| Research Article | ||
Open Vet. J.. 2025; 15(1): 179-186 Open Veterinary Journal, (2025), Vol. 15(1): 179-186 Research Article Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbits modelRaghad Al-Saadi1, Zainab Jamal Mohammed Jawad1, Omar Hussein Khalaf1*, and Siti Nur F. Muhsain21Department of Pathology & Poultry Diseases, College of Veterinary Medicine, University of Baghdad, Baghdad City, Iraq 2Faculty of Pharmacy, Universiti Teknologi MARA, Shah Alam, Malaysia *Corresponding Author: Omar Hussein Khalaf. Department of Pathology & Poultry Diseases, College of Veterinary Medicine, University of Baghdad, Baghdad City, Iraq. Email: omar.h [at] covm.uobaghdad.edu.iq Submitted: 30/08/2024 Accepted: 24/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
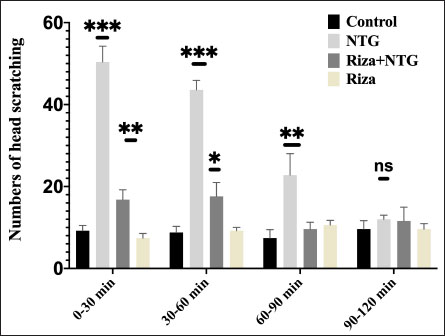
AbstractBackground: Migraine is one of multiple attack neurological conditions that causes moderate to severe headaches with no defined pathophysiology and few animal models. Aim: Establishing an animal model that reproduces migraine-like action is important in medical research to identify the mechanism underlying this disorder. Additionally, it facilitates the availability and reliability of new models that may act as human surrogate models. Method: Rabbits were divided into four groups. Negative group, migraine group, rizatriptan-nitroglycerin group, and rizatriptan group. The frequency of head scratching and the histopathological changes in the brain, liver, kidney, and heart for groups were evaluated in all groups. Results: The behavioral characteristic of head scratching was significantly increased in the NTG group (50.4 ± 3.8) compared with the control group (9.2 ± 1.3) after 30 min of the experiment. Moreover, animals treated with rizatriptan benzoate (Riza) 10 mg/kg/orally for 14 days followed by NTG injection showed a significant decrease in the head scratch action (16.8 ± 2.3 and 17.6 ± 3.3) than the animals of NTG group (50.4 ± 3.8 and 43.6 ± 2.3) after 30 min and 60 min, respectively. Furthermore, animals treated with Riza alone showed no statistical differences in the head scratches (7.8 ± 1.3, 9.2 ± 0.8, 10.6 ± 1.1 and 9.6 ± 1.3, respectively) during the 120 min of the experiment, compared with the control group. Histopathological alterations in the brain of rabbits that received NTG showed severe diffuse dilated and engorged blood vessels. These changes were also recorded in the liver and kidney of this group. This marked vasodilation of blood vessels and central and portal veins confirms the successful induction of migraine in the rabbit model. In contrast, animals treated with Riza for 14 days demonstrated substantially less vascular dilation following NTG injection. No significant pathological lesions were observed in animals treated with Riza. Conclusion: The current study successfully established a rabbit model of migraine using a single dose of NTG to induce migraine-like behavior. Moreover, pre-treatment with rizatriptan benzoate for fourteen days significantly reduced the symptoms of migraine and histopathological changes in different organs. Keywords: Rizatriptan, Migraine, Histopathology, Rabbit model. IntroductionRizatriptan benzoate (Riza) is used to treat the symptoms of the most common neurological conditions known as migraine headaches (Tfelt-Hansen and Messlinger, 2019). Cerebral and meningeal arterial vasodilation during migraine attach reverses by vasoconstriction following rizatriptan benzoate administration by the release of the chemical messenger serotonin (Silberstein,2004; Tardiolo et al., 2019). Rizatriptan a second-generation triptan tablet wafer formulation is absorbed rapidly in the gastrointestinal tract reaching its peak plasma concentration to treat migraine headaches (Tfelt-Hansen and Messlinger, 2019; Yang et al., 2022). Oral administration of Riza at 10 mg has proven its safety and efficacy in treating acute, moderate, or severe migraine (Aubé et al., 2010). The acute potential oral toxicity (LD50) of Riza in mice and rats was 700 mg/kg/B.W and 2227 mg/kg/B.W, respectively, whereas the estimated intravenous LD50 was 89 and 141 mg/kg/B.W, respectively (Product Monograph, 2020). However, the chronic toxicity of Riza was measured following multiple repeated doses for 12 months in dogs and rats and up to 14 weeks in mice and no adverse effects at the suggested therapeutic dosages have been reported (Product Monograph, 2020). A highly lipophilic organic nitrate such as nitroglycerin (NTG) is used in the treatment of coronary artery diseases such as angina pectoris, myocardial infarction, and heart failure. The vasodilatory action of NTG produces headache, photophobia (light sensitivity), and anxiety which are similar to migraineurs (Marsh and Marsh, 2000; Thadani and Ripley, 2007). It was noted that individuals suffering from migraine showed a higher cardiovascular response to NTG compared with control patients (Thadani and Ripley, 2007; Van Oosterhout et al., 2020). The frequent side effect of NTG represented as headache was demonstrated to induce migraine models in humans (Demartini et al., 2019). For decades, migraine was considered one of the most studied headache disorders in humans (Amiri et al., 2022). To study migraine, a variety of rodents, such as mice and rats, have been used to simulate different features of migraine with certain limitations (Erdener and Dalkara 2014; Harriott et al., 2019). However, the use of rabbits as an alternative model for NTG-induced migraine is less commonly reported in the literature. Therefore, the aim of the current study was to investigate the suitability of rabbits as a migraine model following NTG injection by evaluating migraine symptoms and histopathological changes in different organs. Moreover, the effect of pre-treatment of Riza against NTG-induced migraine attacks was also assessed. Material and MethodsExperimental animalsMale, 2-month-old, rabbits (Oryctolagus cuniculus) (n=24), with 450–500 g weight, were divided equally into 4 groups: Negative control group. The migraine rabbit model received 10 mg/kg of NTG (Flagship Biotech International Pvt Ltd) s/c and served as a positive control group. Rizatriptan benzoate group (MSD, UK) (Riza-NTG group) received 10 mg/kg orally for 14 days before migraine induction, then 30 minutes after the last Riza administration, nitroglycerin was injected with 10 mg/kg s/c (Zhang et al., 2017). The Riza group was treated only with 10 mg/kg/orally for 14 days. All animals were monitored daily for any clinical signs associated with NTG or rizatriptan administration. Four hours following migraine induction, animals were anesthetized using xylazine–ketamine intramuscular injection as described by (Holve et al., 2013), and then euthanized by cervical dislocation according to AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Tissue specimens of the brain, liver, kidney, and heart were collected and immediately fixed in 10% neutral buffer formalin. Samples were routinely processed, embedded in paraffin wax, sectioned at 5um thickness, and stained with H&E stain for histopathological evaluation (Khalaf et al., 2019; Abbasa and Jawad, 2023; Shakir et al., 2023). Behavioral testsThe behavioral examination was measured according to previous studies. Briefly, after all rabbits were acclimatized inside the cages, the frequency of head scratching was calculated for 0–120 minutes during the whole 30-minute intervals (Min et al., 2017; Sun et al., 2021). The means of head-scratching behavior in the migraine rabbit model for the control, NTG, Riza+NTG, and Riza groups during 0–120 minutes were counted and analyzed statistically. After NTG injection, the animal demonstrated symptoms of restlessness accompanied by head scratching, which lasted for approximately 90 minutes, and the pain signs of head scratching were relatively obvious, for which rabbits were regarded as positive reactions of migraine. The symptoms of rabbits’ head scratching were recorded continuously by observers who were blinded to all treatment groups every 30 minutes for 0–120 minutes. Statistical analysisGraphPad Prism software (Prism version 8) was used to analyze the data of head-scratching behavior in the migraine rabbit model. One-way ANOVA followed by Tukey’s multiple comparisons test was used to calculate the differences among all the groups. The mean ± SD was considered and p values less than 0.05 were considered as significant results (95% confidence intervals). Ethical approvalRabbit management and handling were carried out in this study with all required permits and performed in accordance with all regulations and recommended procedures approved by the Iraqi Ethics Committee of the College of Veterinary Medicine, University of Baghdad (Protocol No. P.G/1218). All animal care and experimental actions such as the migraine model experiment and anesthetic technique were approved by the Ethics Committee for Animal Experiments at the College of Veterinary Medicine Collage, University of Baghdad, Iraq. We minimized the number of animals to obtain the statistical significance differences (Protocol No. P.G/1218). ResultsHead scratching behaviorIn the current study, rabbits have been used to establish a new model to study migraine (pain attacks). The frequency of head scratching actions was counted based on observation every 30-minute intervals for 0–120 min to provide scientific migraine-like behavior in the rabbits treated with NTG or without NTG injection. The NTG-induced migraine rabbits showed a significantly higher number of head scratches compared with the negative control group. The typical frequency of head scratching started in the NTG-injected rabbits within 5 min and lasted for at least 90 min (Fig. 1). The behavioral characteristic of head scratching was significantly higher in the NTG group (50.4 ± 3.8) compared with the control group (9.2 ± 1.3) following 30 min of s/c injection of NTG.
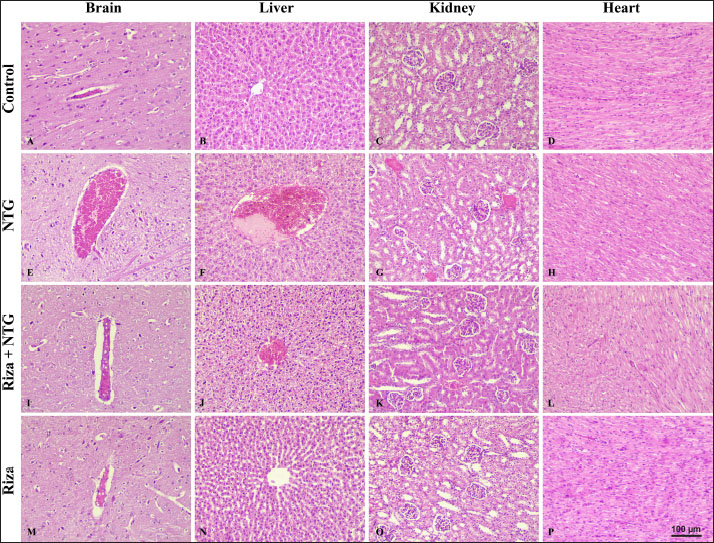
Fig. 1. Frequency of head scratching behavior in migraine rabbit model. Control, NTG, Riza+NTG, and Riza during 0–120 minutes following nitroglycerin injection. Each point represented mean ± SD (n=6), Adjusted *P values < 0.005. Interestingly, animals of the Riza+NTG treated group demonstrated significantly much lower head scratches (16.8 ± 2.3 and 17.6 ± 3.3) than the animals of the NTG group (50.4 ± 3.8 and 43.6 ± 2.3) after 30 min and 60 min, respectively. Whereas, the animals of the Riza group showed no significant differences in the head scratches (7.8 ± 1.3, 9.2 ± 0.8, 10.6 ± 1.1 and 9.6 ± 1.3) after 30 min, 60 min, 90 min, and 120 min, respectively, compared with the negative control. No significant differences were recorded in the number of head scratches behavior following 90–120 min in all treated groups which referred to the successful migraine rabbits model establishment. Histopathology studyHistopathological alterations within the brain of rabbits that received NTG that characterized by severe diffuse dilated and engorged blood vessels (Fig. 2E). Similar changes were also noted in the liver and kidney of this group. Marked vasodilation and congestion of central and portal veins in addition to the blood vessels between the renal tubules (Figs. 2F and G). Interestingly, in comparison with rabbits of the NTG, mild vascular dilation was diffusely observed in the brain, liver, and kidney of Riza+NTG (Figs. 2I, J, and K), suggesting that rabbits can be utilized as a promising sensitive model of migraine. Moreover, no significant histopathological changes were observed in all examined tissues of the rabbits that were treated with Riza for 14 days, as well as in the negative control group (Figs. 2M, N and O), suggesting the safety of Riza when it is administrated at therapeutic does. No significant histological lesions have been observed in the sections of the heart of the control and all treated animals (Figs. 2D, H, L, and P).
Fig. 2. Representative histopathological examinations of different tissue specimens from rabbits injected with NTG, Riza+NTG, and Riza alone. (A-D) Brain, liver, kidney and heart of control group showed normal tissue architecture. (E-G) Brain, liver, and kidney of rabbits injected with10 mg/kg of NTG s/c showed severe diffuse dilated and engorged blood vessels. (I-K) Brain, liver, and kidney of rabbits treated with Riza at dose of 10 mg/kg/orally for 14 days followed by10 mg/kg of NTG s/c showed mild vascular vasodilation and engorgement. (M-O) Brain, liver, and kidney of rabbits treated with Riza alone at dose of 10 mg/kg/orally for 14 days showed no histopathological changes. (D, H, L, and P) Tissue section of hearts of all animals showed no significant lesions detected. H&E stain, Scale bar 100 μm. DiscussionMigraine is a worldwide neurological disorder that affects approximately one billion people (GBD, 2018), and it has been reported that young adults and middle-aged women potentially suffer from migraine (Yang et al., 2022). Although several studies have suggested the pathophysiology of migraine, including abnormal cranial vasodilation, the activation of meningeal afferents, and neurogenic inflammation, the mechanisms of migraine induction remain unclear (Edvinsson et al., 2021). For decades, developing potential animal models to study complex conditions such as migraine-like disorder has been challenging due to the lack of a translational model that reflects most human disorder (Borsook and Burstein, 2012). No doubt that rats and mice have been frequently utilized as models for studying migraine following NTG administration (Bates et al., 2010; Akerman et al., 2019). However, higher or repetitive doses of NTG are required to induce relevant migraine effects in rodents, especially in rats, which are linked to inefficient bioconversion of NTG in the liver in comparison to humans which possibly limits the detection of light avoidance as a headache disorder (Sokołowska et al., 2004; Bates et al., 2010) and because of few available translational animal models (Demartini et al., 2019). Therefore, establishing a new reliable model that reproduces migraine-like behavior is needed to better understand the mechanism of this worldwide neurological disorder and increase the availability and reliability of new models. The present study has several highlights: 1) At the beginning, this study has proved that rabbits develop frequent head scratching and redden ears; 2) NTG administration has successfully induced migraine-like headache in rabbits; 3) Riza reduced NTG-induced migraine when demonstrated as a prophylactic treatment, suggesting that rabbits respond effectively to migraine treatment; 4) no histopathological changes associated with daily administration of Riza in rabbits. To the best of our knowledge, the current study provides valuable initial insights for using rabbits as a model to study migraine-like symptoms. For decades, rabbits have contributed not only as a model to evaluate new therapeutic compounds but also to studying metabolic and infectious diseases as well as CNS disorders in humans (Shiomi, 2009; Close at al., 2019; Shwaish et al., 2024). The opinion of using rabbits as an animal model for migraine rather than other animals was efficient for the purpose of the current study. First, a single s/c dose of NTG induced acute headache symptoms (migraine) in rabbits rather than multiple dosages in rats and mice (Jalgaonkar et al. 2023). Moreover, rabbits act as an excellent species for investigating a number of aspects of human disorders including neuronal disorders. Phylogenetically, rabbits are closer to humans than rodents regarding brain development and neuroanatomical characteristics. In addition, rabbits have an appropriate size and ease of use and care in the laboratory facility, which allows the observation of clinical symptoms and the progression of the disease (Fan et al 2018; Zhang et al, 2022). NTG has been widely utilized as a reliable translational approach to induce typical migraine headaches (Demartini et al., 2019). In the present study, rabbits effectively responded to subcutaneous injection of NTG and immediately showed migraine-like behaviors that lasted for 90 min, such as red ears, frequency head scratching, and restlessness which indicated the successful initial insights of migraine-like behaviors in the rabbit model. The mechanism of NTG-induced migraine has been well studied for decades, and because of its lipophilic features, NTG easily crosses the blood–brain barrier and causes a dual action that parallels the headache response that has been reported in human studies. In addition, NTG activates specific structures in the CNS that cause nausea, photophobia, and phonophobia (Maniyar et al., 2014a,b). Nitric oxide, which is produced following NTG administration, works directly on the wall of blood vessels causing vasodilation and inflammation (Moncada et al., 1991; Chung and Fung, 1993). The current findings are consistent with previous experimental studies in the migraine field that described the importance of using NTG to induce migraine in humans and animals through its direct and indirect action on blood vessels and neurons and consistent ability to produce migraine-like features (Kleschyov et al., 2003; Thatcher et al., 2004). Interestingly, rabbits that received a single dose of 10 mg/kg NTG subcutaneously exhibited migraine-like behaviors that lasted for 90 min, and these behaviors might mimic the symptoms that appear in humans and another animal model that received NTG and developed headache, increased scratching that reflect discomfort in their heads, and uncomfortable feelings caused by pain (Goadsby et al., 2002; Markovics et al., 2012; Sufka et al., 2016; Farajdokht et al., 2017; Dodick, 2018). Certainly, more work remains to be done to identify the relevance of using the rabbit model for migraine experiments and to provide an opportunity to find biomarkers for headache disorders. Several available antimigraine treatments have been used to decrease the incidence and severity of migraine attacks (Silberstein et al., 2012), and among these agents, a selective serotonin (5-hydroxytryptamine [5-HT]) receptor agonist rizatriptan, the second-generation of triptan (Tfelt-Hansen and Messlinger, 2019). Triptans are a family of antimigraine drugs that stimulate 5-HT1B/D receptors, in turn, cause vasoconstriction and inhibit the release of several neuropeptides that cause migraine (Loder, 2010). One of the key findings of the current study was to determine whether the rabbit model would respond to Riza as an antimigraine medication and the hypothesis that rizatriptan could be used as a prevention to reduce migraine symptoms when it is dosing prior attacks. Interestingly, the results suggested that rabbits pretreated with Riza for 14 days followed by NTG injection successfully responded to the pretreatment via attenuation of the severity of migraine-like symptoms. These data are consistent with the ability of rizatriptan to reduce migraine via reduced cerebral blood flow and blood volume from arterial-to capillary (Okazawa et al., 2006), in addition to reducing several factors that induce migraine in humans and different animal models (Asghar et al., 2011; Mason et al., 2017; Yang et al., 2022). The efficacy of rizatriptan 10 mg in acute migraine has been clearly established with or without aura. Clearly, Riza is a drug that is used to treat migraine symptoms with no future prevention of migraine attacks. However, this study suggested that Riza might be minimizing the acute migraine symptoms rather than prevention of migraine. Our results agreed with the results of Yao et al. (2012), who found that Riza minimized the expression of the mRNAs of proenkephalin and substance P, as well as inhibited the analgesic role of the endogenous pain modulatory system in the rat midbrain. Histologically, this work also demonstrated that NTG caused marked vasodilation and engorgement of blood vessels, which were not only observed in the brain but were also seen in the liver and kidney. Histological lesions associated with NTG injection were markedly reduced by the prophylactic use of Riza, indicating the protective effect of Riza against NTG-induced vasodilation. These observations were strengthened by the fact that the NTG administration causes potent vasodilation, which was mediated by the conversion of NTG into nitric oxide in the endothelial layer of blood vessels that triggers a migraine attack (Tassorelli et al., 1999; Sureda et al., 2022). However, consistent with several preclinical studies, our data showed that Riza caused vasoconstriction of blood vessels and reduced cerebral blood flow, which resulted in relief of migraine onset. This result was markedly obvious in histological sections. Furthermore, we evaluated the toxicopathological changes associated with daily use of 10 mg/kg of Riza alone. Interestingly, the data demonstrated that there were no tissue or vascular alterations in the examined tissues, especially in the brain. These findings support the evidence of cerebrovascular safety of Riza based on preclinical experiments (Gori et al., 2005). ConclusionTaken together, this study demonstrates for the first time that the rabbit model might be a more suitable and promising model to study migraine, in addition to identifying the pathogenesis underlying migraine attacks, screening, and testing a new generation of antimigraine medications. Moreover, pretreatment of rizatriptan benzoate would be a drug of choice to minimize the symptoms of migraine. AcknowledgmentThe authors would like to thank Dr. Maulood M. Shather for additional reading and support. Conflict of interestThe authors declare no conflict of interest. Author’s contributionsRA and SM: study’s concept. RA, ZJ, and OK: Practical work, data analysis, tissue sections preparation and interpretation. RA and OK: Writing manuscript draft and editing. All authors revised and prepared the manuscript for publication. FundingThis research received no specific grant. Data availabilityAll data supporting the current study are available within the manuscript. ReferencesAbbasa, M.I. and Jawad, Z.J.M. 2023. Hepatoprotective effect of alcoholic extract of Ficus carica leaves against cypermethrin-induced liver toxicity in male albino rats. Iraqi J. Vet. Med. Vol. 47(2), 64–2. Akerman, S., Karsan, N., Bose, P., Hoffmann, J.R., Holland, P.R., Romero-Reyes, M. and Goadsby, P.J. 2019. Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity. Brain. 142(1), 103–119. Amiri, P., Kazeminasab, S., Nejadghaderi, S.A., Mohammadinasab, R., Pourfathi, H., Araj-Khodaei, M., Sullman, M.J.M., Kolahi, A.A. and Safiri, S. 2022. Migraine: A review on its history, global epidemiology, risk factors, and comorbidities. Front Neurol. 12, 800605. Asghar, M.S., Hansen, A.E., Amin, F.M., Van der Geest, R.J., Koning, P.V., Larsson, H.B., Olesen, J. and Ashina, M. 2011. Evidence for a vascular factor in migraine. Ann Neurol. 69(4), 635–645. Aubé, M., Chouha, F., Vaillancourt, J. and Sampalis, J. 2010. Effectiveness and safety of rizatriptan benzoate 10 mg in the treatment of migraine headaches. Clin. Med. Insights: Ther. 2. Bates, E.A., Nikai, T., Brennan, K.C., Fu, Y.H., Charles, A.C., Basbaum, A.I., Ptácek, L.J. and Ahn A.H. 2010. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 30(2), 170–178. Borsook, D. and Burstein, R. 2012. The enigma of the dorsolateral pons as a migraine generator. Cephalalgia.32(11), 803–812. Chung, S.J. and Fung, H.L. 1993. Relationship between nitroglycerin-induced vascular relaxation and nitric oxide production. Probes with inhibitors and tolerance development. Biochem. Pharmacol. 45(1), 157–63. Close, L.N., Eftekhari, S., Wang, M., Charles, A.C. and Russo, A.F. 2019. Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia 39(3), 428–434. Demartini, C., Greco, R., Zanaboni, A.M., Sances, G., De Icco, R., Borsook, D. and Tassorelli, C. 2019. Nitroglycerin as a comparative experimental model of migraine pain: From animal to human and back. Prog. Neurobiol. 177, 15–32. Dodick, D.W. 2018. Migraine. Lancet. 391(10127), 1315–1330. Edvinsson, L. and Haanes, K.A. 2021. Identifying new antimigraine targets: lessons from molecular biology. Trends. Pharmacol. Sci. 42(4), 217–225. Erdener, S.E. and Dalkara, T. 2014. Modelling headache and migraine and its pharmacological manipulation. Br. J. Pharmacol. 171(20), 4575–4794. Fan, J., Chen, Y., Yan, H., Niimi, M., Wang, Y. and Liang, J. 2018. Principles and applications of rabbit models for atherosclerosis research. J. Atheroscler. Thromb. 25(3), 213–220. Farajdokht, F., Babri, S., Karimi, P., Alipour, M. R., Bughchechi, R. and Mohaddes, G. 2017. Chronic ghrelin treatment reduced photophobia and anxiety-like behaviors in nitroglycerin- induced migraine: role of pituitary adenylate cyclase-activating polypeptide. Eur J Neuroci. 45(6), 763–772. GBD 2016 Headache Collaborators. 2018. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol.17(11), 954–976. Goadsby, P.J., Lipton, R.B. and Ferrari, M.D. 2002. Migraine--current understanding and treatment. N. Engl. J. M. 346(4), 257–270. Gori, S., Morelli, N., Bellini, G., Bonanni, E., Manca, L., Orlandi, G., Iudice, A. and Murri, L. 2005. Rizatriptan does not change cerebral blood flow velocity during migraine attacks. Brain Res. Bull. 65(4), 297–300. Harriott, A.M., Strother, L.C., Vila-Pueyo, M. and Holland, P.R. 2019. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J. Headache Pain. 29;20(1), 91. Holve, D.L., Gum, G.G. and Pritt, S.L. 2013. Effect of sedation with xylazine and ketamine on intraocular pressure in New Zealand white rabbits. JAALAS, 52(4), 488–490. Hou, M., Tang, Q., Xue, Q., Zhang, X., Liu, Y., Yang, S., Chen, L. and Xu, X. 2017. Pharmacodynamic action and mechanism of Du Liang soft capsule, a traditional Chinese medicine capsule, on treating nitroglycerin-induced migraine. J. Ethnopharmacol. 4, 195–231 Jalgaonkar, S., Tripathi, R., Khatri, N., Patankar, R., Gajbhiye, S., Sayyed, M. and Shankar, A. 2023. Analgesic, anti-inflammatory, and antioxidant potential of S-adenosyl L-Methionine on nitroglycerine induced migraine in mice models. J. Pharma. Pharmacol. Res. 6, 20–27. Khalaf, O.H., Chaki, S.P., Garcia-Gonzalez, D.G., Ficht, T.A. and Arenas-Gamboa, A.M. 2019. The nod-scid IL2rγnull mouse model is suitable for the study of osteoarticular brucellosis and vaccine safety. Infect. Immun, 87(6), e00901-18. Kleschyov, A.L., Oelze, M., Daiber, A., Huang, Y., Mollnau, H., Schulz, E., Sydow, K., Fichtlscherer, B., Mülsch, A. and Münzel, T. 2003. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circ. Res. 93(9):e104–12. Loder, E. 2010. Triptan therapy in migraine. N. Engl. J. Med. 363(1), 63–70. Maniyar, F.H., Sprenger, T., Schankin, C. and Goadsby, P.J. 2014. Photic hypersensitivity in the premonitory phase of migraine--a positron emission tomography study. Eur. J. Neurol. 21(9), 1178–83. Maniyar, F.H., Sprenger, T., Schankin, C. and Goadsby, P.J. 2014. The origin of nausea in migraine-a PET study. J Headache Pain. 15(1), 84. Markovics, A., Kormos, V., Gaszner, B., Lashgarara, A., Szoke, E., Sandor, K., Szabadfi, K., Tuka, B., Tajti, J., Szolcsanyi, J., Pinter, E., Hashimoto, H., Kun, J., Reglodi, D. and Helyes, Z. 2012. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol. Dis. 45(1), 633–644. Marsh, N. and Marsh, A. 2000. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin. Exp. Pharmacol. Physiol. 27, 313–319. Mason, B.N., Kaiser, E.A., Kuburas, A., Loomis, M.M., Latham, J.A., Garcia-Martinez, L.F. and Russo, A.F. 2017. Induction of migraine-like photophobic behavior in mice by both peripheral and central cgrp mechanisms. J. Neurosci. 37(1), 204–216. Moncada, S., Palmer, R.M. and Higgs, E.A. 1991. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43(2), 109–142. PMID: 1852778. Okazawa, H., Tsuchida, T., Pagani, M., Mori, T., Kobayashi, M., Tanaka, F. and Yonekura, Y. 2006 Effects of 5-HT1B/1D receptor agonist rizatriptan on cerebral blood flow and blood volume in normal circulation. J. Cereb. Blood Flow Metab. 26(1), 92–98. Product Monograph. 2020. Rizatriptan benzoate tablets, manufacturer’s standard 5 mg and 10 mg rizatriptan (as rizatriptan benzoate) 5-ht1 receptor agonist migraine therapy, available via https://pdf.hres.ca/dpd_pm/00056193.PDF. Shakir, Z.K., Siti-Nur, F.M. and Al-Saadi Raghad N. 2023. Effect of perfluorooctanoic acid on kidney function in diabetic and non- diabetic male guinea pigs. Iraqi J. Vet Med. 47(2), 73–80. Shiomi, M. 2009. Rabbit as a model for the study of human diseases. In: Houdebine, LM., Fan, J. (eds) Rabbit Biotechnology. Dordrecht, Netherland: Springer, pp. 49–63. Shwaish, M.M., Hasan, M.S., Jarad, A.S. and Al-Rekabi F.M.K. 2024. Experimental ivermectin poisoning in rabbits with trial for treatment. Egypt. J. Vet. Sci. 55 (5), 1205–1216. Silberstein, S.D. 2004. Migraine. Lancet; 363(9406), 381–391. Silberstein, S.D., Holland, S., Freitag, F., Dodick, D.W, Argoff, C. and Ashman, E. 2012. Quality Standards subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 78(17), 1337–1345. Sokołowska, M., Bednarski, M., Kwiecień, I., Filipek, B. and Włodek, L. 2004. Bioactivation of nitroglycerin to nitric oxide (NO) and S-nitrosothiols in the rat liver and evaluation of the coexisting hypotensive effect. Fundam. Clin. Pharmacol.18(4), 449–456. Sufka, K.J., Staszko, S.M., Johnson, A.P., Davis, M.E., Davis, R.E. and Smitherman, T.A. 2016. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J. Headache Pain. 17(40). Sun, S., Zheng, G., Zhou, D., Zhu, L., He, X., Zhang, C., Wang, C. and Yuan, C. 2021. Emodin interferes with nitroglycerin-induced migraine in rats through CGMP-PKG pathway. Front. Pharmacol. 20;12, 758026. Sureda-Gibert, P., Romero-Reyes, M. and Akerman, S. 2022. Nitroglycerin as a model of migraine: clinical and preclinical review. Neurobiol. Pain. 12, 100105. Tardiolo, G., Bramanti, P. and Mazzon E. 2019. Migraine: Experimental models and novel therapeutic approaches. Int. J. Mol. Sci. 20(12), 2932. Tassorelli, C., Joseph, S.A., Buzzi, M.G. and Nappi, G. 1999. The effects on the central nervous system of nitroglycerin--putative mechanisms and mediators. Prog. Neurobiol. 57(6), 607–624. Tfelt-Hansen, P. and Messlinger, K. 2019. Why is the therapeutic effect of acute antimigraine drugs delayed? A review of controlled trials and hypotheses about the delay of effect. Br. J. Clin. Pharmacol. 85(11), 487–2498. Thadani, U. and Ripley, T.L. 2007. Side effects of using nitrates to treat heart failure and the acute coronary syndromes, unstable angina and acute myocardial infarction. Expert Opin. Drug Saf. 6(4), 385–96. Thatcher, G.R., Nicolescu, A.C., Bennett, B.M. and Toader, V. 2004. Nitrates and NO release: contemporary aspects in biological and medicinal chemistry. Free Radic. Biol. Med. 37(8), 1122–1143. Van Oosterhout, W.P., Schoonman, G.G., Saal, D.P., Thijs, R.D., Ferrari, M.D. and Van Dijk, J.G. 2020. Abnormal cardiovascular response to nitroglycerin in migraine. Cephalalgia 40(3), 266–277. Yang, S., Chen, C., Liu, X., Kang, Q., Ma, Q., Li, P., Hu, Y., Li, J., Gao, J., Wang, T. and Wang, W. 2022. Xiongshao zhitong recipe attenuates nitroglycerin-induced migraine-like behaviors via the inhibition of inflammation mediated by nitric oxide synthase. Front. Pharmacol. 13, 920201. Yao, G., Man, Y., Luo, X., Yu, T. and Ji, L. 2012. Rizatriptan benzoate influences the endogenous pain modulatory system in a rat model of migraine. Neural Regen. Res, 7(2), 131–135. Zhang, X.F., Zhang, W.J., Dong, C.L., Hu, W.L., Sun, Y.Y., Bao, Y., Zhang, C.F., Guo, C.R., Wang, C.Z. and Yuan, C.S. 2017. Analgesia effect of baicalein against NTG-induced migraine in rats. Biomed. Pharmacother. 90, 116–121. Zhang, Z., Song, Y., La,i L. and Li, Z. 2022. Genome-edited rabbit, a prospective alternative model for neurological diseases. Ageing Neur. Dis. 2, 16. | ||
| How to Cite this Article |
| Pubmed Style Al-saadi R, Jawad ZJM, Khalaf OH, Muhsain SNF. Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model. Open Vet. J.. 2025; 15(1): 179-186. doi:10.5455/OVJ.2025.v15.i1.17 Web Style Al-saadi R, Jawad ZJM, Khalaf OH, Muhsain SNF. Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model. https://www.openveterinaryjournal.com/?mno=217997 [Access: September 04, 2025]. doi:10.5455/OVJ.2025.v15.i1.17 AMA (American Medical Association) Style Al-saadi R, Jawad ZJM, Khalaf OH, Muhsain SNF. Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model. Open Vet. J.. 2025; 15(1): 179-186. doi:10.5455/OVJ.2025.v15.i1.17 Vancouver/ICMJE Style Al-saadi R, Jawad ZJM, Khalaf OH, Muhsain SNF. Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model. Open Vet. J.. (2025), [cited September 04, 2025]; 15(1): 179-186. doi:10.5455/OVJ.2025.v15.i1.17 Harvard Style Al-saadi, R., Jawad, . Z. J. M., Khalaf, . O. H. & Muhsain, . S. N. F. (2025) Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model. Open Vet. J., 15 (1), 179-186. doi:10.5455/OVJ.2025.v15.i1.17 Turabian Style Al-saadi, Raghad, Zainab Jamal Mohammed Jawad, Omar Hussein Khalaf, and Siti Nur Fadzilah Muhsain. 2025. Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model. Open Veterinary Journal, 15 (1), 179-186. doi:10.5455/OVJ.2025.v15.i1.17 Chicago Style Al-saadi, Raghad, Zainab Jamal Mohammed Jawad, Omar Hussein Khalaf, and Siti Nur Fadzilah Muhsain. "Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model." Open Veterinary Journal 15 (2025), 179-186. doi:10.5455/OVJ.2025.v15.i1.17 MLA (The Modern Language Association) Style Al-saadi, Raghad, Zainab Jamal Mohammed Jawad, Omar Hussein Khalaf, and Siti Nur Fadzilah Muhsain. "Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model." Open Veterinary Journal 15.1 (2025), 179-186. Print. doi:10.5455/OVJ.2025.v15.i1.17 APA (American Psychological Association) Style Al-saadi, R., Jawad, . Z. J. M., Khalaf, . O. H. & Muhsain, . S. N. F. (2025) Histopathological effects of repeated 14-day administration of rizatriptan benzoate in a nitroglycerin-induced migraine rabbit model. Open Veterinary Journal, 15 (1), 179-186. doi:10.5455/OVJ.2025.v15.i1.17 |