| Research Article | ||
Open Vet. J.. 2025; 15(1): 211-221 Open Veterinary Journal, (2025), Vol. 15(1): 211-221 Research Article Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbitsRana Suhail AL-Juwary1* and Muntaha Mahmood AL-Kattan21College of Education for Girls/Biology, University of Mosul, Mosul, Iraq 2College of Science/Biology, University of Mosul, Mosul, Iraq *Corresponding Author: Rana Suhail AL-Juwary. College of Education for Girls/Biology, University of Mosul, Mosul, Iraq. Email: ranasuhail [at] uomosul.edu.iq Submitted: 03/09/2024 Accepted: 15/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
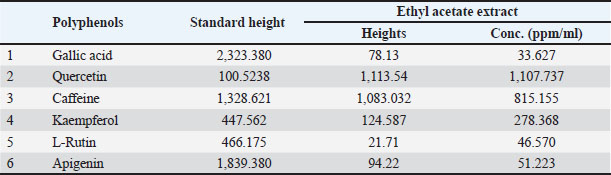
AbstractBackground: Hepatic steatosis is the second most prevalent liver disease following viral hepatitis, and its incidence is increasing rapidly, posing a significant threat to human health. Aim: This research aimed to identify natural treatment methods that reduce the risk of experimental hepatic steatosis with triton by answering the following questions: 1. Do polyphenols extracted from strawberries reduce the risk of experimental hepatic steatosis in rabbits? 2. Do omega-3 extracted from almonds reduce the risk of experimental hepatic steatosis in rabbits? 3. Is giving Omega-3, Vit. E considered the optimal treatment for hepatic steatosis? Methods: This study aimed to assess the effects of strawberry polyphenols, omega-3 from almonds, omega-3, and vitamin E on reducing the risk of Triton-induced liver steatosis. The experiment involved 100 male rabbits (10–12 months old, 1,150–1,350 g) divided randomly into the following 10 groups: control group, Triton (300 mg/kg body mass) group, strawberry polyphenols group, strawberry polyphenols with Triton group, almond Omega-3 group, almond Omega-3 with Triton group, strawberry polyphenols with almond Omega-3 group, strawberry polyphenols with almond Omega-3 with Triton group, Omega-3, Vit. E group, and Omega-3, Vit. E with Triton group. Dosing occurred daily for 4 months. Results: The results of the study indicated a significant increase in the levels of Triglycerides, Total cholesterol, Low-density lipoprotein-cholesterol, and Very low-density lipoprotein-cholesterol in the group of rabbits treated with Triton compared with the other groups, while these parameters decreased in the groups treated with strawberry polyphenols, omega-3 from almonds, omega-3, and vitamin E. In addition, the levels of High-density lipoprotein-cholesterol, Paroxinase-1 activity, and apelin concentration decreased in the group treated with Triton but increased in the groups receiving strawberry polyphenols, omega-3 from almonds, omega-3, and vitamin E, at a probability level (p ≤ 0.01). Conclusion: These results suggest that polyphenols extracted from strawberries, Omega-3 extracted from almonds, and Omega-3, Vit. E is a suitable treatment for hepatic steatosis. Keywords: Almond, Apelin, Fatty liver, PON-1, Strawberry, Triton. IntroductionLiver steatosis is the second most prevalent liver condition following viral hepatitis. The incidence of hepatic steatosis is rising sharply, presenting a major health risk. Hepatic steatosis encompasses various pathological issues, including cirrhosis and liver cancer, and can progress to steatohepatitis. Fortunately, hepatic steatosis is typically regarded as a reversible disease, as it is often possible to return the liver to its normal condition (Khan and Khan, 2022). Hepatic steatosis is a major public health concern, impacting 25% of the global population (Teng et al., 2023). Hepatic steatosis can progress from fat buildup in the liver to more serious conditions, such as nonalcoholic steatohepatitis, cirrhosis, and potentially liver cancer. Moreover, it increases the likelihood of developing a condition characterized by high blood glucose levels and heart-related conditions, contributing to a high overall mortality rate (Ghazanfar et al., 2024). Although insufficient physical exercise, excessive fat accumulation in the abdominal area, insulin resistance, and associated immune response are considered major causes of hepatic steatosis development, our understanding of the causes of this disease and the mechanisms underlying its development remains incomplete, limiting the available therapeutic options (Jeong, 2020). Although only a small percentage (5%) of patients with fatty liver progress to cirrhosis, hepatic steatosis is recognized as the most prevalent underlying factor for chronic liver disorders, largely as a result of the widespread increase in obesity (Han et al., 2023). The liver stores energy as glycogen and triglycerides, but in situations of obesity, overnutrition, or liver-damaging medications, excess fat builds up in liver cells from prolonged fat storage, leading to the formation of hepatic steatosis (Heeren and Scheja, 2021). In relation to liver diseases, oxidative stress-induced loss of liver function encompasses various conditions, including hepatotoxicity (Ramachandran and Jaeschke, 2018), liver ischemia-reperfusion injury (Forman and Zhang, 2021), nonalcoholic fatty liver disease (NAFLD) (Gonzalez et al., 2020), and hepatocellular carcinoma (Li et al., 2023). These conditions are linked to excessive free radical production, which can cause systemic imbalances and disruptions in organ communication, potentially leading to severe outcomes or even death (Asrani et al., 2019). PON1 exhibits lactonase and ester hydrolase activities, has the ability to hydrolyze a variety of organic molecules such as thiolactone, unsaturated aliphatic esters, aromatic carboxylic esters, and carbamates, and has important roles in various biochemical pathways such as protection against oxidative damage and lipid peroxidation, contribution to innate immunity, detoxification of reactive molecules, bioactivation of drugs, modulation of endoplasmic reticulum stress, and regulation of cell proliferation/apoptosis. Due to its ability to perform multiple independent and often unrelated functions, it is considered a “satellite protein” (Kulka, 2016; Taler-Verčič et al., 2020). Apelin is a peptide released from fat tissue that contributes to maintaining energy homeostasis, glucose metabolism, and insulin sensitivity. Recent studies have suggested that apelin could serve as a biological marker for diagnosing or tracking the development of these conditions. It might also be the focus of upcoming treatments intended to enhance metabolism and reduce inflammation associated with NAFLD and diabetes (Murali and Aradhyam, 2023). Materials and MethodsThe study involved 100 male rabbits obtained from local markets in the Kogjali area of Mosul. Their health and disease-free status were verified through periodic examinations by veterinarians. The rabbits were housed in locally made cages measuring 100, 75, and 65 cm in length and width. Ten groups were formed out of them, each consisting of 10 rabbits. The temperature in the environment was maintained between 26°C and 29°C with, a 14-hour light cycle each day. The cages were well-ventilated, and the floors were covered with coarse sawdust to absorb moisture. The sawdust was replaced daily, and continuous sterilization was performed to ensure that the cages remained clean and free from contamination and disease. Rabbits underwent a 1-month acclimation period to adjust to their new environment and the standard rabbit feed. Feed was provided in equal quantities and a fixed sequence, with regular water in special containers fixed to the cage to prevent water leakage. The rabbits were given unrestricted access to food and water. Green, feed was provided continuously throughout the treatment period. Plants used in the study1. Strawberry fruits: Strawberry fruits were collected from the nurseries of Agriculture and Forestry College/Mosul University in February 2024, washed with tap water to get rid of dust with the addition of a little vinegar, dried with a piece of cloth, left for half an hour to dry well at room temperature, cut into thin slices, placed on special paper for drying, placed in an electric oven with a warm air current at a temperature of 27ºC–30ºC and turned from time to time for 24 hours, ground to obtain strawberry powder, and placed in a glass container away from moisture and sunlight until needed. Identification of standard phenolic compounds by high-performance liquid chromatography (HPLC)From the chromatographic analytical charts obtained, the retention time of each of the standard compounds was determined for six compounds including gallic acid with a standard retention time of 4.028 minutes; quercetin with a standard retention time of 4.839 minutes; caffeine with a standard retention time of 5.790 minutes; kaempferol with a standard retention time of 7.925 minutes; L-rutin with a standard retention time of 2.252 minutes; and apigenin with a standard retention time of 3.044 minutes at a wavelength of 280 nm. Table 1 displays the presence of several phenolic compounds in the extract, including Gallic acid with a height of 78.13, Quercetin at 1,113.54, Caffeine at 1,083.032, Kaempferol at 124.587, L-Rutin at 21.71, and Apigenin at 94.22. These measurements highlight the extract’s quantitative and qualitative composition. Identification of a number of standard phenolic compounds from strawberry fruits, quantitatively and qualitativelyFigure 1 displays the phenolic components of strawberry fruits in an ethyl acetate extract. It should be noted that the reference concentrations of the phenolic compounds used in this study are 1,000 ppm. 2. Almonds: Almonds were sourced from the Choman/Haj Omran area in Erbil Governorate. They were ground into powder and stored in a glass container until needed. Preparation of plant extractsPlant extracts were prepared following the method mentioned by Al-Lahibi (2022); Zidane (2023) based on the characteristics of compounds extracted from the plants as well as the kind of solvent used throughout the procedure of separation and by using the sequence of solvents system extraction, as ethyl acetate was used in the extraction process for strawberries, while petroleum ether was used for almonds. Then, a rotary vacuum evaporateator was used to concentrate the extracts at 20ºC lower than the boiling point of each solvent used. Separation and purification of phenols from strawberry fruits by acid hydrolysisPhenolic compounds are typically not present in their free form but are bound to sugars such as glycosides within plants. To obtain pure phenols and accurately identify them, an acid hydrolysis process is necessary to break glycosidic bonds, releasing the phenols from sugar (Arthur, 1972; Al-Daody, 1998). Using this process, phenols isolated and purified from strawberries, and polyphenols were identified using HPLC technology, following the method described by Harborne (1998); Behbahani et al., (2011); Al-Lahibi (2022). The concentration of the phenolic compound is separated by the following law:
Fatty acid extraction from almonds using saponificationSaponification was performed for extraction-free fatty acids (Omega-3) following the method described by Al-Jaghfi (2022). Omega-3 fatty acids were then diagnosed and purified using HPLC according to the technique outlined (Al-Daody, 1998). Experimental designThis is a preliminary study to determine the optimal and most effective dose of polyphenols extracted from strawberries and Omega-3 extracted from almonds daily. We used 100 male Oryctolagus cuniculus rabbits in this research, and they were randomly divided into 10 groups, with each group including 10 rabbits. Triton was acquired from the central store of the University of Mosul, and a Triton solution was prepared at a concentration of 300 mg/kg of body mass.
Table 1. Height and concentrations of phenolic compounds identified using HPLC analysis of strawberry fruit.
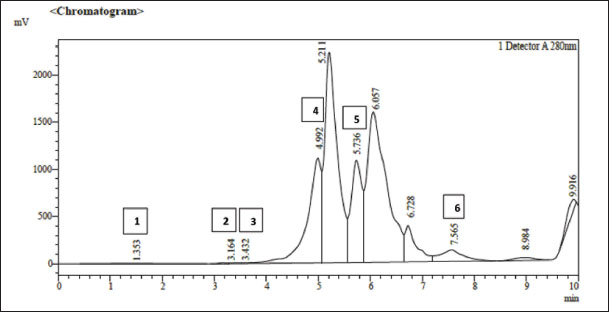
Fig. 1. HPLC chromatography of the ethyl acetate extract of strawberry fruits, which is characterized by distinct peaks corresponding to gallic acid, quercetin, caffeine, kaempferol, l-rutin, and apigenin, where the peak heights and concentrations (ppm/ml) are provided, which illustrates the quantitative and qualitative characteristics of the extract composition. 1. L-Rutin 2. Apigenin 3. Gallic acid 4. Quercetin 5. Caffiene 6. Kaempferol. Sample collectionAfter 4 months of treatment, blood samples were collected from rabbits by performing a cardiac puncture with a 6 ml medical syringe. Blood was placed into tubes without any anticoagulant ingredients and sealed with tight yellow caps. The tubes were then maintained at room temperature for 20 minutes or until blood was clotted (Wilson et al., 1972). Biochemical testsThe total lipid profiles were measured using an automatic chemistry analyzer (Smart-120) at 450 nm for male rabbit blood serum samples, including Triglycerides (TG), Total cholesterol (TC), Low-density lipoprotein-cholesterol (LDL-c), Very low-density lipoprotein-cholesterol (VLDL-c), and High-density lipoprotein-cholesterol (HDL-c). Estimation of PON-1 enzymatic activityThe concentration of Paroxinase-1 (PON-1) enzyme in the serum of treated and untreated rabbits (male) was estimated using a readymade analysis kit from Rel Assay Diagnostics (Clinical chemistry solutions) based on the colorimetric method mentioned in Marchegiani et al. (2008). Hormonal testsThe concentration of apelin in rabbit blood serum was measured using a premade analysis kit from the Chinese BT LAB Bioassay Technology Laboratory, using the ELISA technique. Analytical statisticsThe outcomes of all laboratory examinations were studied to determine the mean and standard deviation according to the simple experimental system and use the complete randomized design. The comparison involving the treated rabbit groups and the control group applied Duncan’s multiple range test with the aid of the statistical analysis system program, as the coefficients took different significant letters at the probability level (p ≤ 0.01) (Antar and Al-Wakaa, 2017). The graphs were drawn using Excel 2010 software.
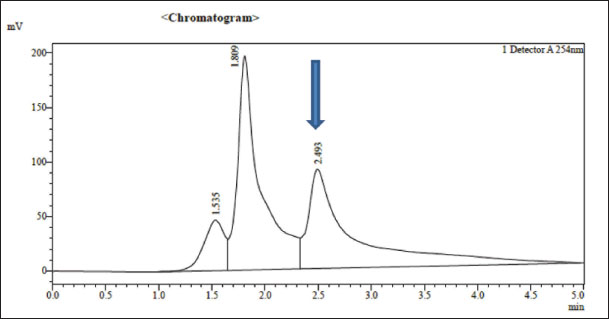
Fig. 2. Standard curve of Omega-3, as determined by HPLC.
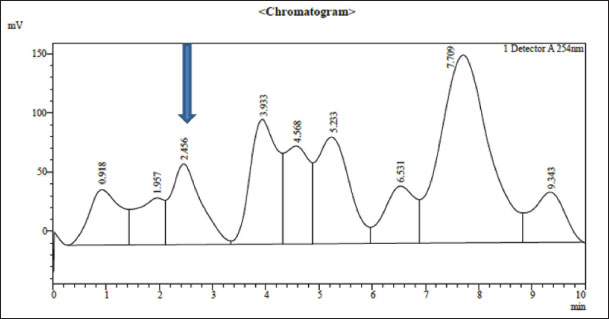
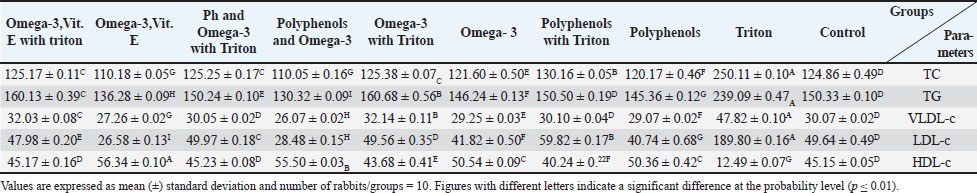
Fig. 3. Omega-3 compound of almond separated from petroleum ether extract and characterized using HPLC. Ethical approvalThe research was approved by the Institutional Animal Ethics Committee (IACUC) at the Mosul University, Veterinary Medicine College, UM.VET.2023.064. ResultsOmega-3 concentration in almonds was determined using HPLC to determine its quantity and qualityUnsaturated fatty acids are easily degraded by the solvent used (petroleum ether). Chromatographic analysis charts were obtained in which the retention time of fatty acid (Omega-3) of the study sample was determined compared with the retention time of the standard sample. The diagnosis showed that Omega-3 in the sample was almost identical to the standard fatty acid as in Figures 2 and 3. Omega-3 appeared with a standard retention time of 2.493 minutes, whereas the retention time of Omega-3 in the study sample was 2.456 minutes in the petroleum ether extract at a wavelength of 254 nm. The Omega-3 concentration of the study sample was 40.34 mg/ml compared with the standard solution concentration of 54 mg/ml. Estimation of TC levels in the blood of white rabbitsTable 2 shows a significant increase in TC concentration of TC in the group of rabbits treated with Triton, with an arithmetic mean of (250.11 ± 0.10) mg100 ml compared to the other groups at a probability degree of p ≤ 0.01, whereas the mean of the rabbits group treated with polyphenols extracted from strawberries with Triton was (130.16 ± 0.05) mg100 ml, followed by the group of rabbits treated with Omega-3 extracted from almonds with Triton, the group of rabbits treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds with Triton, and the group of rabbits treated with Omega-3, Vit. E with Triton, with the arithmetic means of (125.38 ± 0.07), (125.25 ± 0.17), and (125.17 ± 0.11) mg100 ml, respectively, showing no significant differences among these groups. The mean of the control group was (124.86 ± 0.49) mg100 ml, while the mean of the rabbits group treated with Omega-3 extracted from almonds was (121.60 ± 0.50) mg100 ml, and the mean of the rabbits group treated with polyphenols extracted from strawberries was (120.17 ± 0.46) mg100 ml. The lowest mean value was for the rabbit group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds and the rabbit group treated with Omega-3 and Vitamin E (110.05 ± 0.16) and (110.18 ± 0.05) mg100 ml, respectively, and these two groups did not differ significantly from one another. Estimation of triglyceride levels in the blood of white rabbitsTable 2 shows a significant increase in TC concentration in rabbits treated with Triton, with an arithmetic mean of (239.09 ± 0.47) mg100 ml, compared to the other groups at a probability degree of (p ≤ 0.01). The mean for rabbits treated with Omega-3 extracted from almonds with Triton was (160.68 ± 0.56) mg100 ml, whereas for rabbits treated with Omega-3 and Vit. E with Triton, it was (160.13 ± 0.39) mg100 ml. The means for the control group and the group treated with polyphenols extracted from strawberries with Triton were (150.33 ± 0.10) and (150.50 ± 0.19) mg100 ml, respectively, and there was no discernible difference between the two groups. Average of rabbits treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds with Triton was (150.24 ± 0.10) mg100 ml, the mean of the rabbits group treated with Omega-3 extracted from almonds was (146.24 ± 0.13) mg100 ml, and the mean of the rabbits group treated with polyphenols extracted from strawberries was (145.36 ± 0.12) mg100 ml, while the arithmetic mean of the group of rabbits treated with Omega-3,Vit. E was (136.28 ± 0.09) mg100 ml. The lowest mean was for the rabbit group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds at (130.32 ± 0.09) mg100 ml. Estimation of LDL-c in serum of white rabbitsTable 2 shows a significant increase in LDL-c concentration in the rabbit group handled with Triton at a mean of (189.80 ± 0.16) mg100 ml compared with the other groups and at a probability degree (p ≤ 0.01), followed by the group of rabbits treated with polyphenols extracted from strawberries with Triton at an arithmetic mean of (59.82 ± 0.17) mg100 ml, and the mean of rabbit group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds with Triton was (49.97 ± 0.18) mg100 ml, while the mean of the control group and rabbit group treated with Omega-3 extracted from almonds with Triton was (49.64 ± 0.49) and (49.56 ± 0.35) mg100 ml, respectively, and there were no notable variations between these two groups. The mean of the rabbit group treated with Omega-3, Vit. E with Triton was (47.98 ± 0.20) mg100 ml, whereas the mean of the rabbits group treated with Omega-3 extracted from almonds was (41.82 ± 0.50) mg100 ml. The mean of the rabbit group treated with polyphenols extracted from strawberries was (40.74 ± 0.68) mg100 ml, while the mean of the rabbits group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds was (28.48 ± 0.15) mg100 ml, whereas the lowest mean was in the rabbit group treated with Omega-3, Vit. E (26.58 ± 0.13) mg100 ml. Estimation of VLDL-c levels in the serum of white rabbitsTable 2 demonstrates a notable rise in VLDL-c in the rabbit group treated with Triton, at a mean of (47.82 ± 0.10) mg100 ml relative to the other groups with a probability degree (p ≤ 0.01), followed by the rabbit group treated with Omega-3 extracted from almonds with Triton, at an arithmetic mean of (32.14 ± 0.11) mg100 ml, while the mean of rabbit group treated with Omega-3, Vit. E with Triton was (32.03 ± 0.08) mg100 ml. The mean of the control group, the rabbit group treated with polyphenols extracted from strawberry with Triton, and the rabbit group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds with Triton were (30.07 ± 0.02), (30.10 ± 0.04), and (30.05 ± 0.02) mg100 ml, respectively, and these groups did not differ significantly from one another. Table 2. The effect of treatment with polyphenols extracted from strawberries, Omega-3 extracted from almonds, and Omega-3 with Vit. E on (TC, TG, LDL-c, VLDL-c, HDL-c) (mg100 ml) in serum of healthy male New Zealand white rabbits and those with experimentally induced liver steatosis.
The mean of the rabbit group treated with Omega-3 extracted from almonds was (29.25 ± 0.03) mg100 ml, while the mean of the rabbit group treated with polyphenols extracted from strawberries was (29.07 ± 0.02) mg100 ml, whereas the mean of the rabbit group treated with Omega-3,Vit. E was (27.26 ± 0.02) mg100 ml. Finally, the lowest mean was for the rabbit group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds, where its amount reached (26.07 ± 0.02) mg100 ml. Estimation of HDL-c in serum of white rabbitsTable 2 illustrates a significant increase in HDL-c in rabbits treated with Omega-3, Vit. E at an arithmetic mean of (56.34 ± 0.10) mg100 ml compared to the other groups and at a probability degree (p ≤ 0.01), followed by the group of rabbits treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds at an arithmetic mean of (55.50 ± 0.03) mg100 ml and then the rabbits group treated with polyphenols extracted from strawberries and rabbits group treated with Omega-3 extracted from almonds with the arithmetic means of (50.36 ± 0.42) and (50.54 ± 0.09) mg100 ml, respectively, and these two groups did not differ significantly from one another. The mean of the control group and the treated group of rabbits with polyphenols extracted from strawberries and Omega-3 extracted from almonds with Triton and the mean of the rabbits group treated with Omega-3,Vit. E with Triton are (45.15 ± 0.05) and (45.23 ± 0.08) and (45.17 ± 0.16) mg100 ml, respectively, and between these three groups, there were no discernible differences. The mean of the rabbit group treated with Omega-3 extracted from almonds with Triton was (43.68 ± 0.41) mg100 ml, and then, the group of polyphenols extracted from strawberries with Triton had an arithmetic mean of (40.24 ± 0.22) mg100 ml, while the lowest mean was in the rabbit group treated with Triton was (12.49 ± 0.07) mg100 ml. Estimation of PON-1 enzyme activity in the serum of white rabbitsTable 3 shows a significant increase in PON-1 activity in the rabbits group given polyphenols extracted from strawberries and Omega-3 extracted from almonds, as well as the group of rabbits treated with Omega-3, Vit. E, with the arithmetic averages of (35.48 ± 0.22) and (35.60 ± 0.10) IUl, respectively, compared to the other groups at a probability degree (p ≤ 0.01), and there were not any notable variations between these two groups, followed by rabbits group given Omega-3 extracted from almonds at an arithmetic average of (32.07 ± 0.44) IUl, while the arithmetic average for the group of rabbits treated with polyphenols extracted from strawberries was (31.62 ± 0.96) IUl. After that, the rabbits group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds with Triton followed by the group of rabbits treated with Omega-3, Vit. E with Triton with arithmetic means (29.18 ± 0.57) and (28.95 ± 0.03) IUl, respectively, these two groups did not differ significantly from one another, while mean of control group was (28.75 ± 0.14) IUl, and mean of rabbits group given polyphenols extracted from strawberries with Triton and the group of rabbits treated with Omega-3 extracted from almonds with Triton was (28.13 ± 0.04) and (28.12 ± 0.03) IUl, respectively, these two groups did not differ significantly from one another, whereas lowest mean was in the group of rabbits treated with Triton an arithmetic mean of (20.41 ± 0.23) IUl. Table 3. Effect of treatment with polyphenols extracted from strawberries and almonds, and Omega-3, Vit. E on the activity of the PON-1 (IUl) in serum of healthy male New Zealand white rabbits and experimentally induced hepatic steatosis.
Apelin levels in the serum of white rabbitsTable 4 illustrates a notable increase in apelin in the rabbit group given polyphenols extracted from strawberries and Omega-3 extracted from almonds with an arithmetic mean of (252.35 ± 3.53) ng/l compared with the rest of the groups at probability degree (p ≤ 0.01). The arithmetic mean of the control group was (175.82 ± 2.13) ng/l, followed by the rabbits treated with polyphenols extracted from strawberries, rabbits given omega-3 extracted from almonds, and rabbits given omega-3, Vit. E with means of (170.13 ± 0.80), (170.90 ± 0.02), and (170.34 ± 0.06) ng/l, respectively, between these prior groups, there were no notable differences, while the mean of rabbit group treated with polyphenols was strawberry extract with Triton (134.94 ± 2.38) ng/l, while the arithmetic mean in the rabbit group treated with Omega-3 extracted from almonds with Triton was (132.77 ± 1.07) ng/l. While the arithmetic mean for the rabbit group treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds with Triton was (170.13 ± 0.02) ng/l, the mean of the rabbit group given Omega-3, Vit. E with Triton was (131.83 ± 0.83) ng/l, and the lowest arithmetic mean was for the group of rabbits treated with Triton, which amounted to (130.44 ± 0.18) ng/l. DiscussionThe observed increase in TG, TC, LDL-c, and VLDL-c but decrease in the concentration of HDL-c in rabbits treated with Triton at the probability degree (p ≤ 0.01) may be due to the mechanism by which Triton works, as it acts as a toxic substance for cells and works to accumulate free radicals. As a non-ionic surfactant, it accelerates cholesterol synthesis by stimulating the enzyme fatty acyl-CoA oxidase, initiating breakdown of fatty acids, and enhancing the absorption of intestinal fats through the emulsification process (Devi and Singh, 2017), As it inhibits the activity of the enzyme lipase and prevents lipoproteins absorption from the bloodstream by tissues outside of the liver (Kumar et al., 2010), it causes the amount of fat in the blood to rise. It is used to raise blood cholesterol and is a risk factor for vascular disorders (Berberich and Hegele, 2022). Table 4. Effect of treatment with polyphenols extracted from strawberries, Omega-3 extracted from almonds, and Omega-3, Vit. E on apelin (ng/l) in serum of healthy male New Zealand white rabbits and experimentally induced hepatic steatosis.
The levels of TG, TC, LDL-c, and VLDL-c were inversely correlated with the levels of HDL-c. The concentration of HDL-c falls as the concentrations of TG, TC, LDL-c, and VLDL-c increase. The ability of polyphenols extracted from strawberries to lower TG, TC, LDL-c, and VLDL-c levels while increasing HDL-c levels might be attributed to their capacity to facilitate cholesterol excretion from the intestine and promote the oxidation of cholesterol into bile acids for excretion in bile juice (Martin et al., 1996; Al-Saadon & Al-Hadi, 2013). A similar effect is seen with Omega-3 extracted from almonds, which acts as anticancer and anti-inflammatory agents, improving cardiovascular health due to their antioxidant properties. Omega-3 works synergistically with Vit. E and enhances antioxidant defense capacity. Vit. E and polyphenols protect LDL-c from Cu+2-induced oxidation to a greater extent, potentially offering more significant oxidative protection than anticipated (Singar et al., 2024). The ability of Omega-3 and Vit. E to decrease TG, TC, LDL-c, and VLDL-c levels can be attributed to their antioxidant properties. When used together, Omega-3 and Vit. E acts synergistically as an antioxidant that scavenges free radicals. Omega-3 has unique properties that offer significant health benefits. After esterification, Omega-3 can integrate into the phospholipid bilayer, where it interacts with surrounding phospholipids, leading to changes in lipid rafts, affecting oxidation rates and signal transduction pathways. This process helps reduce the accumulation of cholesterol and triglycerides in the cell membrane and blood serum, reflecting lower concentrations of VLDL-c and LDL-c. There is an inverse relationship between these variables and HDL-c, where there is a rise in HDL-c levels because of treatment with Omega-3 and Vitamin E results in decreased levels of TC, TG, LDL-c, VLDL-c, and Omega-3 with Vit. The function of Vit. E is similar to that of eicosapentaenoic acid, leading to the reduced levels of TG,TC, LDL-c, and VLDL-c and increased HDL-c (Al-Hadidy et al.,2008; Mason et al., 2016). Vit. E also contains an unsaturated isoprenoid side chain, enhancing its antioxidant activity. This side chain allows Vit. E enables it to partially move through lipid membranes and accept electrons more readily (Galli et al., 2022). The significant increase observed in the rabbit group treated with polyphenols extracted from strawberries as well as in Omega-3 extracted from almonds for the same group at a probability level (p ≤ 0.01) is that polyphenols play an inhibitory role in the translation and expression of the gene responsible for the enzyme in liver cells by increasing the hepatic mRNA, thus increasing the enzyme (PON-1) by stimulating the aryl-hydrocarbon receptor, as it increases its concentration as well as increasing its effectiveness, as polyphenols such as quercetin, myrictin, and kaempferol and as substances containing an aromatic hydroxyl group, are responsible for their antioxidant activity, especially fats (Lv et al., 2021) by scavenging this enzyme and protecting it from oxidative stress, as these compounds give a hydrogen atom to free radicals and thus inhibit their effectiveness (Carrillo-Marlinez et al., 2024) This applies to the group of rabbits treated with Omega-3, Vit. E. As for the reason for the decrease in the effectiveness of the PON-1 enzyme, Triton may have inhibited the concentration of PON-1-mRNA in the liver because Triton caused an increase in free radical production, which causes an imbalance in the number of HDL-c molecules associated with the PON-1 enzyme. Consequently, these free radicals affect the stability of the association between PON-1 and HDL-c (Ikhlef et al.,2017). This enzyme is mainly produced in the liver, appears in blood serum, and is present in high-density lipoproteins. Treatment with Triton led to the accumulation of free radicals, which negatively affected the efficacy of this enzyme (Taler-Verčič et al., 2020). The cause of the increase in apelin in rabbits treated with polyphenols extracted from strawberries and Omega-3 extracted from almonds may be due to the cumulative effect of these compounds for both strawberries and almonds, as phenols are considered antioxidants. They have many biological effects because they may scavenge free radicals and inhibit oxidation as they have strong antioxidant properties. These compounds give a hydrogen atom to the free radical, thus inhibiting its effectiveness (Lv et al., 2021), and in synergy with Omega-3 extracted from almonds, which acts as an antioxidant that provides protection for the membranes of cells secreting apelin, thus increasing its secretion. Treatment with Omega-3, Vit. E contributes greatly to increasing the concentration of hormones in serum. Omega-3 has a unique chemical composition that gives it distinctive properties that make it a free radical scavenger and enhance the secretion of the hormone apelin in synergy with vitamin E (Sherratt et al., 2021), which plays a major role as an antioxidant, and has therapeutic benefits because of its molecular mobility through lipid membranes and its easy acceptance of electrons (Galli et al., 2022). The reduced apelin concentration in rabbits treated with Triton could be attributed to its tendency to accumulate free radicals and elevate oxidative stress (Al-Mashhadini & Al-Hayali, 2020). This process damages the cell membranes of hormone-secreting cells and disrupts other cellular organelles, ultimately inhibiting hormone secretion from fat cells. Apelin, an adipokine produced by fat cells, lowers sugar and fat levels and promotes pancreatic beta-cell production of insulin (Soltan et al., 2023). ConclusionWe conclude that treatment with Triton significantly contributes to the development of hepatic steatosis, whereas polyphenols extracted from strawberries, Omega-3 extracted from almonds, and Omega-3, Vit. E had a key influence on reducing the treatment effects of Triton and improving antioxidant status. AcknowledgmentThe authors express their deep gratitude to the Deanship of the College of Science for their support and contributions, which were instrumental in the completion of this research. Conflict of interestThe authors declare no conflict of interest. FundingNone. Authors’ contributionsAll authors contributed equally to this research. Data availabilityAll data were provided in the manuscript. ReferencesA-Ani, Z. 2023. Protective effects of methionine on some physiological and biochemical parameters of triton-stressed male rats. Pak. J. Med. Health Sci. 17(03), 271. Al-Daody, A.C. 1998. Chemical study on some Iraqi plants. Ph. D. Thesis, Mosul University, Mosul, Iraq. Al-Hadidy, A. A., Abdul-Fattah, J. H. and Al-Kattan, M. M. 2008. Effect of Zingiber officinale Rhizomes powder on some physiological, Histological and Biochemical features in white Male Rabbits. Rafidain J. of Sci., 19(2 A). Al-Jaghfi, Z.N. 2022. Chromatographic separation of some natural compounds from the seeds of the Iraqi cumin plant Cuminum cyminum L. plant and studying its inhibitory effect on two types of bacteria. M. S. thesis, Mosul University, Mosul, Iraq. Al-Lahibi, A.H. 2022. Qualitative and quantitative separation of many active compounds from tubers of Cyperus rotundus L. plant and studying its antibacterial effect on some types of bacteria. M. S. thesis, Mosul University, Mosul, Iraq. Al-Mashhadini, T. and Al-Hayali, H. L. 2020. Biochemical and physiological study of the effect of sesame seeds on quail males exposed to thermal stress. Indian J. Pub. Hea. Res Dev, 11(4), 1077–1083. Al-Saadon, M. B. and Al-Hadi, R. D. 2013. Isolating and Studying Effect of Tannins, Oil, Flavonoids, Proteinous and non-proteinous Compounds from (Agrimonia Eupatoria) on Mice Exposed to Oxidative Stress. Rafidain J. of Sci., 24(2A). Antar, S.H. and Al-Wakaa, A.H.A. 2017. Statistical analysis of agricultural experiments using SAS program. Baqubah: Diyala University Central Press. Arthur, I.V. 1972. Practical organic chemistry including qualitative organic analysis. 3rd ed, Hoboken, NJ: Prentice Hall. p: 445. Asrani, S.K., Devarbhavi, H., Eaton, J. and Kamath, P.S. 2019. Burden of liver diseases in the world. J. Hepatol. 70(1), 151–171. Behbahani, M., Shanehsazzadeh, M. and Hessami, M.J. 2011. Optimization of callus and cell suspension cultures of Barringtonia racemosa (Lecythidaceae family) for lycopene production. Sci. Agric. 68(1), 69–76. Berberich, A.J. and Hegele, R.A. 2022. A modern approach to dyslipidemia. Endocr. Rev. 43(4), 611–653. Carrillo-Martinez, E.J., Flores-Hernández, F.Y., Salazar-Montes, A.M., Nario-Chaidez, H.F., and Hernández-Ortega, L.D. 2024. Quercetin, a flavonoid with great pharmacological capacity. Molecules 29(5), 1000. Devi, S. and Singh, R. 2017. Assessment of lipid lowering effect of Nepeta hindostana herb extract in experimentally induced dyslipidemia. J. Nutr. Intermed. Metab. 9, 17–23 Forman, H.J. and Zhang, H. 2021. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. J. Nat. Rev. Drug Discov. 20(9), 689–709. Galli, F., Bonomini, M., Bartolini, D., Zatini, L., Reboldi, G., Marcantonini, G., Gentile, G., Sirolli, V. and Di Pietro, N. 2022. Vit. E (alpha-tocopherol) metabolism and nutrition in chronic kidney disease. J. Antioxidants. 11(5), 989. Ghazanfar, H., Javed, N., Qasim, A., Zacharia, G.S., Ghazanfar, A., Jyala, A., Shehi, E. and Patel, H. 2024. Metabolic dysfunction-associated steatohepatitis and progression to hepatocellular carcinoma: a literature review. Cancers 16(6), 1214. Gonzalez, A., Huerta-Salgado, C., Orozco-Aguilar, J., Aguirre, F., Tacchi, F. and Simon, F. 2020. Role of oxidative stress in hepatic and extrahepatic dysfunctions during nonalcoholic fatty liver disease (NAFLD). Oxid. Med. Cell. Longev. 2020, 1617805. Han, S.K., Baik, S.K. and Kim, M.Y. 2023. Non-alcoholic fatty liver disease: definition and subtypes. Clin. Mol. Hepatol. 29(Suppl), S5. Harborne, J.B. 1998. Photochemical methods a guide to modern techniques of plant analysis. 5th ed. London: Chapman and Hall, pp: 21–72. Heeren, J. and Scheja, L. 2021. Metabolic-associated fatty liver disease and lipoprotein metabolism. J. Mol. Metab. 50, 101238. Ikhlef, S., Berrougui, H., Kamtchueng Simo, O., Zerif, E. and Khalil, A. 2017. Human paraoxonase 1 overexpression in mice stimulates HDL cholesterol efflux and reverse cholesterol transport. PLoS One 12(3), e0173385. Jeong, S.W. 2020. Nonalcoholic fatty liver disease: a drug revolution is coming. Diabetes Metab. J. 44(5), 640–657. Khan, S.U. and Khan, M.U. 2022. Molecular developments in cell models of fatty liver disease. DYSONA-Life Sci. 3(1), 16–29. Kulka, M. 2016. A review of paraoxonase 1 properties and diagnostic applications. Pol. J. Vet. Sci. 19(1), 225–232. Kumar, V., Khan, M.M., Khanna, A.K., Singh, R., Singh, S., Chander, R., Mahdi, F., Mahdi, A.A., Saxena, J.K. and Singh, R.K. 2010. Lipid lowering activity of Anthocephalus indicus root in hyperlipidemic rats. J. Evid. Based Complement. Altern. Med. 7, 317–322. Li, Y., Yu, Y., Yang, L. and Wang, R. 2023. Insights into the role of oxidative stress in hepatocellular carcinoma development. J. Front. Biosci. Landmark. 28(11), 286. Lv, Q.Z., Long, J.T., Gong, Z.F., Nong, K.Y., Liang, X.M., Qin, T., Huang, W. and Yang, L. 2021. Current state of knowledge on the antioxidant effects and mechanisms of action of polyphenolic compounds. J. Nat. Prod. Commun. 16(7), 1934578X211027745. Marchegiani, F., Marra, M., Olivieri, F., Cardelli, M., James, R. W., Boemi, M. and Franceschi, C. 2008. Paraoxonase 1: genetics and activities during aging. J. Rejuvenation Res. 11(1), 113–127. Martin, D., Murray, R., Granner, D., Mayes, P. and Rodwell, W. 1996. Harper’s biochemistry, 24th ed. Taipei, Canada: Lang Medical Publication. Mason, R.P., Jacob, R.F., Shrivastava, S., Sherratt, S.C. and Chattopadhyay, A. 2016. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. J. Biochim. Biophys. Acta Biomembr. 1858(12), 3131–3140. Murali, S., and Aradhyam, G. K. 2023. Structure–function relationship and physiological role of Apelin and its G protein coupled receptor. J. Biophys. Rev. 15(1), 127–143. Ramachandran, A. and Jaeschke, H. 2018. Oxidative stress and acute hepatic injury. J. Curr. Opin. Toxicol. 7, 17–21. Sherratt, S.C., Juliano, R.A., Copland, C., Bhatt, D.L., Libby, P. and Mason, R.P. 2021. EPA and DHA containing phospholipids have contrasting effects on membrane structure. J. Lipid Res. 62, 100106. Singar, S., Kadyan, S., Patoine, C., Park, G., Arjmandi, B. and Nagpal, R. 2024. The effects of almond consumption on cardiovascular health and gut microbiome: a comprehensive review. Nutrients 16(12), 1964. Soltan, O.I., Gazwi, H.S., Ragab, A.E., Mahmoud, M.E., Fudllalah, F.M., Alqahtani, M.M., Alasmari, A., Ghazzawy, H.S. and Hikal, D.M. 2023. Antihyperlipidemic effect of bread fortified with potato peel powder against Triton X-100-induced hyperlipidemia in male albino rats. J. Funct. Foods. 108, 105725. Taler-Verčič, A., Goličnik, M. and Bavec, A. 2020. The structure and function of paraoxonase-1 and its comparison to paraoxonase-2 and-3. J. Mol. 25(24), 5980. Teng, M.L., Ng, C.H., Huang, D.Q., Chan, K.E., Tan, D.J., Lim, W.H., Yang, J.D., Tan, E. and Muthiah, M.D. 2023. Global incidence and prevalence of nonalcoholic fatty liver disease. J. Clin. Mol. Hepato. 29(Suppl), S32. Verwer, C.M., van Amerongen, G., van den Bos, R. and Hendriksen, C.F. 2009. Handling effects on body weight and behavior of group-housed male rabbits in a laboratory setting. J. Appl. Anim. Behav. Sci. 117(1–2), 93–102. Wilson, S., Gullan, R. and Hocker, E. 1972. Studies of the stability of 18 chemical constituent serum. J. Clin. Chem. 18(2), 1498–1503. Zidane, S.R. 2023. Separation and identification of a number of natural products from the flowers of the Iraqi Hibiscus sabdariffa L. plant and their reflection on the growth of two types of pathogenic bacteria. M. S. thesis, Mosul University, Mosul, Iraq. | ||
| How to Cite this Article |
| Pubmed Style Al-juwary RS, Al-kattan MM. Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits. Open Vet. J.. 2025; 15(1): 211-221. doi:10.5455/OVJ.2025.v15.i1.20 Web Style Al-juwary RS, Al-kattan MM. Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits. https://www.openveterinaryjournal.com/?mno=218614 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.20 AMA (American Medical Association) Style Al-juwary RS, Al-kattan MM. Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits. Open Vet. J.. 2025; 15(1): 211-221. doi:10.5455/OVJ.2025.v15.i1.20 Vancouver/ICMJE Style Al-juwary RS, Al-kattan MM. Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 211-221. doi:10.5455/OVJ.2025.v15.i1.20 Harvard Style Al-juwary, R. S. & Al-kattan, . M. M. (2025) Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits. Open Vet. J., 15 (1), 211-221. doi:10.5455/OVJ.2025.v15.i1.20 Turabian Style Al-juwary, Rana Suhail, and Muntaha Mahmood Al-kattan. 2025. Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits. Open Veterinary Journal, 15 (1), 211-221. doi:10.5455/OVJ.2025.v15.i1.20 Chicago Style Al-juwary, Rana Suhail, and Muntaha Mahmood Al-kattan. "Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits." Open Veterinary Journal 15 (2025), 211-221. doi:10.5455/OVJ.2025.v15.i1.20 MLA (The Modern Language Association) Style Al-juwary, Rana Suhail, and Muntaha Mahmood Al-kattan. "Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits." Open Veterinary Journal 15.1 (2025), 211-221. Print. doi:10.5455/OVJ.2025.v15.i1.20 APA (American Psychological Association) Style Al-juwary, R. S. & Al-kattan, . M. M. (2025) Effects of experimental hepatic steatosis on apelin and various biochemical parameters in male white rabbits. Open Veterinary Journal, 15 (1), 211-221. doi:10.5455/OVJ.2025.v15.i1.20 |





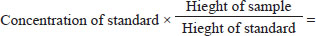 (ppm/ml) Conc. of sample
(ppm/ml) Conc. of sample