietary supplements on growth performance, body composition, blood health, immune response, antioxidant capacity, transcriptomic response, liver histomorphology, and intestine morphometry in Nile tilapia. Materials and MethodsFish husbandryThe Nile tilapia (Oreochromis niloticus) used in this research was obtained from the New Hope Egypt Aquatic fish farm located in the Baltem district of Kafr El-Sheikh governorate, Egypt. Fish were housed in concrete tanks for a period of two weeks to acclimate to the specific conditions of the ponds, including a temperature of 26.48°C ± 1.07°C, pH of 7.8 ± 0.31, dissolved oxygen (DO) levels at 7.55 ± 0.74 mg/l, and total ammonia nitrogen (TAN) at 0.045 ± 0.006 mg/l. The duration of light and dark periods was adjusted to 12 hours each in the photoperiod; 2,700 fish, weighing an average of 30 ± 1.069 g each, were randomly placed in triplicates in a 21 m3 concrete pond (7×3×1 m) at a rate of 150 fish per each pond. The fish were placed haphazardly among 18 cement ponds. Every pond had access to a constant supply of aeration provided by air stones. Approximately 30% of the pond’s water volume was refreshed every 2 days to maintain a consistent water level. Fish were given experimental diets until they were full twice a day at 8:00 and 14:00 for a period of 90 days. The uneaten food in every pond was gathered, dried, and measured following each feeding. Fish that died in every pond were promptly gathered and the daily mortality data were reported. Experimental design and diet preparationThe research included two sets of variables: one control group (C) and one experimental group (BA). The control group was divided into three additional subgroups, each given a standard diet with different fat levels (5%, 7%, and 9%) and 0 g BA. More precisely, sub-group C1 was given a basal diet containing 5% fat and 0 g BA, sub-group C2 was given a basal diet containing 7% fat and 0 g BA, and sub-group C3 was given a basal diet containing 9% fat and 0 g BA. The ingredients and composition of each feed type are reported in Table 1. The study used BA from Shangdong Longchang Animal Health Product Co., Ltd., in Jinan, China, with a purity level of 30%. The BA mixture included 17% CDCA, 77% HDCA, and 5% CA. Conversely, the control group was given a basic diet with matching fat levels (5%, 7%, and 9%), along with an extra 0.4 g/kg of BA supplementation. This group was further categorized into three sub-groups: BA1 got a basal diet with 5% fat and 0.4 g/kg BA; BA2 got a basal diet with 7% fat and 0.4 g/kg BA; and BA3 got a basal diet with 9% fat and 0.4 g/kg BA. There are three replicates for each subgroup. Table 1. Composition and chemical analysis of experimental basal diets.
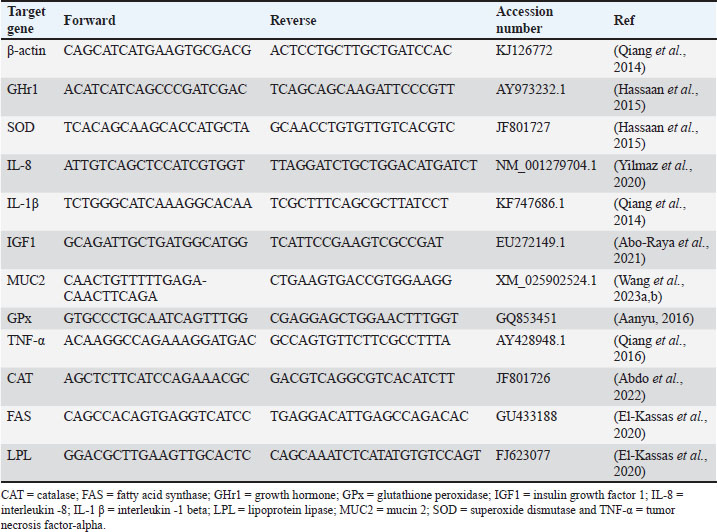
Fish growth performance, feed utilization efficiency, and biometric indicesTo assess the growth of the fish, they were caught with appropriate nets after 90 days and sedated with clove oil (Merck, Germany, 50 μl/ l). The fish’s final weight (FW) was determined by individually weighing each one. Each fish’s total length (l) was measured with a measuring board from the front tip of the mouth to the extended tip of the tail fin. The growth performance was evaluated, and feed utilization, as well as biometric indices, was determined in the following manner:The formula for body weight gain (BWG) is calculated by subtracting the initial body weight (W0) from the final body weight (W1). The formula for weight gain rate (WG %) is equal to the difference between the FW (W1) and the initial weight (W0), divided by the initial weight (W0) and multiplied by 100. Specific growth rate (SGR % /day)=100 × (lnW1−lnW0) / the experimental period (days). Protein efficiency ratio is calculated as the ratio of weight gain (in grams) to protein intake (in grams). The formula for feed conversion ratio (FCR) is equal to feed intake (in grams) divided by BWG (in grams). The condition factor (K)=100 × (W1/l3). Blood sampling and separation of the serumAt the end of the experiment, blood samples were collected from the caudal vein of 15 fish in each experimental group and stored in vacuum tubes. Two clean tubes were used for collecting the obtained blood. The first type was designed for hematological testing and included ethylenediaminetetraacetic acid as an anticoagulant. The second type included tubes without anticoagulant that were used for collecting blood serum. Following a 15 minutes centrifugation at 4°C and 3,000 rpm using an Eppendorf centrifuge (SIGMA 4-16 K refrigerated centrifuge, Sigma Laborzentrifugen, Germany), the serum supernatant was separated and preserved in plastic Eppendorf tubes at −20°C for further examination. Hematological analysisThrall et al. (2004) employed a method to assess the overall white blood cell (WBC) and RBC count, Hb content through the cyanomet Hb method with Drabkin’s solution, and packed cell volume (PCV) using the microhematocrit method. Differential leukocytic count (lymphocyte, heterophil, and monocyte) was determined by preparing thin blood films, drying them in air, fixing with methanol for 3–5 minutes, staining with Gimsa stain for 8–10 minutes, and finally allowing to dry. One hundred WBCs were counted in every blood smear as described by Thrall et al. (2004). Serum biochemical analysisAspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured via the colorimetric method at 540 nm using kits following the manufacturer’s instructions (Reitman and Frankel, 1957). Serum urea and creatinine levels were measured using the colorimetric method with commercially available kits (Heinegård and Tiderström, 1973). Serum triglyceride and total cholesterol levels were assessed using the GPO-PAP and CHOD-PAP methods with commercial clinical kits as per the manufacturer’s instructions. Trinder (1969) stated that serum glucose levels were assessed by using commercially available glucose enzymatic PAP kits from Bio-Merieux, France. Protein and albumin levels were analyzed using the method outlined by Doumas et al. (1981). Mathematical calculation was used to determine the level of globulin in the sample. Digestive enzymes activityAs per the manufacturer’s guidelines, diagnostic reagent kits from Cusabio Biotech Co., Ltd., Wuhan, Hubei, China, were used to assess the digestive enzyme activity in serum (9 fish/ group). The functions of the digestive enzymes (lipase and amylase) were investigated following the methods described in Abdel-Tawwab et al. (2018). Moss and Henderson (1999) conducted colorimetric tests for lipase and amylase activity at 660 and 580 nm, respectively. Antioxidant capacityThe activity of catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA) levels were assessed in the serum of nine fish per treatment using ELISA kits. Specific kit for each enzyme (CAT, SOD, and MDA kits) from Inova Biotechnology, China, was used for the estimation of each specific enzyme following the manufacturer’s instructions. A microplate ELISA reader (BIO-RAD 680 Microplate Reader) at a wavelength of 450 nm was used to read and interpret data following the protocol described by Abdel-Tawwab et al. (2018). Immune responseTo examine how BA and fat percentage in diet impact the immune response of fish, nine fish per treatment were selected at random way for this study. As per the technique outlined by Demers and Bayne (1997), serum lysozyme activity was measured using ELISA at 450 nm using a microplate ELISA reader. Histological observationTo conduct histopathological examination, tissue samples from the anterior, middle, and posterior parts of the intestine, along with the hepatopancreas, were collected from five fish per treatment while under deep anesthesia induced by 40% ethyl alcohol. The specimens were immersed in a 10% formaldehyde solution for 24–48 hours and subsequently dried out with increasing levels of ethyl alcohol (from 70% to pure alcohol). The dried specimens were clarified using xylene and encased in paraffin wax. Sections measuring 4–5 µm were stained with hematoxylin and eosin (H&E) for histopathological and morphometric analysis as described by Moustafa et al. (2020). Two pathologists carried out the evaluation of histopathological liver lesions. Intestinal villi length and width, as well as crypt depth, were assessed with image analysis software (NIH, Bethesda, MD). RNA extraction, cDNA synthesis, and quantitative real-time PCRRNA extraction was conducted according to the manufacturer’s instructions with the TRIzol reagent. The RNA was converted into cDNA through reverse transcription with the MultiScribe RT enzyme kit. The cDNA was analyzed using real-time triplication Polymerase Chain Reaction (PCR) with Power SYBR Green PCR Master Mix on a 7,500 Real-Time PCR System from Applied Biosystems in Foster City, California, USA. To compare the mRNA expression fold changes of various genes, the Ct values of the target genes were compared with those of a control sample. Normalization of the fold change was conducted with respect to the housekeeping gene β-actin expression. The researchers used the 2-∆∆Ct method, where they compared the Ct values of the target gene to the β-actin housekeeping gene (Livak and Schmittgen, 2001). Table 2 presents the primer sequences and gene accession number used in the research. Water quality analysisBoth the OxyGuard handy Polaris DO and temperature meter and the HACH PHC725-pH meter were used to monitor DO, temperature, and pH levels in each pond twice per day (9 am and 4 pm). With a portable photometer (Martini MI 405), samples of water (20 ml) were collected from the middle point of each pond in order to determine the TAN once per day (1 pm). 10 ml of the water sample was used in the measurement of TAN in the photometer glass tube with the addition of five drops of Nessler reagents A and B. The reaction takes place within 3 minutes and the value of TAN could be obtained from the photometer monitor. Unionized ammonia (UIA) was calculated mathematically with reference to pH and temperature values for each measurement (https://www.svl.net/unionized-amonia-calculator/). Table 2. The sequences of applicable primers used for real-time q-PCR investigation of gene expression.
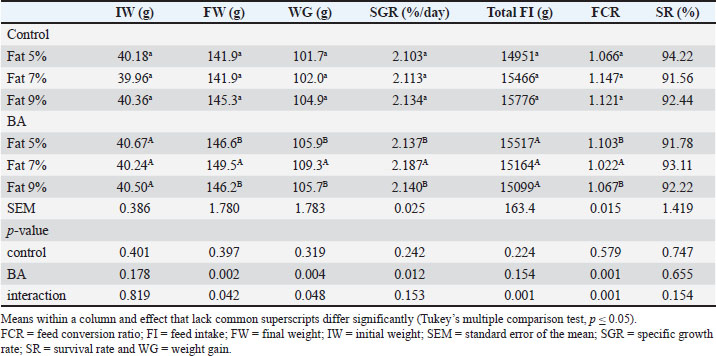
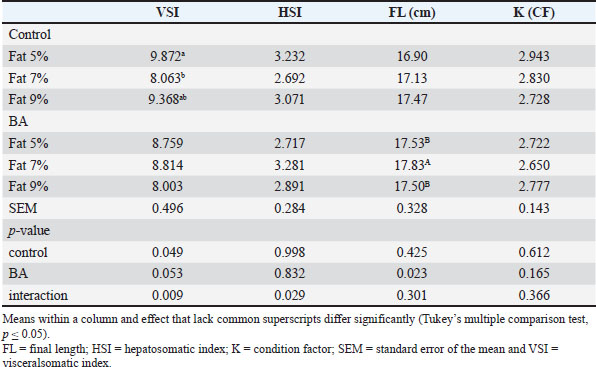
Statistical analysisThe normality of the data was assessed, and it was validated through analysis of the residuals. Percentage data were processed after applying an arcsine transformation. Graph Pad Prism 6 (Graph Pad Prism v6.0, San Diego, CA) was used to conduct statistical analysis of the data. Two-way ANOVA was used to compare the main impacts of fat percentage and BA. Tukey’s multiple comparison test was used to analyze the interaction of the two factors in cases where it was relevant. The significance level was determined as p <0.05. Ethical approvalThe Institutional Aquatic Animal Care and Use in Research Committee at the Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Egypt reviewed and approved the protocol for this study (approval number: IAACUC-KSU-0161-2022). ResultsFish growth performance, feed utilization efficiency, and biometric indicesFish that were given diets with BA supplementation showed significantly higher FW and WG compared to the control group (p < 0.05). Likewise, an elevated fat diet led to increased FW and WG. There was a noteworthy interaction detected between dietary fat level and BA supplementation (p < 0.05). Dietary fat levels significantly affected SGR, while BA supplementation did not have a significant impact. BA supplementation and dietary fat levels both had a significant impact on feed intake (p < 0.05), showing increased intake in diets with BA supplementation and higher fat levels (Table 3). It is noteworthy that fish given the control diet containing 5% fat exhibited a notably higher viscerosomatic index (VSI), hepatosomatic index (HSI), and final length (FL) compared to fish fed diets containing 7% and 9% fat (p < 0.05) (Table 4). There was no significant variation (p > 0.05) in the condition factor (K) between the different treatment groups. Table 3. Growth performance and feed utilization efficiency of Nile tilapia feed different fat percentage with or without BA for 90 days.
Hematological analysisTable 4. Biometric indices of Nile tilapia feed different fat percentage with or without BA for 90 days.
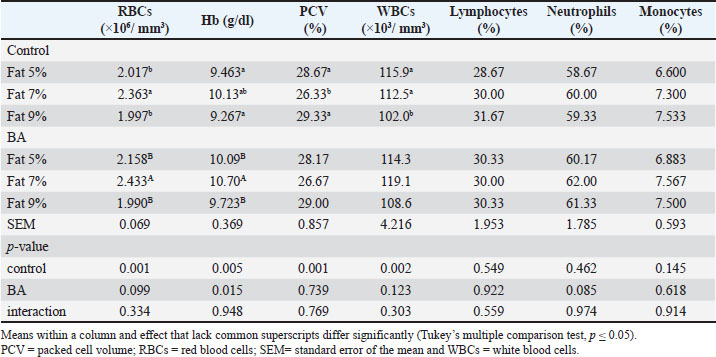
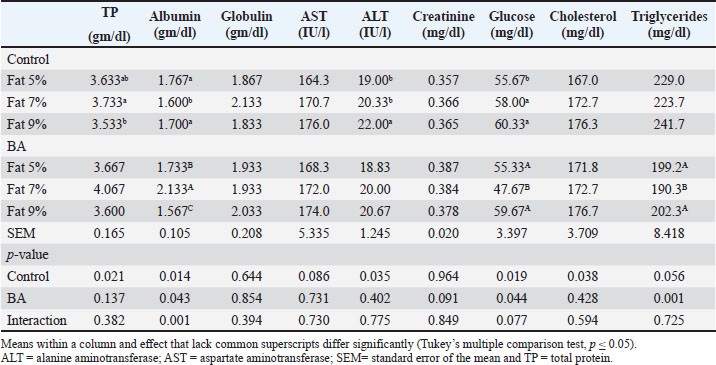
The research investigated how different levels of dietary fat and supplementation of BA affect the hematological parameters of fish. The outcomes are displayed in Table 5. Findings indicated that fish given a diet containing 7% fat displayed notably elevated levels of RBCs, Hb concentration, PCV, and WBCs compared to the control group, with a statistical significance (p < 0.05). Nonetheless, there were no significant differences (p > 0.05) in lymphocyte, neutrophil, or monocyte proportions across any of the treatment categories. Serum biochemical analysisThe research investigated how dietary fat levels and BA supplementation affect the serum biochemical parameters of fish. The findings revealed that fish given a diet with 7% fat supplemented with BA had notably elevated levels of total protein (TP), albumin, and ALT activity (p < 0.05). Still, there were no significant differences (p > 0.05) observed in globulin, AST activity, creatinine, glucose, cholesterol, or triglyceride levels. The control diets showed elevated glucose levels, decreased cholesterol levels, and reduced triglyceride levels. There were no notable variations (p > 0.05) in levels of creatinine, glucose, cholesterol, or triglycerides across the treatment groups. The findings are shown in Table 6. Table 5. Hematological analysis of Nile tilapia feed different fat percentage with or without BA for 90 days.
Table 6. Serum biochemical analysis of Nile tilapia feed different fat percentage with or without BA for 90 days.
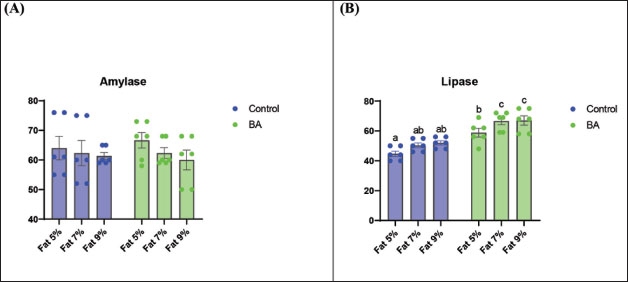
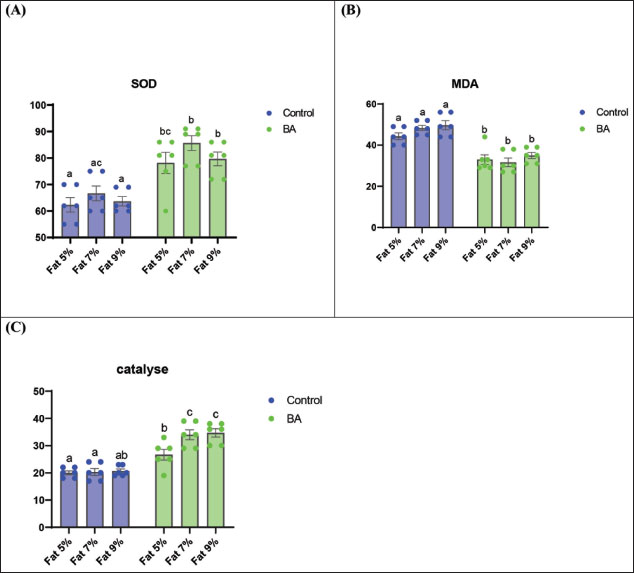
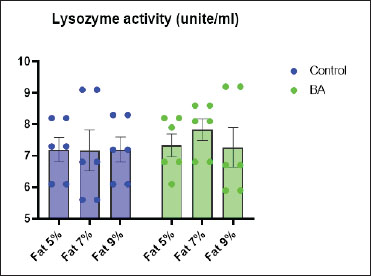
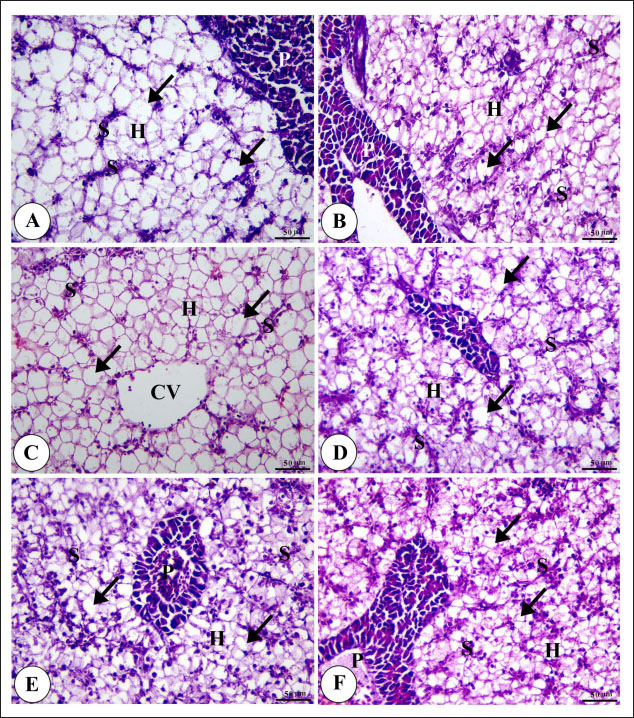
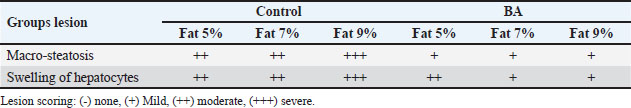
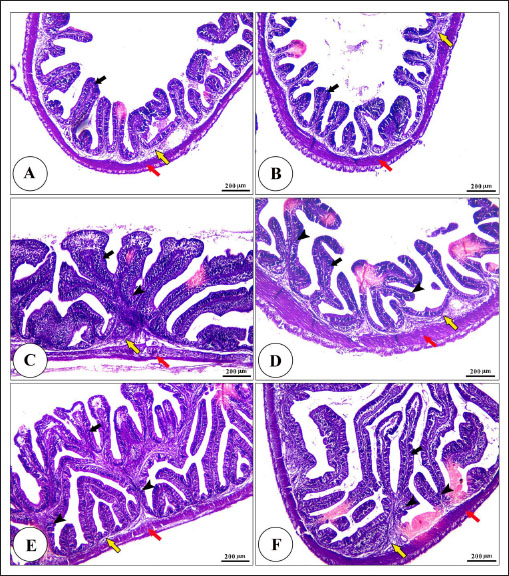
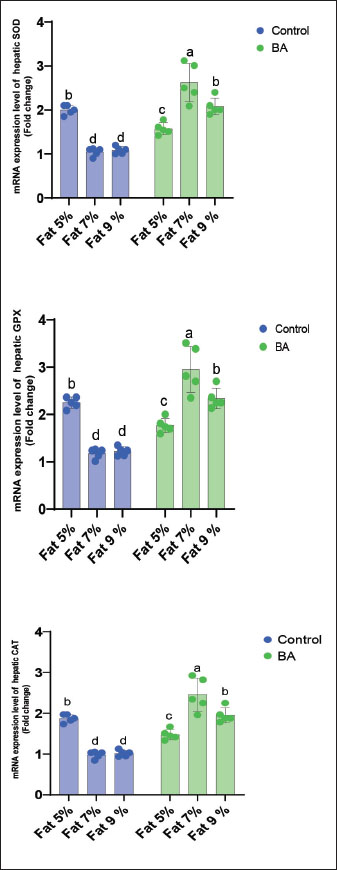
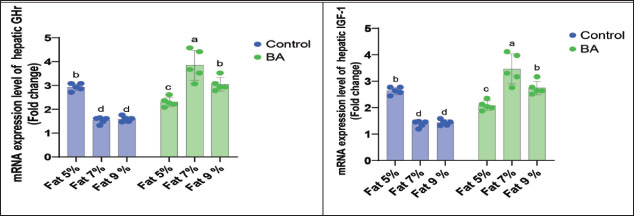
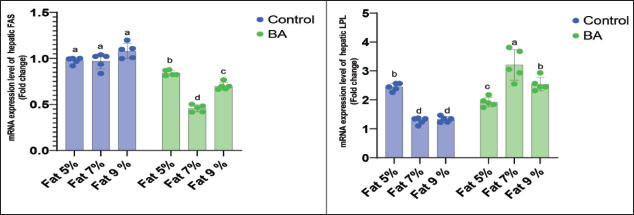
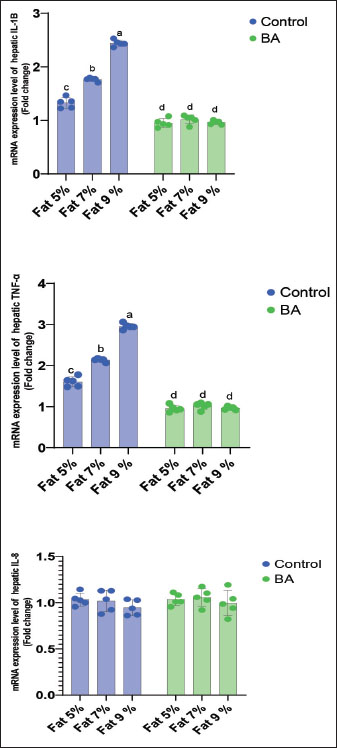
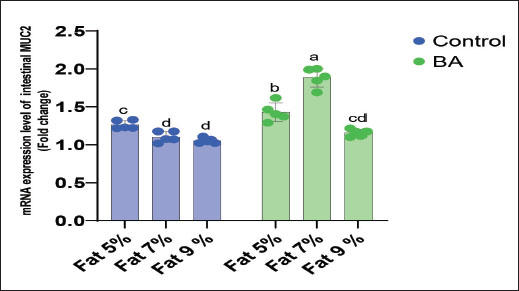
Digestive enzymes activityThis research explored how the activity of digestive enzymes in O. niloticus is affected by different levels of dietary fat and supplementation with BA. Figure 1A and B displays the findings. Fish that were given the BA-supplemented diet with 5% fat showed a notably higher level of amylase activity (Fig. 1A) compared to all other groups (p < 0.05). Fish fed the control diet containing 5% fat showed a significantly higher amylase activity compared to the group fed the control diet with 9% fat (p < 0.05). Fish that were fed the BA-supplemented diet containing 7% fat had noticeable higher levels of lipase activity (Fig. 1B) compared to all other groups (p < 0.05). Antioxidant capacityThis research investigated how different levels of dietary fat and dietary inclusion of BA affect the antioxidant capacity of O. niloticus. The findings are displayed in Figure 2A–C. Fish fed a diet containing 5% fat and BA supplement showed significantly higher SOD activity compared to the control group with 7% fat (p < 0.05). Fish fed diets containing 5% and 7% fat supplemented with BA showed significantly lower MDA concentration compared to the control group with 5% fat (p < 0.05). Fish fed diet with 5% fat and BA supplementation had significantly higher CAT activity than the control group with 9% fat (p < 0.05). Immune responseThis research analyzed how different levels of dietary fat and the addition of BA impact lysozyme activity in O. niloticus (Fig. 3). Fish fed the diet with 7% fat supplemented with BA had significantly higher lysozyme activity compared to all other groups (p < 0.05). Histological observationThe liver parenchyma of fish of all groups is primarily composed of polyhedral hepatocytes with central nuclei with glycogen and fat deposition. The hepatocytes were radiating from the central vein in a fan-shaped pattern. The hepatic sinusoids are lined with endothelial cells forming a very thin cytoplasmic sheet. The exocrine pancreatic tissue consists of clusters of pyramidal cells organized in acini. The pancreatic cells have a dark basophilic cytoplasm, distinct basal nuclei, and many large eosinophilic zymogen granules. The liver of all groups showed steatosis which ranged from mild degrees in the BA1, BA2, and BA3 groups to severe steatosis and vacuolation in the C3 group. The hepatocytes of the C1, C2, and C3 groups appeared swollen (Fig. 4). The scoring of histopathological lesions was summarized in Table 7. An analysis of the intestine’s tissue showed that a layer of simple columnar epithelium with goblet cells lines the intestinal wall, while the core of intestinal villi consists of loose connective tissue with a lamina muscularis and tunica serosa on the exterior. The length and branching of intestinal villi were markedly noticed in BA2, BA3, C3, BA1, C1, and C2 groups, respectively, especially in the anterior, middle, and posterior parts of the intestine as displayed in Figures 5–7. The data of morphological analysis of different parts of the intestine are presented in Table 8. Transcriptomic responseThe mRNA gene expression of genes linked to antioxidants, the BA supplementation groups in contrast to the control groups (C2 and C3), BA2, and BA3 showed significant hepatic overexpression of SOD, glutathione peroxidase (GPX), and CAT. BA2 group fed a diet containing 7.0% fat and 0.4 mg/kg BA had the highest value (p < 0.05), while BA1 showed lower mRNA levels of SOD, GPX, and CAT than the control group (C1) (p < 0.05) (Fig. 8). Additionally, as shown in Figure 9, the growth-related genes growth hormone (GHr1) and insulin growth factor 1 (IGF1) were significantly upregulated in the BA2 and BA3 groups supplemented with 7.0% and 9.0% fat and 0.4 mg/kg BA compared to the control groups (C2 and C3) (p < 0.05). In contrast, BA1 showed lower mRNA levels of GHr1 and IGF1 than the C1 group (p < 0.05). Genes associated with lipid metabolism showed that the control groups (C1, C2, and C3) had significantly greater levels of fatty acid synthase (FAS) mRNA expression compared to the groups supplemented with BA BA1, BA2, and BA3 (p < 0.05). Conversely, fish fed diets supplemented with BAs showed greater levels of lipoprotein lipase (LPL) mRNA expression, particularly in the BA2 and BA3 groups, compared to the control groups (p < 0.05). On the other hand, BA1 had lower mRNA levels of (LPL) than the C1 group (p < 0.05). as shown in Figure 10. Figure 11 shows a decrease in liver mRNA expression of interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α) in all BA-supplemented groups compared to the control groups (p < 0.05), while IL-8 had no significant changes in mRNA expression across all BA-supplemented and control groups (p > 0.05). In Figure 12, the levels of intestinal mucin (MUC2) mRNA were higher in the BA1, BA2, and BA3 groups supplemented with 5.0%, 7.0%, and 9.0% fat and 0.4 mg/kg BA compared to the control groups C1, C2, and C3 (p < 0.05), with the BA2 group exhibiting the greatest amount of gene expression (p < 0.05).
Fig. 1. Effects of dietary lipid levels and BA on digestive enzymes activity in O. niloticus. Where amylase (A) and lipase (B). Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the (p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05).
Fig. 2. Effects of dietary lipid levels and BA on antioxidant capacity in O. niloticus. Where (A) SOD and (B) MDA. Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05).
Fig. 3. Effects of dietary lipid levels and BA on lysozyme activity in O. niloticus. Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05).
Fig. 4. Photomicrograph of H&E stained panel of hepatopancreas of C1 “Fat 5%” (A) C2 “Fat 7%” (B), C3 “Fat 9%” (C), BA1 “Fat 5%” (D), BA2 “Fat 7%” (E), and BA3 “Fat 9%” (F) groups showing normal hepatic architecture with CV, polyhedral shaped hepatocytes (H) arranged in cord-like pattern and separated by blood sinusoids (S) in addition to pancreatic acini (P), steatosis of hepatocytes (arrows). Table 7. Scoring of histopathological lesions of hepatopancreas.
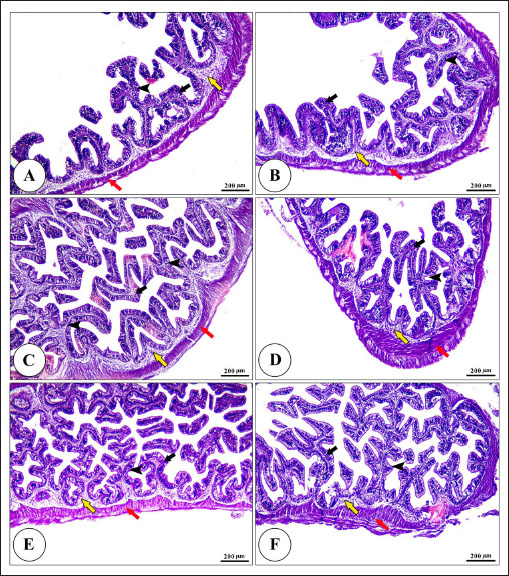
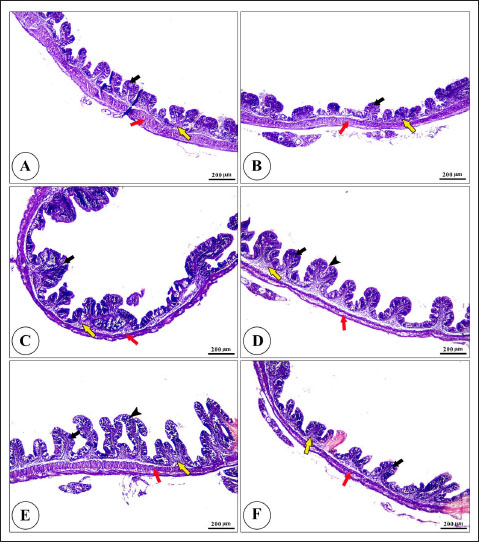
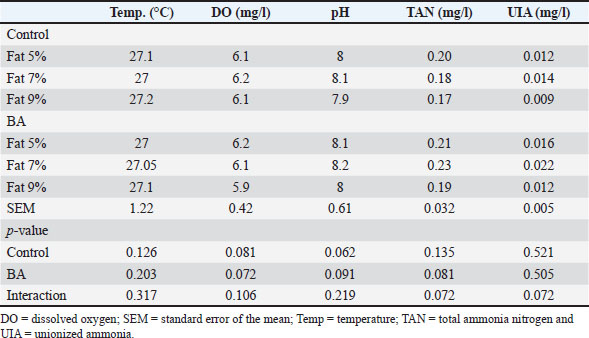
Fig. 5. Photomicrograph of H&E stained panel of anterior part of intestine of C1 “Fat 5%” (A), C2 “Fat 7%” (B), C3 “Fat 9%” (C), BA1 “Fat 5%” (D), BA2 “Fat 7%” (E), and BA3 “Fat 9%” (F) groups showing intestinal wall composed tunica mucosa of simple columnar epithelium (black arrows) which have finger-like intestinal villi which show branching in some groups (arrow heads), lamina propria (yellow arrows) and lamina muscularis (red arrows). Water quality analysisThis study examined how dietary fat levels and BA supplementation impacted the water quality parameters in fish ponds (Table 9). There were no notable differences in temperature, DO, pH, TAN, or UIA across all treatment groups. DiscussionFats and BAs are essential in fish diets, aiding in energy storage, cell membrane formation, and fat-soluble vitamin absorption. According to Tocher (2003), fats are a concentrated energy source for fish that supply vital fatty acids needed for development and breeding. BAs help in the breakdown and uptake of fats by turning them into smaller droplets that are better absorbed by the lining of the intestines (Macierzanka et al., 2019). Recognizing the significance of fats and BAs in fish nutrition is crucial for creating good quality diets that support healthy growth and overall fish welfare in aquaculture industries.
Fig. 6. Photomicrograph of H&E stained panel of middle part of intestine of C1 “Fat 5%” (A), C2 “Fat 7%” (B), C3 “Fat 9%” (C), BA1 “Fat 5%” (D), BA2 “Fat 7%” (E), and BA3 “Fat 9%” (F) groups showing intestinal wall composed tunica mucosa of simple columnar epithelium (black arrows) which have finger-like intestinal villi which show branching in some groups (arrow heads), lamina propria (yellow arrows) and lamina muscularis (red arrows). The results of this study demonstrate that dietary BA inclusion may enhance the growth performance and feed utilization of fish. This is likely due to the positive effects of BA on gut health and nutrient absorption (Zhao et al., 2024). BA promotes the development and maintenance of the intestinal epithelium, leading to increased nutrient absorption and improved growth (Ticho et al ., 2019). The observed increase in FI in fish-fed BA-supplemented diets may be due to the increased metabolic demands associated with faster growth (Liu et al., 2024b). However, the improved FCR suggests that BA supplementation allows fish to use feed more efficiently even with increased intake. The lack of significant interaction between BA supplementation and dietary fat level on most parameters suggests that the positive effects of BA are independent of dietary fat content. The biometric indices data suggest that BA supplementation may also have some positive effects on the internal organ development and metabolism of fish. This may be attributed to when fish are fed diets supplemented with BAs, it can lead to improved lipid digestion and absorption efficiency, which in turn can promote better growth and development of internal organs such as the liver, pancreas, and intestines (Li et al., 2023). Overall, the findings of this study suggest that BA supplementation can be a valuable tool for improving the growth performance, feed utilization, and potentially the general health of fish in aquaculture.
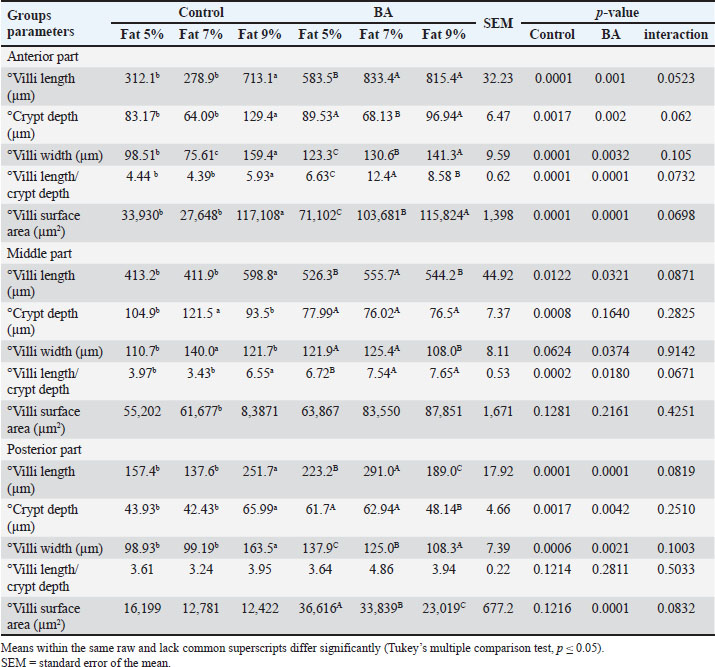
Fig. 7. Photomicrograph of H&E stained panel of posterior part of intestine of C1 “Fat 5%” (A), C2 “Fat 7%” (B), C3 “Fat 9%” (C), BA1 “Fat 5%” (D), BA2 “Fat 7%” (E), and BA3 “Fat 9%” (F) groups showing intestinal wall composed tunica mucosa of simple columnar epithelium (black arrows) which have finger-like intestinal villi which show branching in some groups (arrow heads), lamina propria (yellow arrows) and lamina muscularis (red arrows). Hematological parameters can provide valuable insights into the health and nutritional status of fish. RBCs, Hb, and PCV are all involved in oxygen transport, while WBCs are involved in immune function. The higher RBC count and Hb concentration observed in fish fed the BA-supplemented diet with 7% fat may indicate improved oxygen-carrying capacity in these fish (Wells et al., 2003). This could be beneficial for growth and performance, as oxygen is essential for cellular respiration (Mallya, 2007). The higher WBC count observed in fish fed the BA-supplemented diet with 7% fat may indicate enhanced immune function in these fish (Jin et al., 2019). This could help to protect them from disease and infection. This result is in line with Jin et al. (2019) who showed enhanced immunity with a BA-supplemented diet of seabream (Acanthopagrus schlegelii). The lack of significant differences in lymphocyte, neutrophil, and monocyte percentages suggests that BA supplementation and dietary fat level did not have a major impact on the relative proportions of different types of WBCs. Overall, the results of this study suggest that BA supplementation may have some positive effects on the hematological parameters of fish, particularly when combined with a diet containing 7% fat. Serum biochemical parameters offer valuable information on the fish’s health and nutritional status. Proteins including TP, albumin, and globulin play a crucial role in regulating blood osmotic pressure and facilitating the transport of nutrients (Mathew et al., 2023). AST and ALT are enzymes that are discharged into the blood when hepatocytes are injured (Contreras-Zentella and Hernández-Muñoz, 2016). Creatinine is a byproduct generated from muscle metabolism and eliminated from the body through the kidneys (Kashani et al., 2020). According to Perego et al. (2019), glucose serves as a primary source of energy for cells, while cholesterol and triglycerides play a crucial role in the structure and function of cell membranes. The increased levels of protein and albumin in fish consuming the BA-supplemented diet with 7% fat suggest the important function of BA in fat and fat-soluble vitamin digestion and absorption in the gut (Di Ciaula et al., 2018). Adding BAs to the diet improves the digestion and absorption of fat, increasing the utilization of dietary proteins (Yin et al., 2021). The enhanced breakdown of fats can lead to a higher production of proteins and, consequently, elevated levels of TP and albumin in the fish. Fish fed a diet with 7% fat and supplemented with BA showed lower ALT activity, potentially suggesting better liver health in these fish (Naiel et al., 2023). Fish that were fed BA-supplemented diets showed lower levels of cholesterol and triglycerides, suggesting enhanced lipid metabolism in these fish which coincided with previous research (Naiel et al., 2023). This may be advantageous in lowering the risk of heart disease. This finding aligns with the study by Yin et al. (2021), which showed that BA can improve certain serum biochemical parameters in juvenile largemouth bass (Micropterus salmoides). Overall, these findings suggest that BA supplementation may have some positive effects on the serum biochemical parameters of fish, particularly when combined with a diet containing 7% fat. Table 8. Morphometric analysis of the intestine of Nile Tilapia that feed different fat doses, with or without BA for 90 days.
Fig. 8. Effects of dietary lipid levels and BA on the relative expression of antioxidant enzymes such as SOD, GPX, and CAT in O. niloticus. Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05). Digestive enzymes are essential for the breakdown and absorption of nutrients from food. Amylase breaks down carbohydrates, while lipase breaks down fats (Kumar and Chakravarty, 2018). The higher amylase activity observed in fish fed the BA-supplemented diet with 5% fat suggests that BA supplementation may improve carbohydrate digestion in these fish (Liu et al., 2024a). This could be beneficial for growth and performance, as carbohydrates are a major energy source for fish. The higher lipase activity observed in fish fed the BA-supplemented diet with 7% fat suggests that BA supplementation may improve fat digestion in these fish (Liu et al., 2024a). These could also be beneficial for growth and performance, as fats are a high-energy nutrient source. This results are in line with Liu et al. (2024a) where dietary supplementation of BAs enhances digestive enzyme activities of spotted seabass (Lateolabrax maculatus). The results of this study suggest that BA supplementation may improve the activity of digestive enzymes in fish and that the optimal dietary fat level for BA supplementation may differ depending on the enzyme in question. Antioxidants play a crucial role in safe-guarding cells against harm triggered by free radicals. Highly reactive molecules called free radicals can harm DNA, proteins, and lipids (Tvrdá and Benko, 2020). SOD, MDA, and CAT play crucial roles as antioxidants. SOD changes superoxide radicals into hydrogen peroxide, and CAT then changes it into water and oxygen (Weydert and Cullen, 2010). MDA serves as an indicator for lipid peroxidation, a harmful process that can result in damage to cellular membranes (Całyniuk et al., 2016). The results show that adding BA to fish diets may enhance their antioxidant capacity, as evidenced by increased SOD and CAT activities and decreased MDA levels. BAs may decrease free radical production and boost fish antioxidant capacity by enhancing lipid digestion and absorption to reduce lipid oxidation in the gut. This finding is consistent with a previous study (Li et al., 2024) that showed how adding BAs to the diet of fish can improve their antioxidant levels. The same results were reported in O. niloticus by Bhusare et al. (2023) indicating that high dietary lipids might generate more reactive oxygen species (ROS) which probably could be responsible for cellular damage and for neutralizing this and the enzymatic antioxidant defense system of the body probably could be activated with BAs which increased activities of SOD and CAT enzymes. The findings of this research indicate that adding BA supplements could enhance the antioxidant capabilities of fish. Lysozyme is a protein that makes lysis to the cell membranes of bacteria. It plays a crucial role in the innate immune system, the body’s initial protection against infections (Ferraboschi et al., 2021). The increased lysozyme activity seen in fish given the 7% fat BA-supplemented diet indicates that BA supplementation could enhance immune function in these fish. BAs, when added to fish feed, can increase the production of antimicrobial peptides in the intestine, which are crucial in the fish’s immune response (Wang et al., 2023a,b). These peptides kill or inhibit pathogens, thus boosting the fish’s immune system and protecting them from infections (Valero et al., 2020). This could help to protect them from disease and infection. This result is similar to Abdel-Tawwab et al. (2023) where BAs, when added to fish feed, enhance the immunity of thinlip mullet, Liza ramada. The results of this study suggest that BA supplementation may be a valuable tool for improving the immune function of fish.
Fig. 9. Effects of dietary lipid levels and BA on the relative expression of growth such as GHr and IGF-1 in O. niloticus. Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05).
Fig. 10. Effects of dietary lipid levels and BA on the relative expression of lipid metabolism such as FAS and LPL in O. niloticus. Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05). The intestine is crucial for fish to take in and absorption of nutrients. The growth and development of fish are influenced by the integrity of gut structure. The research showed that boosting the height of intestinal folds in salmon can amplify the surface area for digestive enzyme and nutrient interaction, leading to improved absorption and utilization of nutrients (Refstie et al., 2000). Previous studies on fish showed that a diet high in fat can harm gut health by affecting intestinal structures and normal microbiota but adding external BA could reduce the negative impact caused by the high-fat diet (Ma et al., 2018; Peng et al ., 2019). Additionally, Yin et al. (2021) found that BA could enhance the morphology and digestive functions of the fish intestine. The current findings indicate that adding BA supplements can significantly enhance the height of intestinal folds when compared to control groups, aligning with the previous experimental results. Therefore, the growth of the fish fed 7% fat with the BA group may be due to enhanced intestinal health, leading to better absorption and utilization of lipids. The current findings showed that the liver of all groups showed steatosis which ranged from mild degree in BA supplemental groups to severe steatosis, and vacuolation and swelling in the groups lack BA supplementation; these results were similar to that described by Jin et al. (2019), while the outcomes are different to those reported in striped catfish (Pangasianodon hypophthalmus) (Adam et al., 2023). These results validated that high levels of dietary BA supplementation are harmful to hepatocytes, but the exact mechanism is still unclear. According to some research studies (Dai et al., 2019; Guo et al., 2019), a diet high in fat may change how energy is metabolized, potentially leading to the storage of fat, oxidative damage to lipids, and ultimately triggering inflammation, cell death, and the generation of ROS in both fish and mammals. Boosting the levels of antioxidant enzymes such as SOD, GPX, and CAT is the main approach to combatting harmful ROS production and protecting the organism (Lesser, 2006). During this research, it was observed that a high-fat diet caused a reduction in the expression of SOD, GPX, and CAT genes, while an additional intake of BA increased this expression. These results indicated that the antioxidant system of O. niloticus was affected by a high-fat diet and that increasing BA intake helped counteract the negative effects. Comparable results were seen in large yellow croakers given high-fat diets, with dietary BA also improving the fish’s antioxidant capacity (Ding et al., 2020; Yin et al., 2021). To corroborate our finding, similar results were found by Yin et al. (2021) that in large largemouth bass fed high-lipid diets, the hepatic gene expression of SOD and GSH-Px was decreased with the high-fat diet and increased with supplemental BA, indicating that the antioxidant system of largemouth bass was damaged by high-fat diet, and the damage was alleviated by supplemental BA. In addition, the same results were detected in large yellow croaker fed high-lipid diets, in which dietary BAs also could promote the antioxidant capacity of fish (Ding et al., 2020). Therefore, the improved capacity to fight oxidation could account for the strong growth performance. The GHr and IGF-1 genes in the liver of O. niloticus showed increased expression levels in all groups given BA compared to control groups. This upregulation can be attributed to the impact of BA supplementation on weight gain. Similar outcomes were reported by Guo et al. (2019) and Romano et al. (2022), who found that dietary BA may help reduce hepatic inflammation and provide protection, as observed in M. salmoides. This research also discovered that by adding BA to the diet, there was a noteworthy increase in the mRNA expression of LPL in the liver. The findings confirmed earlier research in fish and mammals indicating that LPL serves as a significant indicator of lipid metabolism capacity (Wang and Eckel, 2009; Ding et al., 2020) and may also hinder the build-up of too much cholesterol in non-liver tissues. Lowering FAS mRNA levels decreases liver FAS, which, in turn, slows liver lipid accumulation. An elevated FAS expression reduces adipogenesis. The results were similar to those documented by Ning et al. (2016) and Ding et al. (2020). Genes linked to inflammation, such as IL-1β and TNF-α, were less active in the BA-supplemented groups than in the control groups fed a fat-supplemented diet, mirroring findings in the study by Jin et al. (2019). In this study, it was discovered that adding BA to the diet resulted in higher levels of intestinal mucin (MUC2) expression in all fish groups compared to control. This finding aligns with previous research by Sveen et al. (2017) and Wang et al. (2023a), suggesting the significance of this gene in regulating mucus production in various environmental conditions, and also, our findings were matched with Wang et al. (2023b) that found the increase of MUC2 expression in O niloticus.
Fig. 11. Effects of dietary lipid levels and BA on the relative expression of inflammatory response such as IL-1β, TNF-α, and IL-8 in O. niloticus. Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05). The measured water quality parameters are all crucial for the health and well-being of fish in this study. Temperature and DO are essential for fish metabolism and respiration, while pH affects the toxicity of ammonia and other chemicals (Parvathy et al., 2023). TAN and UIA are waste products produced by fish, and high levels can be harmful (Gyamfi et al., 2024). The lack of significant differences in water quality parameters among the treatment groups suggests that neither dietary fat level nor BA supplementation had a major impact on the water quality in the fish pond. This is a positive finding, as it indicates that these dietary interventions can be used without compromising the health of the fish or the quality of the water. BAs are biodegradable compounds that can be broken down by bacteria present in aquatic environments (Neves et al., 2020; Doden et al., 2021). This means that when fish excrete BAs into the water after consuming feed containing them, these compounds can be naturally degraded over time. Additionally, the dilution effect of large volumes of water in fish ponds helps to further minimize any potential negative impact of BAs on water quality.
Fig. 12. Effects of dietary lipid levels and BA on the relative expression of mucin (MUC2) in O. niloticus. Data are presented as the means ± SEM. Different letters above the bars denote significant differences among the groups at the p < 0.05. Bars bearing the same letters are not significantly different (p > 0.05). Table 9. Water quality analysis of Nile tilapia feed different fat percentage with or without BA for 90 days.
ConclusionAdding BA to the diet of Nile tilapia can improve their growth rate, food efficiency, gut health, immune system, and ability to fight off oxidative stress. This is because of its beneficial impacts on the health of the digestive system, absorption of nutrients, and metabolism. BA supplementation increased digestive enzyme activity, leading to improved nutrient absorption and growth. It also enhanced immune function by increasing lysozyme activity and antioxidant capacity. Gene expression analysis showed that BA supplementation upregulated genes involved in antioxidant defense, growth, and intestinal health while downregulating genes involved in FAS and inflammation. Therefore, it is recommended to use BA as a dietary supplementation for improving tilapia growth and immune-physiological responses. AcknowledgmentsThe authors would like to thank the Department of Aquaculture, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Egypt, for providing facilities to carry out this work. Conflict of interestThe authors declare that they have no conflict of interest. FundingThis study was not financially supported by any funding organization. Authors’ contributionsAll authors contributed equally to this manuscript. Data availabilityAll relevant data are available from the authors upon request. ReferencesAanyu, M. 2016. Effects of phytogenic compounds on growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Philosopher Doctor thesis, Institute of Aquaculture, School of natural sciences, University of Stirling, Stirling, Scotland, United Kingdom, chapter2, p. 59. Abdel-Tawwab, M., Abdel-Latif, H.M., Basuini, M.F.E., El-Nokrashy, A.M., Khaled, A.A., Kord, M., Soliman, A.A., Zaki, M., Nour, A.E., Labib, E.M. and Khalil, H.S. 2023. Effects of exogenous bile acids (BAs) on growth, lipid profile, digestive enzymes, and immune responses of thinlip mullet, Liza ramada. Sci. Rep. 13(1), 22875. Abdel-Tawwab, M., Samir, F., Abd El-Naby, A.S. and Monier, M.N. 2018. Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish Immunol. 74, 19–25; doi:10.1016/j.fsi.2017.12.033 Abdo, S. E., El-Nahas, A. F., Abdelmenam, S., Elmadawy, M. A., Mohamed, R., Helal, M. A., and El-Kassas, S. 2022. The synergetic effect of Bacillus species and Yucca shidigera extract on water quality, histopathology, antioxidant, and innate immunity in response to acute ammonia exposure in Nile tilapia. Fish Shellfish Immunol. 128, 123–135. Abo-Raya, M. H., Alshehri, K. M., Abdelhameed, R. F., Elbialy, Z. I., Elhady, S. S., and Mohamed, R. A. 2021. Assessment of growth-related parameters and immune-biochemical profile of nile tilapia (Oreochromis niloticus) fed dietary ulva fasciata extract. Aquac. Res, 52(7), 3233–3246. Adam, A.H., Verdegem, M., Soliman, A.A., Zaki, M., Khalil, R.H., Nour, A.E.M., Khaled, A.A., El Basuini, M.F. and Khalil, H.S. 2023. Effect of dietary bile acids: growth performance, immune response, genes expression of fatty acid metabolism, intestinal, and liver morphology of striped catfish (Pangasianodon hypophthalmus). Aquac. Rep. 29, 101510. Ahmedou, A., Al-Hawary, I.I., Alsayadi, M., Yassine, T., Elshafey, A. and Elbialy, Z.I. 2025. Assessment of quality and safety of some imported and locally sourced fish in the Egyptian markets. Egypt. J. Vet. Sci. 56(2), 273–287; doi:10.21608/ejvs.2024.271906.1869 Arenas, M., Álvarez-González, C.A., Barreto, A., Sánchez-Zamora, A., Suárez-Bautista, J., Cuzon, G. and Gaxiola, G. 2021. Physiological and metabolic protein-sparing effects of dietary lipids on common snook Centropomus undecimalis (Bloch, 1792) juveniles. Aquac. Nutr. 27(4), 1089–1102; doi:10.1111/anu.13250 Bhusare, S., Sardar, P., Sahu, N.P., Shamna, N., Kumar, P., Paul, M., Jana, P., Raghuvaran, N. and Bhavatharaniya, U. 2023. Bile acid improves growth, lipid utilization and antioxidative status of genetically improved farmed tilapia (Oreochromis niloticus) fed with varying protein-lipid diets reared in inland saline water. Anim. Feed Sci. Technol. 303, 115677; doi:10.1016/j.anifeedsci.2023.115677 Borges, P., Oliveira, B., Casal, S., Dias, J., Conceição, L. and Valente, L.M.P. 2009. Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. Br. J. Nutr. 102(7), 1007–1014; doi:10.1017/S0007114509345262 Boujard, T., Gélineau, A., Covès, D., Corraze, G., Dutto, G., Gasset, E. and Kaushik, S. 2004. Regulation of feed intake, growth, nutrient and energy utilisation in European sea bass (Dicentrarchus labrax) fed high fat diets. Aquaculture 231(1), 529–545; doi:10.1016/j.aquaculture.2003.11.010 Całyniuk, B., Grochowska-Niedworok, E., Walkiewicz, K.W., Kawecka, S., Popiołek, E. and Fatyga, E. 2016. Malondialdehyde (MDA)–product of lipid peroxidation as marker of homeostasis disorders and aging. Ann. Acad. Med. Siles. 70, 224–228. Contreras-Zentella, M.L. and Hernández-Muñoz, R. 2016. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid. Med. Cell. Longev. 2016(1), 3529149; doi:10.1155/2016/3529149 Dai, Y.J., Cao, X.F., Zhang, D.D., Li, X.F., Liu, W.B. and Jiang, G.Z. 2019. Chronic inflammation is a key to inducing liver injury in blunt snout bream (Megalobrama amblycephala) fed with high-fat diet. Dev. Comp. Immunol. 97, 28–37. Demers, N.E. and Bayne, C.J. 1997. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 21(4), 363–373. Di Ciaula, A., Garruti, G., Baccetto, R.L., Molina-Molina, E., Bonfrate, L., Portincasa, P. and Wang, D.Q. 2018. Bile acid physiology. Ann. Hepatol. 16(1), 4–14. Ding, T., Xu, N., Liu, Y., Du, J., Xiang, X., Xu, D., Liu, Q., Yin, Z., Li, J., Mai, K. and Ai, Q. 2020. Effect of dietary bile acid (BA) on the growth performance, body composition, antioxidant responses and expression of lipid metabolism-related genes of juvenile large yellow croaker (Larimichthys crocea) fed high-lipid diets. Aquaculture 518, 734768; doi:10.1016/j.aquaculture.2019.734768 Doden, H.L., Wolf, P.G., Gaskins, H.R., Anantharaman, K., Alves, J.M. and Ridlon, J.M. 2021. Completion of the gut microbial epi-bile acid pathway. Gut Microbes. 13(1), 1907271. Doumas, B.T., Bayse, D.D., Carter, R.J., Peters Jr, T. and Schaffer, R. 1981. A candidate reference method for determination of total protein in serum. I. development and validation. Clin. Chem. 27(10), 1642–1650; doi:10.1093/clinchem/27.10.1642 Elkaradawy, A., Abdel-Rahim, M.M. and Mohamed, R.A. 2022. Quillaja saponaria and/or linseed oil improved growth performance, water quality, welfare profile and immune-oxidative status of Nile tilapia, Oreochromis niloticus fingerlings. Aquac. Res. 53(2), 576–589. El-Naggar, K., Mohamed, R., El-katcha, M.I., Abdo, S.E. and Soltan, M.A. 2021. Plant ingredient diet supplemented with lecithin as fish meal and fish oil alternative affects growth performance, serum biochemical, lipid metabolism and growth-related gene expression in Nile tilapia. Aquac. Res. 52(12), 6308–6321. El-Kassas, S., Abdo, S.E., Abosheashaa, W., Mohamed, R., Moustafa, E.M., Helal, M.A. and El-Naggar, K., 2020. Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex Nile tilapia fed Moringa oleifera leaf powder. Aquac. Rep. 18, p.100422. FAO Report. 2024. Global fisheries and aquaculture production reaches a new record high. The Food and Agriculture Organization (FAO), Rome. Available via www. Fao.org/ Ferraboschi, P., Ciceri, S. and Grisenti, P. 2021. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. J. Antibiot. 10(12), 1534. Guo, J.l., Zhou, Y.l., Zhao, H., Chen, W.Y., Chen, Y.J. and Lin, S.M. 2019. Effect of dietary lipid level on growth, lipid metabolism and oxidative status of largemouth bass, Micropterus salmoides. Aquaculture 506, 394–400. Gyamfi, S., Edziyie, R.E., Obirikorang, K.A., Adjei-Boateng, D. and Skov, P.V. 2024. Nile tilapia (Oreochromis niloticus) show high tolerance to acute ammonia exposure but lose metabolic scope during prolonged exposure at low concentration. Aquat. Toxicol. 271, 106932. Hassaan, M. S., Soltan, M. A., and Abdel-Moez, A. M. 2015. Nutritive value of soybean meal after solid state fermentation with Saccharomyces cerevisiae for Nile tilapia, Oreochromis niloticus. J. animal feed sci. technol. 201, 89–98. Heinegård, D. and Tiderström, G. 1973. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta 43(3), 305–310; doi:10.1016/0009-8981(73)90466-X Hofmann, A.F., Hagey, L.R. and Krasowski, M.D. 2010. Bile salts of vertebrates: structural variation and possible evolutionary significance [S]. J. Lipid Res. 51(2), 226–246. Jiang, M., Wen, H., Gou, G.W., Liu, T.L., Lu, X. and Deng, D.F. 2018. Preliminary study to evaluate the effects of dietary bile acids on growth performance and lipid metabolism of juvenile genetically improved farmed tilapia (Oreochromis niloticus) fed plant ingredient-based diets. Aquac. Nutr. 24(4), 1175–1183; doi:10.1111/anu.12656 Jin, M., Pan, T., Cheng, X., Zhu, T.T., Sun, P., Zhou, F., Ding, X. and Zhou, Q. 2019. Effects of supplemental dietary l-carnitine and bile acids on growth performance, antioxidant and immune ability, histopathological changes and inflammatory response in juvenile black seabream (Acanthopagrus schlegelii) fed high-fat diet. Aquaculture 504, 199–209; doi:10.1016/j.aquaculture.2019.01.06 Kashani, K., Rosner, M.H. and Ostermann, M. 2020. Creatinine: from physiology to clinical application. Eur. J. Intern. Med. 72, 9–14; doi:10.1016/j.ejim.2019.10.025 Kumar, S. and Chakravarty, S. 2018. Amylases. In Enzymes in human and animal nutrition. Eds., Nunes, C.S. and Kumar, V. pp: 163–180. Lee, S.M., Jeon, I.G. and Lee, J.Y. 2002. Effects of digestible protein and lipid levels in practical diets on growth, protein utilization and body composition of juvenile rockfish (Sebastes schlegeli). Aquaculture 211(1), 227–239; doi:10.1016/S0044-8486(01)00880-8 Lesser, M.P. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 68, 253–278. Li, J., Wang, Z., Cao, X., Wang, J., Gong, Y., Wang, X., Lai, W., Bu, X., Zheng, J., Mai, K. and Ai, Q. 2023. Effects of supplemental mixed bile acids on growth performance, body composition, digestive enzyme activities, skin color, and flesh quality of juvenile large yellow croaker (Larimichthys crocea) in soybean oil based diet. Front. Mar. Sci. 10. p.1149887. doi:10.3389/fmars.2023.1149887 Li, L., Liu, T., Li, J., Yang, Y., Liu, H. and Zhang, P. 2024. Effectiveness of bile acids as a feed supplement to improve growth performance, feed utilization, lipid metabolism, digestive enzymes, and hepatic antioxidant status in aquaculture animals: a meta-analysis. Aquac. Rep. 36, 102121. Li, T. and Apte, U. 2015. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv. Pharmacol. 74, 263–302; doi:10.1016/bs.apha.2015.04.003 Liu, Y., Li, X., Lin, J., Song, K., Li, X., Wang, L., Zhang, C. and Lu, K. 2024a. Effects of dietary supplementation of bile acids on growth, glucose metabolism, and intestinal health of spotted seabass (Lateolabrax maculatus). J. Anim. 14(9), 1299. Liu, Y., Li, X., Lin, J., Song, K., Li, X., Wang, L., Zhang, C. and Lu, K. 2024b. Effects of dietary supplementation of bile acids on growth, glucose metabolism, and intestinal health of spotted seabass (Lateolabrax maculatus). J. Anim. 14(9), 1299. Liu, H., Zhang, X., Li, K., Fang, Q., Liu, X., Sun, L., Guo, G., An, S., Li, M., Wang, G. and Kong, Y. 2024. Effects of dietary of bile acid on growth performance, antioxidant capacity, liver and intestinal health and immune function in loach (Misgurnus anguillicaudatus). Aquac. Rep. 39, 102455. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4), 402–408. Ma, Q., Li, L.Y., Le, J.Y., Lu, D.L., Qiao, F., Zhang, M.L., Du, Z.Y. and Li, D.L. 2018. Dietary microencapsulated oil improves immune function and intestinal health in Nile tilapia fed with high-fat diet. Aquaculture 496, 19–29. Macierzanka, A., Torcello-Gómez, A., Jungnickel, C. and Maldonado-Valderrama, J. 2019. Bile salts in digestion and transport of lipids. Adv. Colloid Interface Sci. 274, 102045; doi:10.1016/j.cis.2019.102045 Mallya, Y.J. 2007. The effects of dissolved oxygen on fish growth in aquaculture. The United Nations University Fisheries Training Programme, Final Project. Martins, D.A., Valente, L.M.P. and Lall, S.P. 2007. Effects of dietary lipid level on growth and lipid utilization by juvenile Atlantic halibut (Hippoglossus hippoglossus, L.). Aquaculture 263(1), 150–158; doi:10.1016/j.aquaculture.2006.10.017 Mathew, J., Sankar, P. and Varacallo, M. 2023. Physiology, blood plasma. Treasure Island, FL: StatPearls Publishing. Moss, D. and Henderson, A. 1999. Digestive enzymes of pancreatic origin. Tietz textbook of clinical chemistry, 3rd ed. Philadelphia, PA: WB Saunders Company, pp: 689–708. Moustafa, E.M., Dawood, M.A., Assar, D.H., Omar, A.A., Elbialy, Z.I., Farrag, F.A., Shukry, M. and Zayed, M.M. 2020. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 515, 734589. Naiel, M.A., Negm, S.S., Ghazanfar, S., Shukry, M. and Abdelnour, S.A. 2023. The risk assessment of high-fat diet in farmed fish and its mitigation approaches: a review. J. Anim. Physiol. Anim. Nutr. 107(3), 948–969. Neves, A.R., Almeida, J.R., Carvalhal, F., Camara, A., Pereira, S., Antunes, J., Vasconcelos, V., Pinto, M., Silva, E.R. and Sousa, E. 2020. Overcoming environmental problems of biocides: synthetic bile acid derivatives as a sustainable alternative. Ecotoxicol. Environ. Saf. 187, 109812. Ning, L.J., He, A.Y., Li, J.M., Lu, D.L., Jiao, J.G., Li, L.Y., Li, D.L., Zhang, M.L., Chen, L.Q. and Du, Z.Y. 2016. Mechanisms and metabolic regulation of PPARα activation in Nile tilapia (Oreochromis niloticus). Biochim. Biophys. Acta. 1861(9), 1036–1048. Parvathy, A.J., Das, B.C., Jifiriya, M.J., Varghese, T., Pillai, D. and Rejish Kumar, V.J. 2023. Ammonia induced toxico-physiological responses in fish and management interventions. Rev. Aquac. 15(2), 452–479. Peng, X.R., Feng, L., Jiang, W.D., Wu, P., Liu, Y., Jiang, J., Kuang, S.Y., Tang, L. and Zhou, X.Q. 2019. Supplementation exogenous bile acid improved growth and intestinal immune function associated with NF-κB and TOR signalling pathways in on-growing grass carp (Ctenopharyngodon idella): enhancement the effect of protein-sparing by dietary lipid. Fish Shellfish Immunol. 92, 552–569. Perego, C., Da Dalt, L., Pirillo, A., Galli, A., Catapano, A.L. and Norata, G.D. 2019. Cholesterol metabolism, pancreatic β-cell function and diabetes. Biochim. Biophys. Acta. 1865(9), 2149–2156; doi:10.1016/j.bbadis.2019.04.012 Ponzoni, R.W., Nguyen, N.H., Khaw, H.L., Hamzah, A., Bakar, K.R.A. and Yee, H.Y. 2011. Genetic improvement of Nile tilapia (Oreochromis niloticus) with special reference to the work conducted by the worldfish center with the GIFT strain. Rev. Aquac. 3(1), 27–41; doi:10.1111/j.1753-5131.2010.01041.x Qiang, J., He, J., Yang, H., Wang, H., Kpundeh, M., Xu, P., Zhu, Z. 2014. Temperature modulates hepatic carbohydrate metabolic enzyme activity and gene expression in juvenile GIFT tilapia (Oreochromis niloticus) fed a carbohydrate-enriched diet. J. Therm. Biol. 40, 25–31. Refstie, S., Korsøen, Ø.J., Storebakken, T., Baeverfjord, G., Lein, I. and Roem, A.J. 2000. Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture 190(1–2), 49–63. Reitman, S. and Frankel, S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28(1), 56–63; doi:10.1093/ajcp/28.1.56 Romano, N., Fischer, H., Rubio-Benito, M.M., Overtuf, K., Sinha, A.K. and Kumar, V. 2022. Different dietary combinations of high/low starch and fat with or without bile acid supplementation on growth, liver histopathology, gene expression and fatty acid composition of largemouth bass, Micropterus salmoides. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 266, 111157. Romano, N., Kumar, V., Yang, G., Kajbaf, K., Rubio, M.B., Overturf, K., Brezas, A. and Hardy, R. 2020. Bile acid metabolism in fish: disturbances caused by fishmeal alternatives and some mitigating effects from dietary bile inclusions. Rev. Aquac. 12(3), 1792–1817; doi:10.1111/raq.12410 Sallam, A.E., Mansour, A.T., Srour, T.M. and Goda, A.M.A. 2017. Effects of different carotenoid supplementation sources with or without sodium taurocholate on growth, feed utilization, carotenoid content and antioxidant status in fry of the European seabass, Dicentrarchus labrax. Aquac. Res. 48(7), 3848–3858; doi:10.1111/are.13212 Sveen, L.R., Grammes, F.T., Ytteborg, E., Takle, H. and Jørgensen, S.M. 2017. Genome-wide analysis of Atlantic salmon (Salmo salar) mucin genes and their role as biomarkers. PLos One 12(12), e0189103. Thrall, M., Baker, D. and Lassen, E. 2004. Veterinary haematology and clinical chemistry. Philadelphia, PA: Lippincott Williams and Wilkins. Tibbetts, S.M., Lall, S.P. and Milley, J.E. 2005. Effects of dietary protein and lipid levels and DP DE−1 ratio on growth, feed utilization and hepatosomatic index of juvenile haddock, Melanogrammus aeglefinus L. Aquac. Nutr. 11(1), 67–75; doi:10.1111/j.1365-2095.2004.00326.x Ticho, A.L., Malhotra, P., Dudeja, P.K., Gill, R.K. and Alrefai, W.A. 2019. Intestinal absorption of bile acids in health and disease. Compr. Physiol. 10(1), 21–56; doi:10.1002/cphy.c190007 Tocher, D.R. 2003. Metabolism and functions of lipids and fatty acids in Teleost Fish. Rev. Fish. Sci. 11(2), 107–184; doi:10.1080/713610925 Trinder, P. 1969. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 6(1), 24–27. Tvrdá, E. and Benko, F. 2020. Free radicals: what they are and what they do. In Pathology. Ed. Preedy, V.R. Amsterdam, The Netherlands: Elsevier, pp:3–13. Valero, Y., Saraiva-Fraga, M., Costas, B. and Guardiola, F.A. 2020. Antimicrobial peptides from fish: beyond the fight against pathogens. Rev. Aquac. 12(1), 224–253. Vourakis, M., Mayer, G. and Rousseau, G. 2021. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 22(15), 8074. Wang, H. and Eckel, R.H. 2009. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297(2), E271–E288. Wang, L., Sagada, G., Wang, C., Liu, R., Li, Q., Zhang, C. and Yan, Y. 2023a. Exogenous bile acids regulate energy metabolism and improve the health condition of farmed fish. Aquaculture 562, 738852; doi:10.1016/j.aquaculture.2022.738852 Wang, T., Zhou, N., He, J., Hao, Z., Zhou, C., Du, Y. and Du, Z. 2023b. Xylanase improves the intestinal barrier function of Nile tilapia (Oreochromis niloticus) fed with soybean (Glycine max) meal. J. Anim. Sci. Biotechnol. 8, 1–15; doi:10.1186/s40104-023-00883-8 Wells, R.M.G., Baldwin, J., Seymour, R.S., Baudinette, R.V., Christian, K. and Bennett, M.B. 2003. Oxygen transport capacity in the air-breathing fish, Megalops cyprinoides: compensations for strenuous exercise. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 134(1), 45–53; doi:10.1016/S1095-6433(02)00179-4 Weydert, C.J. and Cullen, J.J. 2010. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 5(1), 51–66. Yeo, X.Y., Tan, L.Y., Chae, W.R., Lee, D.Y., Lee, Y.A., Wuestefeld, T. and Jung, S. 2023. Liver’s influence on the brain through the action of bile acids. Front. Neurosci. 17, 1123967; doi:10.3389/fnins.2023.1123967 Yilmaz, E. 2020. Effect of dietary carob (Ceratonia siliqua) syrup on blood parameters, gene expression responses and ammonia resistance in tilapia (Oreochromis niloticus). Aquac. Res, 51(5), 1903–1912. Yin, P., Xie, S., Zhuang, Z., He, X., Tang, X., Tian, L., Liu, Y. and Niu, J. 2021. Dietary supplementation of bile acid attenuate adverse effects of high-fat diet on growth performance, antioxidant ability, lipid accumulation and intestinal health in juvenile largemouth bass (Micropterus salmoides). Aquaculture 531, 735864. Zhao, Y., Wang, C., Li, Y.L., Li, X.J., Ding, H.Y., Liu, C.N., Zhang, L.M., Ji, X.S., Yu, L.P., Qiu, Z.W., Meng, L.D., Huang, Q.B. and Wang, H. 2024. Effects of bile acids supplementation in feed on digestive capacity, antioxidant capacity, lipid metabolism, and intestinal microorganisms in Yellow River carp (Cyprinus carpio). Aquac. Rep. 36, 102096; doi:10.1016/j.aqrep.2024.102096 | ||
| How to Cite this Article |
| Pubmed Style Yuan Y, Omar AA, Emam W, Mohamed RA. Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus). Open Vet. J.. 2025; 15(1): 222-243. doi:10.5455/OVJ.2025.v15.i1.21 Web Style Yuan Y, Omar AA, Emam W, Mohamed RA. Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus). https://www.openveterinaryjournal.com/?mno=218725 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.21 AMA (American Medical Association) Style Yuan Y, Omar AA, Emam W, Mohamed RA. Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus). Open Vet. J.. 2025; 15(1): 222-243. doi:10.5455/OVJ.2025.v15.i1.21 Vancouver/ICMJE Style Yuan Y, Omar AA, Emam W, Mohamed RA. Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus). Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 222-243. doi:10.5455/OVJ.2025.v15.i1.21 Harvard Style Yuan, Y., Omar, . A. A., Emam, . W. & Mohamed, . R. A. (2025) Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus). Open Vet. J., 15 (1), 222-243. doi:10.5455/OVJ.2025.v15.i1.21 Turabian Style Yuan, Yi, Amira A. Omar, Wasseem Emam, and Radi A. Mohamed. 2025. Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus). Open Veterinary Journal, 15 (1), 222-243. doi:10.5455/OVJ.2025.v15.i1.21 Chicago Style Yuan, Yi, Amira A. Omar, Wasseem Emam, and Radi A. Mohamed. "Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus)." Open Veterinary Journal 15 (2025), 222-243. doi:10.5455/OVJ.2025.v15.i1.21 MLA (The Modern Language Association) Style Yuan, Yi, Amira A. Omar, Wasseem Emam, and Radi A. Mohamed. "Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus)." Open Veterinary Journal 15.1 (2025), 222-243. Print. doi:10.5455/OVJ.2025.v15.i1.21 APA (American Psychological Association) Style Yuan, Y., Omar, . A. A., Emam, . W. & Mohamed, . R. A. (2025) Impact of dietary inclusion of bile acid and fat percent on growth, intestinal histomorphology, immune-physiological and transcriptomic responses of Nile tilapia (Oreochromis niloticus). Open Veterinary Journal, 15 (1), 222-243. doi:10.5455/OVJ.2025.v15.i1.21 |