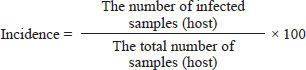| Research Article | ||
Open Vet. J.. 2025; 15(1): 187-197 Open Veterinary Journal, (2025), Vol. 15(1): 187-197 Research Article Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattleTeuku Zahrial Helmi1, Farida Farida2, Aisyah Kasturi3, Ella Prisa Lusisia Rahmah3, Winaruddin Winaruddin2 and Muhammad Hanafiah2*1Laboratory of Biochemistry, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 2Laboratory of Parasitology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 3Students in Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia *Corresponding Author: Muhammad Hanafiah. Laboratory of Parasitology at Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Email: hanafi_2015 [at] usk.ac.id Submitted: 02/09/2024 Accepted: 31/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
AbstractBackground: The Setaria nematode, classified within the order Spirurida and family Setariidae, has predilection in the peritoneal cavity of its host, with mosquitoes acting as vectors for transmitting microfilariae to cattle, its primary host. Aim: This study investigates the morphology and molecular characteristics of Setaria digitata in Aceh cattle and evaluates the effectiveness of three staining solutions lactophenol, glycerol, and lugol 2% for visualizing adult worms. Methods: A total of 124 blood serum and adult worm samples were collected from Aceh cattle at slaughterhouses in Banda Aceh and Bireuen. Microfilariae detection was conducted using the Knott test, while worm morphology was assessed via polymerase chain reaction amplification of the 12S rDNA and cox1 genes. Results: Each staining solution enhanced worm morphology visualization with varying benefits. The Lugol 2% solution offered a complete view of morphology but lacked detail for internal structures. Lactophenol improved the visibility of both morphology and internal organs, though sample integrity declined quickly. Glycerol displayed both the morphology and internal structures with clarity, making it the most effective option overall. Molecular amplification results showed a 450 bp band for the 12S rDNA gene and a 680 bp band for the cox1 gene in S. digitata. Conclusion: Glycerol proved superior for maintaining morphology and visualizing the internal structures of S. digitata. Molecular profiling using the 12S rDNA and cox1 genes effectively confirmed the identity of S. digitata, underscoring the value of these genetic markers for species identification. Keywords: Morfology, Molecular, Setaria, Aceh cow, Staining solutions. IntroductionAceh cow is one of the most bred livestock types chosen by farmers in Aceh, Indonesia. This cow has diverse body shapes, physical attributes, genetic composition, and good adaptive ability in environments with limitations. Thus, they need to be protected, preserved, and have their advantages improved (Mukhtar et al., 2015). Aceh cow has been designated as a cow breed from Indonesia based on the decree of the Ministry of Agriculture of the Republic of Indonesia number 2907/Kpts/OT.140/6/2011 (Makmur et al., 2020). Aceh cow has also been assigned Indonesia National Standard (Standar Nasional Indonesia–SNI) by National Standard Agency number 7651.3:2013 as a meat-producing and working breed (Ikhsanuddin et al., 2018). Aceh cow is a cross of Bos indicus and Bos sondaicus, which have smaller statures compared to other cows. The males commonly have hump and horns. The male and female Aceh cow coat colors that have been assigned in the SNI are brick red and light brown (Mukhtar et al., 2015). Aceh cow is also one of the ruminant livestock with high economic value among the farmers (Mukhtar et al., 2015). Thus, understanding the rearing condition of Aceh cows and the presence of serious health problems such as worm infestation is crucial. Parasitic disease is a factor that may lower livestock productivity. The presence of worms in livestock is often dismissed by farmers despite having a large influence on livestock health and productivity (Purwaningsih et al., 2018). Filaria nematodes are parasites in the tissue and tissue chamber of all vertebrates. Among these filaria nematodes, Setaria sp. worm is a parasite residing in the cow peritoneal cavity where it lives, grows, and sexually reproduces. The male Setaria sp. worm has a small and thin body while the female worm has a longer and thicker body than the male. Setaria sp. worm is a type of worm that commonly causes health problems in cows (Senanayake et al., 2020) Setaria sp. worm has a round long form shaped like a soft thread which can be seen with the naked eye when the cow’s abdomen is opened (Madani et al., 2021). Setaria sp. worm looks like a milky white thin thread, getting thinner by its posterior with an average length of 62.8 ± 9.89 mm (ranging between 40 and 80 mm), when found in adult form and does not cause some kind of pathogenicity to the cow (Dehkordi et al., 2014). The cow is a natural host of Setaria sp. worm. This parasite generally does not cause disease in its natural host. Infective larvae (L3) transmission via mosquitoes as vectors into aberrant hosts such as goats, sheep, or horses and causes serious neuropathological disorders and is often fatal (Senanayake et al., 2020). Mosquitoes are infected by microfilaria upon consuming microfilaria containing blood from a host. These microfilariae grow into infective larvae at the mosquito’s chest muscle within 2 to 3 weeks. Infected mosquitoes transmit these infective larvae to another vulnerable host during their blood feeding (Rafee and Amarpal, 2016). Worm identification was carried out by microscopic observation of the parasite and one of the methods is by observing the worm’s morphology. Worms found in cows usually still cannot be identified macroscopically with the naked eye, so the staining solution should be used to identify the worm with clarity and easier identification. This research used several staining solutions, lugol 2%, lactophenol, and glycerol. According to Hastuti and Haryatmi (2021), these solutions can produce a clear background and clear worm morphology which make the worms transparent. Other than identification with different staining solutions, molecular character analysis is also required to ensure that the species found matches the desired target, which in this case was Setaria digitata worm. Therefore, The objective of this research is to conduct morphological and molecular characterization of S. digitata worms in Aceh cows and to determine a good staining solution for identifying S. digitata worms from cows slaughtered in Banda Aceh and Bireuen slaughterhouse. Materials and MethodsThe blood and worm sample collection was obtained through direct methods from slaughtered cows and extracted innards. Setaria sp. worms located in the peritoneum and other cow organs were directly taken from the slaughtered cows. Setaria sp., shaped like a milky white thin thread, could be seen by the membrane covering the abdomen in the peritoneum of Setaria sp. infected cows. Worm samples were washed and stored in sample bottles filled with 70% alcohol by using dressing tweezers. Samples were transported and observed in the Parasitology Laboratory of the Veterinary Medicine Faculty, Universitas Syiah Kuala. Setaria sp. microfilaria presence detection in Aceh cow by Knott testOne hundred twenty-four Aceh cow blood samples were analyzed for microfilaria by Knott’s test. The whole blood sample was obtained from the jugular vein when the cows were culled. The blood samples were mixed with EDTA for the Knott Test performed in the Parasitology Laboratory. As much as 1 ml of blood was taken and mixed with 9 ml of 2% formalin and then centrifuged at 1,500 rpm. The supernatant was discarded and the pellet was dripped with Methylene blue 10%. One drop of the mixture was smeared on an object glass to observe the presence of microfilaria by a binocular microscope (Watanabe et al., 2004). Identification of adult Setaria sp. in Aceh cowLugol 2% and lactophenol stainingAdult worms found in Aceh cow’s duodenum serous cavity and peritoneum cavity during necropsy were collected and stored in bottles filled with alcohol 70%. The samples were then fixed with Lugol 2% and lactophenol staining and were left alone for around 5–10 minutes. Samples were then put on a petri dish and observed under a microscope. Glycerol stainingAdult worms found in the peritoneum cavity of Aceh cow during necropsy were collected and stored in alcohol 70% solution, before being moved into a glass bottle and fixed with glycerol before being observed under a light microscope. Species identificationMorphological observation of Setaria sp. worm was performed by binocular light microscope Olympus CX 21 and a capture system according to the method explained by Pinheiro et al. (2019) by using HDMI+USB 1080P/2m SIGMA microscope camera. The incidence rate was determined based on the observation by using the following equation:
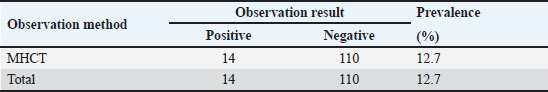
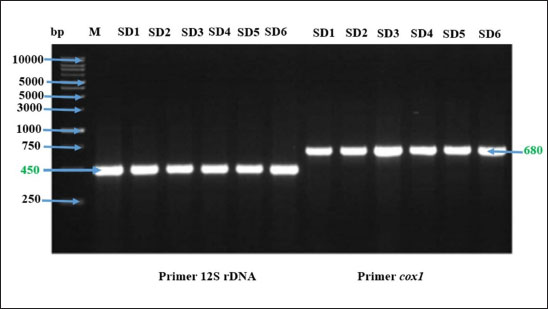
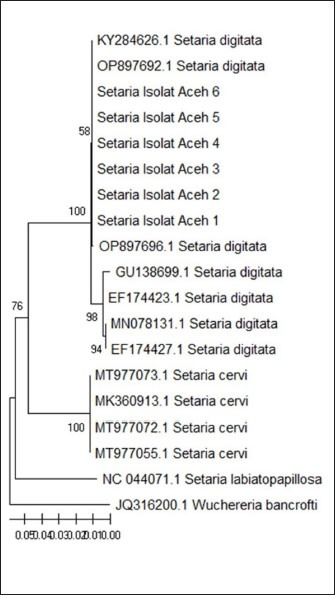
Molecular identification of Setaria spp. wormDNA extractionThe DNA extraction from samples Setaria worm in Aceh cow was conducted using a DNA extraction kit. The procedure started by combining 200 mg of the sample with 320 µl of lysis buffer, 16 µl of RNase A, 16 µl of proteinase K, and 3.2 µl of DTT in a 2 ml tube. The mixture was incubated at 65°C for 1 hour. After incubation, the sample was centrifuged at 16,099 × g for 11 minutes. Following this, 320 µl of binding buffer was added, and the mixture was vortexed and incubated again at 65°C for 10 minutes. The incubated mixture was then rinsed with 320 µl of 100% ethanol, 500 µl of wash buffer, and 500 µl of 75% ethanol. Finally, the DNA was eluted in 100 µl of elution buffer at 65°C. The primer characteristic used in this research is presented in Table 1. Phylogenetic analysisPolymerase chain reaction products of cox1 and 12s rDNA were purified using Gel recovery kit (Vivantis, Malaysia) and sequenced on both strands using Big Dye Terminator V.3.1 Cycle Sequencing kit in an ABI 3130 Genetic Analyzer (Applied Biosystems, USA). Sequences were aligned together using ClustalW software (Chowdhury and Garai, 2017) to determine the consensus sequences. Consensus 12S rDNA and cox1 sequences were subjected to BLASTn analysis (http://blast.ncbi.nlm.nih.gov) and compared to all nucleotide sequences of Setaria species available in the current databases. Sequence identities (in %) were calculated by pairwise comparisons. Subsequently, the consensus sequences were aligned with a selected subset of closely related sequences of the genus Setaria. Phylogenetic relationships were inferred based on analyses employing the Neighbor-Joining (NJ) method using MEGA7. The topological stability of the tree was evaluated by 1000 bootstrap replications. Analysis dataData are presented in the form of figure and table, and then data obtained are analyzed descriptively. Ethical approvalThe experimental protocol was approved by the Animal Care and Use Committee of Faculty Veterinary Medicine, Syiah Kuala University No. 2.KEH.080.07.2023. ResultsSetaria sp. microfilaria detection in Aceh cow using Knott TestThe blood were taken from 124 Aceh cows slaughtered in Banda Aceh and Bireun Slaughterhouse, Aceh to detect Setaria sp. microfilaria using Microhematocrit Centrifugation Technique (MHCT) (Rafee and Amarpal, 2016). The results can be seen in Figure 1 and Table 2. Identification of adult Setaria sp. in Aceh cowThe observation of adult Setaria sp. from 124 cows slaughtered in Banda Aceh and Bireuen, Aceh, through direct observation could be seen in Figure 2 and Table 3 below. Identification result of adult S. digitata worm by using three staining solution to evaluate their morphology can be seen in Figure 3 below. The molecular identification of Setaria spp. wormThe DNA amplification result of Setaria spp from Banda Aceh and Bireuen slaughterhouse used the forward and reverse primer for cox1 and the forward and reverse primer for 12S rDNA with master mix PCR Go Tag which specifically attached to the template samples SD1, SD2, SD3, SD4, SD5 and SD6 then produced 450 bp for 12S rDNA and 680 bp for cox1 (Fig. 4). The 12S rRNA gene analysis result of Setaria. spThe Blast result of 12S rRNA gene nucleotide from Setaria sp. worm samples from Aceh cows (Fig. 5). The genetic distance tableThe genetic distance of S. digitata found in Aceh cow with those data from GenBank is presented in Table 4. Phylogenetic tree analysis based on COX 1 geneThe phylogenetic tree analysis result for COX 1 gene of S. digitata seen on Figure 6. The phylogenetic analysis result 450 bp, with maximum parsimony method in Mega program version 11.0, and Kimura 2 parameter 1,000× bootstraps showed that the Setaria worm samples from this research are still grouped as S. digitata, although it has different clade with other comparison isolates from the genbank sequence data. The Setaria genus in this research formed two groups, which are the S. digitata and Setaria labiatopapilosa group. The S. digitata group is divided into three groups where the S. digitata worm in this research is within their own group along with S. digitata isolate from China, different from other S. digitata and from Dirofilaria and Wucheria worms as the out-group representatives. DiscussionTable 2 revealed that Setaria sp. incidence rate in Aceh cow was 12.7%. This is lower compared to the result from Sundar and D’Souza (2013) who reported the prevalence of Setaria sp. in cows in India ranged between 77% and 95% with 187 out of 500 livestock samples found positive for Setaria sp. However, This result is lower compared to the incidence rate observed by Rodrigues et al. (2021) who reported that 136 nematodes found in cows and water buffaloes in Brazil with the prevalence rates were 24% and 25%, respectively. Based on the results, it indicated that for livestock in the Aceh area the cases are still low, but attention needs to be given to eliminate the Setariosis cases in livestock and to broad the treatment coverage beyond anthelmintic drugs. Table 1. The PCR condition and primer sequences for tandem repeat DNA, cox1 and 12Sr.
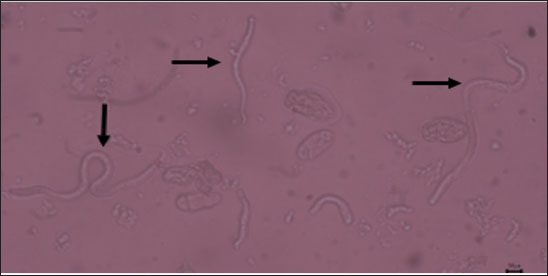
Fig. 1. Sheathed microfilaria (L1) of S. digitata (40x) (arrow). Table 2. Microfilaria prevalence in Aceh cow blood analyzed with MHCT (n=124 cows).
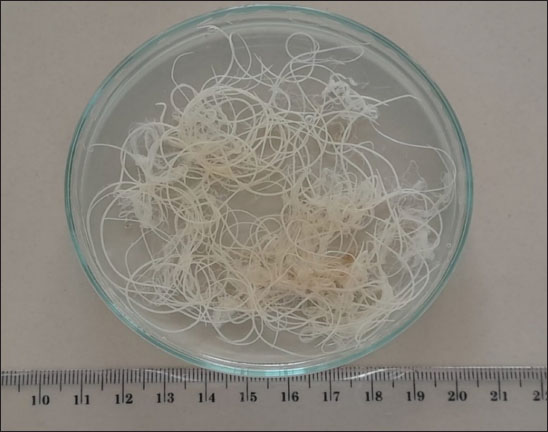
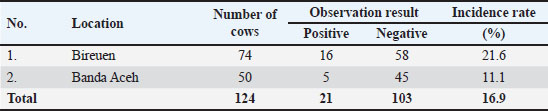
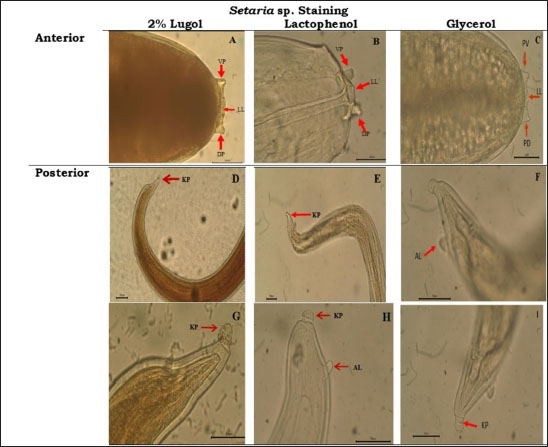
Fig. 2. Setaria sp. worm (40x). Table 3 showed that the incidence rate of Setaria sp. worm in Aceh was 16.9%. The incidence rate in this research is lower compared to the research performed by Shin et al. (2002) in Korea where the prevalence rate of Setaria sp. in cows was 70%. Although these worms grow into adulthood in the abdominal cavity and thus mostly are not dangerous for livestock, serious pathogenic results may occur in animals such as goats, sheep, and horses where the Setaria sp. larvae may migrate randomly into the central nervous system. The difference in nematode prevalences in several territories is influenced by several factors: the infectious agent, age, sex, breed, feed, and reading management. The rearing and enclosure management by the farmers and overall farm management greatly influence parasite infection prevalence (Tolistyawati et al., 2016). Blood is a body liquid that distributes oxygen to all body tissue and sends the nutrition needed by the cells. This allows Setaria sp. worm larvae to grow in their host’s body. Definitive diagnosis in setariosis cases can be done by complete ophthalmic testing, showing worm movement inside the eye, or by Knott test method to detect Setaria microfilariae (Jayathilake et al., 2019). Knott test is a technique to detect microfilaria by hemolysis and blood sample concentration to detect Setaria species microfilariae (Rafee and Amarpal, 2016). The morphological observation to identify Setaria sp. worm found in the peritoneum cavity of Aceh Cow from Banda Aceh and Bireuen, Aceh, was performed by glycerol, Lugol 2%, and lactophenol solutions (Fig. 3). The morphological observation of the worms with lactophenol staining solution in this research (Fig. 3D, E, H) resulted in clearer and easier-to-identify worm morphology, but the sample did not last as the worm would be damaged. Whereas the use of Lugol 2% (Fig. 3A, D, G) and glycerol (Fig. 3C, F, I) staining solution took a longer time to be clearly seen but lasted longer and the morphology appeared clear. According to Asarina and Haeruni (2019), the three solutions have each’s advantages and disadvantages in identifying worm morphology. Glycerol solution is hygroscopic so it absorbs water molecules. Samples added with glycerol in all concentrations are proven to last longer and not dry. Glycerol is a colorless odorless viscous chemical often used in drug formulation. Glycerol is generally used for preservation media or long-term storage as well as a medium to move microorganisms (Seniati et al., 2020). Glycerol can be used as a medium because glycerol can protect antimicrobial activity by improving the structural stability of the microbe’s natural protein thus preventing the protein from damage from thermal process and aggregation (Setiaji et al., 2015). Lugol solution is often used in microscopic parasite observation, among them is worm morphology observation. Lugol solution staining gives a clear background with a yellow color on the worm (Hastuti and Haryatmi, 2021). Lactophenol staining gives a clear view of worm morphology where the worm turns transparent, but this staining may lower the elasticity thus hindering the identification process (Beraldo and Pascotto, 2014) or even at times damaging the worm in a moderately long storage span (Hanafiah et al., 2023). From Figure 3, the anterior and posterior parts of S. digitata by several staining solutions could be seen. Figure 3A–C depicted the anterior of S. digitata, and Figure 3D–I showed the anterior part stained by Lugol 2%, lactophenol, and glycerol. The anterior part (Fig. 3A–C) showed that S. digitata worm has peribuccal crown with central “helmet” at the end of its head and triangle-shaped lateral labia (LL). Figure 3D–I showed that the tail ends with a smooth knob with oval lateral appendages (ALs). Differences with Setaria sp. worm morphology could be seen at the anterior tip. The anterior tip of S. digitata worm is round with two similar projections. The posterior tip of S. digitata has a smooth or slightly rough knob/dull tip with surface papilae (Rodrigues et al, 2021). Setaria sp. morphology observation revealed that S. digitata morphology differs between sexes. The female worm has a peribuccal crown or “helm” in the anterior tips shaped like a triangle with two projecting thorns showing the dorsal projection (DP) and ventral projection (VP) at the peribuccal crown. The posterior of the female S. digitata has a smooth round terminal knob with ALs. Meanwhile, the male S. digitata has a similar anterior to the female worm and a wavy cuticle. The posterior end of the male worm has a thick and sturdy right side with a narrow proximal end while the left spicula has a stem and a rod (Sundar and D’Souza, 2015). Table 3. Setaria sp worm incidence in several observation sites.
Fig. 3. (A–C) S. digitata Anterior: DP; VP; and LL at the peribuccal crown. (D–I) S. digitata Posterior: the posterior end of S. digitata thinned with smooth knob (KP) and AL.
Fig. 4. The DNA amplification result of Setaria spp. adult worm in agarose gel 1%. M=Marker, SD1, SD2, SD3, SD4, SD5 and SD6=S. digitata worm. Setaria sp. in cows can cause fibrinous peritonitis. This worm can live and reproduce in cows by using the blood circulation system as a source of nutrition for their growth. Moreover, infective larvae transmission through mosquito vectors into hosts such as sheep and horses can cause serious neuropathological problems. Setaria sp. worm can easily infect cows, especially in areas with high mosquito populations (Hanafiah et al., 2023).
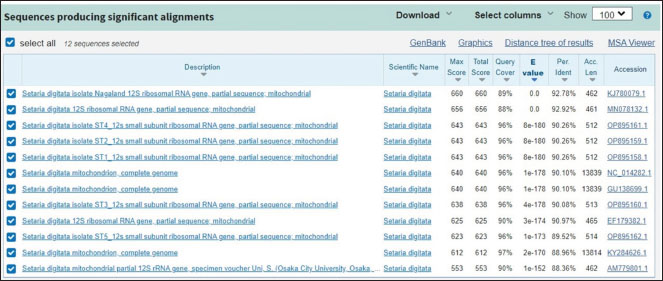
Fig. 5. The Blast result of 12S rRNA gene nucleotide from Setaria sp. worm samples from Aceh cows. Table 4. The genetic distance of S. digitata in Aceh cow.
According to Maharana et al. (2020), morphological characteristic identification is not enough to detect and differentiate S. digitata with other species like S. equina, S. labiatopapillosa, and S. cervi. This is due to the morphological characteristic similarities between species which makes species identification difficult. Thus, molecular characterization is required to identify S. digitata species and determine the phylogenetic position of S. digitata from Aceh cows in Indonesia within the genus as well as other filaria nematodes genus position based on the analysis of the two genes COX1 and 12S rDNA. Setaria digitata is the most reported in tropical Asian countries, where its vector is generally found (Sundar and Sundar, 2015; Maharana et al., 2020). South Korea is the only non-tropical country in high latitude where S. digitata has been reported in a horse (Shin et al., 2017; Kim et al., 2018). In this research, S. digitata is identified and confirmed by molecular method in a horse on a farm in North China, where the latitude line is close to South Korea. This incidental case most likely occurred due to the presence of a cow farm in the surrounding environment. Thus, this finding indicates that cows in north China possibly carried S. digitata. In 2017, S. digitata was first identified with molecular method in a water buffalo carcass in South China, but not in living water buffalo or cow (Liu et al., 2017). Setariasis is an ophthalmology disorder known in horse, with S. digitata reported to be the most common cause (Nabie et al., 2017). S. digitata is regarded as a primary health hazard in animals and causes economic losses to the owners.
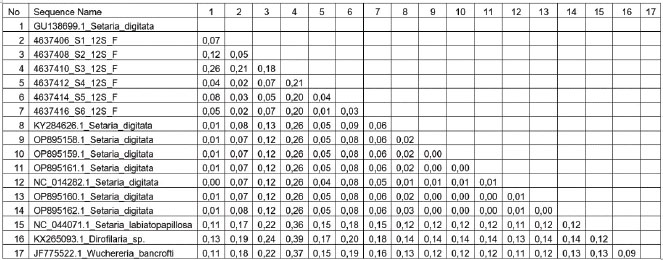
Fig. 6. The phylogenetic analysis of S. digitata based on COX 1 gene of S. digitata. Although traditional identification has been used to differentiate Setaria sp. species, according to Perumal et al. (2016) and Maharana et al. (2020) this is insufficient because S. digitata worm has similar morphology with S. equina, S. labiatopapillosa and S. cervi. Yatawara et al. (2010) and Liu et al. (2017) have conducted research by nucleic acid (DNA) based detection method to ensure the species of filaria isolated from a mare. The result was obtained and confirmed to be S. digitata when compared to other sequences in NCBI GenBank database by BLAST. The genetic sequence extracted from the worm has 99% similarities with S. digitata isolated from cows and water buffaloes in Sri Lanka and from water buffaloes in Greater China. The Blast result of the nucleotide sequences (Fig. 5) in this research used the 12S rRNA gene from Setaria sp Aceh isolate and showed 92.78% similarity with S. digitata Nagaland isolate (Accession no. KJ780079.1) and has the most distant similarity with S. digitata isolate from Osaka City University (Accession no. AM779801.1) with 88.36% similarity. When compared with Chamuah et al. (2015), the sequence analysis of ribosomal DNA 12S, 28S, and ITS-2 showed 98% similarity with S. digitata, while the mitochondrial gene Cox 1 showed 99% similarity with S. digitata Sri Lanka isolate (Acc. No.EF179382), and 87% similar with S. labiatopapillosa. The ITS-2 region showed 98% similarity with Sri Lanka isolate (Accession no. EF196088), 96% similarity with Brugia pahangi (Accession no. EU373652), 95% similarity with B. malayi (EU37361) and 96% similarity with Volvulus onchocerca (AF228572). In Chamuah et al. (2015) the ribosomal DNA sequence analysis of 12S, 28S, and ITS-2 region showed 98% identity with S. digitata, while mitochondrial gene Cox 1 showed 99% similarity with S. digitata Sri Lanka isolate (Acc. No. EF179382,) and 87% similarity with S. labiatopapillosa. The ITS-2 region showed 98% similarity with Sri Lanka isolates (Accession no. EF196088), 96% similarity with Brugia pahangi (Accession no. EU373652), 95% similarity with B. malayi (EU37361), and 96% similarity with Onchocerca volvulus (AF228572). Based on the genetic distance in this research, SD1, SD2, SD3, SD4, SD5, and SD6, samples have 1 to 12 % genetic distance compared to available S. digitata isolate from GenBank (Accession no. GU138699.1), while SD3 samples have 26 genetic distance value compared to S. digitata isolate from the GenBank (Accession no. GU138699.1), and has 18 to 21 % genetic distance when compared to the other research samples. This analysis result also showed the genetic distance value of Setaria sp sample Aceh isolate (SD1-SD6) with other parasitic nematodes out of the Setaria group with 37% against Wuchereria bancrofti (Accession no. JF775522.1) and 39% against Dirofilaria sp (Accession no. KX2650933.1). The phylogenetic tree rooted at further mind point confirmed that the S. digitata isolated in this research is part of the same large clade as those isolated from Japan, India, and Sri Lanka. S. equina and S. tundra are formed on different clusters. S. digitata and S. labiatopapillosa appeared to be sister species with a bootstrap value of 67% (Fig. 3). The 12S rDNA sequence is a useful marker for phylogenetic analysis because it has slower evolution compared with other protein-coding mitochondrial genes (Peng et al., 2017). The phylogenetic analysis of S. digitata showed that the isolate sequence from different host species produces closely related clades with high bootstrap support within the Setaria genus. Nucleotide sequence variation observation is few. This finding indicates that the S. digitata isolates detected worldwide have similar molecular characteristics (Maharana et al., 2010).
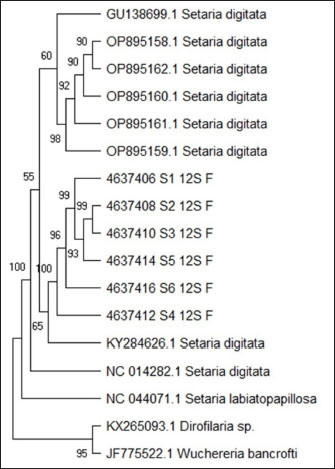
Fig. 7. The phylogenetic tree analysis result of 12S rDNA gene. The phylogenetic tree analysis result of 12S rDNA gene is shown in Figure 7. The phylogenetic analysis result 680 bp, using the maximum parsimony method in Mega Program version 11.0, with Kimura 2 parameter model with 1,000× bootstrap, showed that the Setaria worm sample of this research is still grouped S. digitata species, despite having different similarity level with other isolates taken from genebank as a comparison. The Setaria genus in this research formed two groups, which are the S. digitata and S. labiatopapilosa group. The S. digitata group is divided into two big groups, the S. digitata and Setaria cervi group. The S. digitata worm in this research is in its own group with S. digitata isolates from China and Thailand, different from other S. digitata which are isolates from Sri Lanka and India as well as with Wucheria worm as an out-group representative. The phylogenetic analysis of S. digitata based on COX 1 gene and 12 S rDNA gene has showed the isolate and sequence from different host species has produced separate clade with high bootstrap support in Setaria genus and the nucleotide sequence variation is observed. This showed that the S. digitata isolates have molecular characteristic similarities worldwide. The trend of wide molecular phylogenetics which appears in this analysis (based on 12S rDNA and COX I gene sequence) was similar to previous findings (Casiraghi et al., 2004; Yatawara et al., 2007; Huang et al., 2009; Peng et al., 2019). Moreover, the phylogeny appears to be partly consistent with the classification based on morphological and biological characteristics of filaria nematodes (Junsiri et al., 2023). Analysis of Setaria sp from Aceh cattle using the COX 1 gene showed the presence of S. digitata and S. labiatopapilosa. These results are expected to contribute to the additional information regarding the Setaria species that infect cattle, and can also be applied in terms of prevention and control of setariosis in livestock. In addition, considering that humans can act as accidental hosts for S. digitata and S. labiatopapillosa parasites, it is also necessary to anticipate whether this parasite is zoonotic or not. Considering that microfilariae and adult S. digitata worms have been found in Aceh cattle, further studies need to be carried out both on other ruminant livestock and mosquitoes as vectors that carry setariaosis. ConclusionThe identification of S. digitata through the cox1 and 12S rDNA genes produced 450 bp and 680 bp fragments, respectively. To observe these worms effectively, three staining agents lactophenol, 2% lugol, and glycerol were tested. While each staining method has specific benefits, glycerol proved to be the most advantageous, offering the clearest visualization and enhancing the ease of detailed examination. This makes glycerol a highly suitable choice for reliable identification, balancing both practicality and clarity in studying the morphology of S. digitata. AcknowledgmentsThe author would acknowledge Parasitology Tim of the Faculty of Veterinary Medicine Universitas Syiah Kuala, Aceh, for kind assistance during parasite detection using modified Knott’s Technique and hematocrit microcapillary method. The authors also acknowledge the National Research and Innovation Agency (BRIN) for providing the facilities, scientific, and technical support for SEM analysis. This study was funded by the Ministry of Research Technology and Higher Education of the Republic of Indonesia through Grant No.141/UN11.2/SPK/ PNBP/2022). Conflicts of interestThe authors declare that there is no conflict of interest. Author’s contributionsMHF drafted the manuscript. FAR and WIN revised and edited the manuscripts. WIN took part in preparing and critical checking this manuscript. TZH performed molecular identification. AIS and ELA performed examination and identification of Setaria worms. MHF, TZH, FAR, AIS, ELA, and WIN edited the references. All authors read and approved the final manuscript. Data availabilityAll references are open access, so data can be obtained from the online web. Reference Asarina, S. and Haeruni, N. 2019. Evaluation on the use of glycerol in preparing semi-permanent worm egg slide. J. Edu. Lab. Manag. 1(2), 37–40. Azhar, A., Akmal, M., Hambal, M., Sabri, M., Rosa, T.S., Rahmaddiansyah, T.S., Purwantar, B. and Dasrul, D. 2020. Physical quality assessment of Aceh cattle meat and their relationship with meat types and hair color differences. In Proceedings of the E3S Web of Conferences 1st ICVAES, Banda Aceh, Indonesia, Vol. 151, pp. 1–4. https://doi.org/10.1051/e3sconf/202015101004 Beraldo, P., Massimo, M. and Pascotto, E. 2014. Analysis of the helminthofauna of European wild cat in Friuli Venezia Giulia. In: Litografia La Ducale srl, editor. In Proceedings of XXVIII Congresso Nazionale Società Italiana Parassitologia. Rome, Italy, pp: 225–227. Casiraghi, M., Anderson, T.J.C., Bandi, C., Bazzocchi, C. and Genchi, C. 2001. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasit. 122, 93–103. Chamuah, J.K., Sakhrie, A, Lama, S., Chandra, S., Chigure, GM., Bauri, RK. and Jacob, S.S. 2015. Molecular characterization of Setaria digitata from Mithun (Bos frontalis). Acta Parasit. 60(3), 391– 394. Chowdhury, B. and Garai, G. 2017. A review on multiple sequence alignment from the perspective of genetic algorithm. Genomics 109(5–6), 419–431. Dehkordi, Z.S., Heidari, H. and Halajian, A. 2015. Case report ofadult Setaria digitata in sheep, Hamedan province, Iran. Comp. Clin. Patho. 24, 185–187. Hastuti, P. dan Haryatmi, D. 2021. Effectiveness of soaking teak leaves (Tectona grandis Linn. f) in coloring the egg stage of STH parasites (Soil Transmitted Helminth). J. Pharmacy, 10(2): 41–47. Hanafiah, M., Athaillah, F., Helmi, T.Z. and Sutriana, A. 2023. Morphology of Setaria spp. (Setariidae; Nematoda) in Aceh cattle, Indonesia. Biodiversitas 24(7), 4151–4160. Huang, J., Huang, Y., Wu, X., Du, W., Yu, X. and Hu, X. 2009. Identification, expression, characterization, and immunolocalization of lactate dehydrogenase from Taenia asiatica. Parasitol. Res. 104, 287–293. Ikhsanuddin, V.M.A. and Nurgiartiningsih, K. 2018. Production performance of aceh cattle at one day of age, weaning age, and one year old. J. Trop. Anim. Vet. Sci. 5(3), 67–72. Jayathilake, W.M.N.K., Nizanantha, K. and De Silva, L.N.A. 2019. Management of ocular setariasis in ponies with local ocular ivermectin injection. J. Adv. Vet. 9(3), 130–132. Junsiri, W., Kamkong, P., Chinkangsadarn, T., Ouisuwan, S. and Taweethavonsawat, T. 2023. Molecular identification and genetic diversity of equine ocular setariasis in Thailand based on the COI, 12S rDNA, and ITS1 regions. Infect. Gen. Evol. 110(6), 3–11. Kaur, D., Ganai, A., Parveen, S., Borkataki, S., Yadav, A., Katoch, R. and Godara, R. 2015. Occurrence of Setaria digitata in a cow. J. Parasit. Dis. 39(3), 477–478. Kim, H.C., Ahn, D.C., Yu, D.H., Chae, J.S., Yoo, J.G., Choi, KS. and Park, BK. 2018. Ocular setariasis by Setaria digitata in a horse in Korea. Korean J. Vet. Serv. 41(1), 15–19. Liu, G., Li, J. and Zhu, X. 2017. Characterization of the complete mitochondrial genome of Setaria digitata (Nematoda: Setariidae) from China. J. Helminth. 91(6), 772–776; doi:10.1017/ S0022149X16000912. Madani, I., Apsari, I. A. P., & Oka, I. B. M. 2021 Identification and prevalence of strongyle worms in an integrated Bali cattle husbandry system in Mengwi, Badung, Bali. Indonesia Medicus Veterinus : 10(2): 223–232. Maharana, B.R., Potliya, S., Ganguly, A., Bisla, R.S., Mishra, C. and Ganguly, I. 2020. First report of the isolation and phylogenetic characterization of equine Setaria digitata from India based on mitochondrial COI, 12S rDNA, and nuclear ITS2 sequence data. Parasitol. Res. 119(2), 473–481. Makmur, A., Abdullah. and Sari, EM. 2020. Reproduction characteristic of female aceh cattle in Pantan Cuaca, Gayo Lues District. Indo. J. Anim. Sci. 22(3), 306–312. Mrifag, R., Lemrabott, M.A., El Kharrim, K.E., Belghyti, D. and Basco, L.K. 2021. Setaria labiatopapillosa (Filarioidea, Nematoda) in Moroccan cattle: atypical localization and morphological characterization of females and microfilaria by light and scanning electron microscopy. Parasitol. Res. 120(3), 911–918. Mukhtar, J. and Hendra. S. 2015. Phenotypic diversity of female aceh cattle in BPTU-HPT Indrapuri. J. Ilmu Pet. 3, 34–38. Nabie, R., Spotin, A. and Rouhani. 2017. Subconjunctival setariasis due to Setaria equina infection; a case report and a literature review. Parasit. Intl. 66, 930–932. Peng, T.L., Armiladiana, M., Mimi, Ruhil, H.H., Maizan, M. and Choong, S.S. 2019. First report of equine Setaria digitata (von Linstow 1906) infestation in Malaysia. Vet. Parasitol. 17(1), 1–5. Perumal, A.N.I., Gunawardene, Y.I.N.S. and Dassanayake, R. 2015. Setaria digitata in advancing our knowledge of human lymphatic filariasis. J. Helminth. 90(2), 129–138. Pinheiro, RHS., Furtado, AP., Santos, JN. and Giese, EG. 2019. Contracaecum larvae: morphological and morphometric retrospective analysis, biogeography and zoonotic risk in the amazon. Rev. Bras. Parasitol. Vet. 28(1), 12–32. Punina, N.V., Zotov, V.S., Parkhomenko, AL., Parkhomenko, TU. and Topunov, AF. 2013. Genetic diversity of Bacillus thuringiensis from different geo ecological regions of Ukraine by analyzing the 16S rRNA and gyrB genes and by AP-PCR and saAFLP. Acta Natur. 5(1), 90–100. Purwaningsih, P., Noviyanti, P. and Sambodo, P. 2017. Gastrointestinal helminth infestation on ettawa crossbreed goat in Amban sub district Manokwari Barat district Manokwari Regency West Papua Province. J. Ilm. Pet. Ter. 5(1), 8–12. Rafee, M.A. and Amarpal A. 2016. Equine ocular setariasis and its management. J. Exp. Bio. Agr. Sci. 4, 1–6. Raju, L.K. and Udaya, M.K. 2018. A simple method of harvesting microfilaria of Setaria digitata. Pharma. Inno. J. 7(4), 657–660. Rodrigues, R.A.R., Conga, D.M.F., Santos, J.N., Gonçalves, E.C., Pinheiro, R.H.S. and Giese, E.G. 2021. Morphological diagnosis of Setaria labiatopapillosa in domestic bovids from Marajó Island, Brazil. Braz. J. Vet. Parasitol. 30(3), 1–7. Senanayake, K.S., Söderberg, J., Põlajev, A., Malmberg, M., Karunanayake, EH., Tennekoon, KH. and Niazi, A. 2020. The genome of Setaria digitata: a cattle nematode closely related to human filarial parasites. Gen. Bio. Evo. 12(2), 3971–3976. Seniati, S., Mulyani, R., dan Syahruddin, S. (2020). Viability Test of Aeromonas hydrophila Bacteria Using Frozen Storage Method in TSB and Glycerol Media. Lutjanus, 25(2): 41–48. Setiaji, J., Johan, T.I. and Widantari, M. 2015. Effect of glycerol in tryptic soy broth (TSB) media on the viability of aeromonas hydrophila bacteria. Dyn. Agr. 30(1), 83–91. Shin, J., Ahn, K.S., Suh, G.H., Kim, H.J., Jeong, H.S., Kim, B.S., Choi, E. and Shin, S.S. 2017. First blindness cases of horses infected with Setaria digitata (Nematoda: Filarioidea) in the Republic of Korea. Korean J. Parasit. 55(6), 667–671. Shin, S.S., Cho, K.O. and Wee, S.H. 2002. Ocular infection of cattle with Setaria digitata. J. Vet. Med. Sci. 64, 7–10. Sundar, S.T. and D’Souza, P.E. 2015. Morphological characterization of Setaria worms collected from cattle. J. Parasit. Dis. 39(3), 572–576. Tolistiawati, I., Widjaja, J.K., Taruk, L.L. and Isnawati, R. 2016. Gastrointestinal parasites in livestock in slaughterhouse. BALABA J. Balitbang Pengen Peny, Sigi District, Central Sulawesi; pp: 71–78. https://doi.org/10.22435/balaba.v12i2.4569.71–78 Tung, K.C., Cheng, F.P., Lai, C.H., Wang, K.S. and Lee, W.M. 2004. Demonstration of vector competence of Culex quinquefasciatus (Diptera: Culicidae) for Setaria digitata. Vet. Parasit. 123(3–4), 279–284. Watanabe, Y., Yang, C.H., Tung, K.C. and Ooi, H.K. 2004. Comparison of microfilaria concentration method for Setaria digitata infection in cattle and for Dirofilaria immitis infection in dogs. J. Vet. Med. Sci. 66(5), 543–545. Yatawara, L., S. Wickramasinghe, R. P. V. J. Rajapakse & T. Agatsuma. (2010). The complete mitochondrial genome of Setaria digitata (Nematoda: Filarioidea): Mitochondrial gene content, arrangement and composition compared with other nematodes. Molecular and Biochemical Parasitology, 173: 32–38. Zahran, F., Nassar, D.A. and Hanafy, M.A. 2022. Combined lactophenol methylene blue-blue glycerol jelly technique as a new approach for helminths staining. Parasit. United J. 15(2), 190– 194. Zargar, S. M. and Shah, A.A. 2022. Advances in nematode identification: a journey from fundamentals to evolutionary aspects. Diversity 14(7), 536. | ||
| How to Cite this Article |
| Pubmed Style Helmi TZ, Farida F, Kasturi A, Rahmah EPL, Winaruddin W, Hanafiah M. Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle. Open Vet. J.. 2025; 15(1): 187-197. doi:10.5455/OVJ.2025.v15.i1.18 Web Style Helmi TZ, Farida F, Kasturi A, Rahmah EPL, Winaruddin W, Hanafiah M. Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle. https://www.openveterinaryjournal.com/?mno=218261 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.18 AMA (American Medical Association) Style Helmi TZ, Farida F, Kasturi A, Rahmah EPL, Winaruddin W, Hanafiah M. Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle. Open Vet. J.. 2025; 15(1): 187-197. doi:10.5455/OVJ.2025.v15.i1.18 Vancouver/ICMJE Style Helmi TZ, Farida F, Kasturi A, Rahmah EPL, Winaruddin W, Hanafiah M. Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 187-197. doi:10.5455/OVJ.2025.v15.i1.18 Harvard Style Helmi, T. Z., Farida, . F., Kasturi, . A., Rahmah, . E. P. L., Winaruddin, . W. & Hanafiah, . M. (2025) Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle. Open Vet. J., 15 (1), 187-197. doi:10.5455/OVJ.2025.v15.i1.18 Turabian Style Helmi, Teuku Zahrial, Farida Farida, Aisyah Kasturi, Ella Prisa Lusisia Rahmah, Winaruddin Winaruddin, and Muhammad Hanafiah. 2025. Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle. Open Veterinary Journal, 15 (1), 187-197. doi:10.5455/OVJ.2025.v15.i1.18 Chicago Style Helmi, Teuku Zahrial, Farida Farida, Aisyah Kasturi, Ella Prisa Lusisia Rahmah, Winaruddin Winaruddin, and Muhammad Hanafiah. "Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle." Open Veterinary Journal 15 (2025), 187-197. doi:10.5455/OVJ.2025.v15.i1.18 MLA (The Modern Language Association) Style Helmi, Teuku Zahrial, Farida Farida, Aisyah Kasturi, Ella Prisa Lusisia Rahmah, Winaruddin Winaruddin, and Muhammad Hanafiah. "Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle." Open Veterinary Journal 15.1 (2025), 187-197. Print. doi:10.5455/OVJ.2025.v15.i1.18 APA (American Psychological Association) Style Helmi, T. Z., Farida, . F., Kasturi, . A., Rahmah, . E. P. L., Winaruddin, . W. & Hanafiah, . M. (2025) Unraveling the morphological and molecular profile of Setaria digitata in Aceh cattle. Open Veterinary Journal, 15 (1), 187-197. doi:10.5455/OVJ.2025.v15.i1.18 |