| Research Article | ||
Open Vet. J.. 2025; 15(1): 171-178 Open Veterinary Journal, (2025), Vol. 15(1): 171-178 Original Article Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, NigeriaEmmanuel Nnabuike Ugbo1, Mustofa Helmi Effendi2, Agatha Ifunanya Ugbo3, Wiwiek Tyasningsih4*, Bernard Nnabuife Agumah1, Hartanto Mulyo Raharjo4, Aswin Rafif Khairullah5, Rebecca Chinenye Ogba6, Fitrine Ekawasti5, Sheila Marty Yanestria7, Ikechukwu Benjamin Moses1 and Katty Hendriana Priscilia Riwu81Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 2Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Department of Microbiology and Parasitology, David Umahi Federal University of Health Sciences, Uburu, Ebonyi State, Nigeria 4Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Department of Science Laboratory Technology, Federal Polytechnic Ohodo, Enugu State, Nigeria 7Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 8Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia *Corresponding Author: Wiwiek Tyasningsih. Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: wiwiek-t [at] fkh.unair.ac.id Submitted: 28/08/2024 Accepted: 02/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
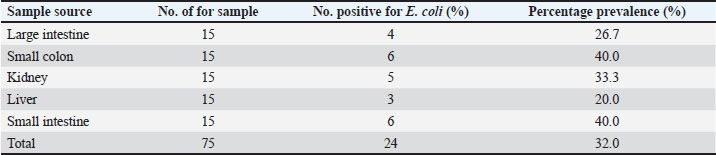
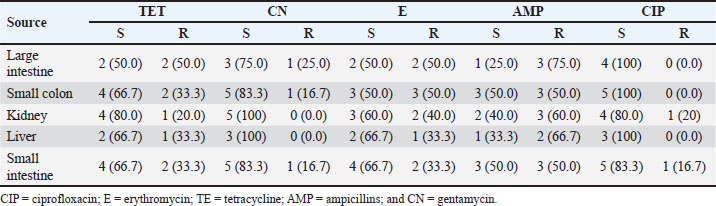
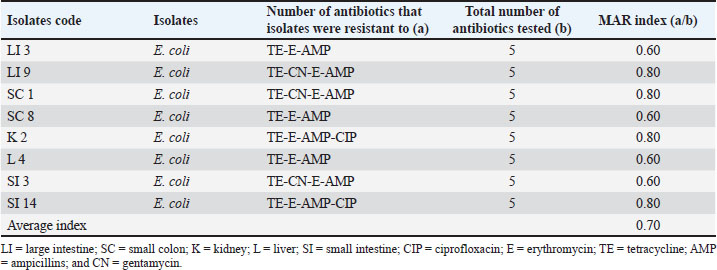
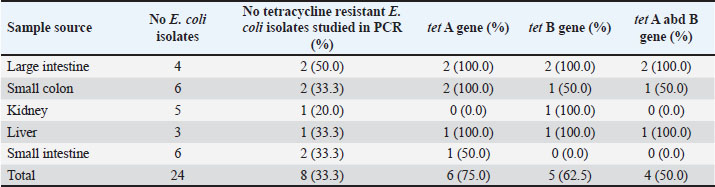
AbstractBackground: Swine is one of the major sources of protein to humans worldwide; antimicrobial-resistant Escherichia coli has become a global public health challenge affecting both humans and livestock due to the presence of tetracycline resistance genes. Aim: This study focused on molecular identification of tetracycline resistance genes (tet A and B) in E. coli isolates from internal organs of swine sold in a slaughterhouse at Abakaliki, Ebonyi State, Nigeria. Methods: A total of 75 internal organs of swine samples were collected from slaughterhouses. Standard microbiological procedures were employed to evaluate the samples bacteriologically. Using the disk diffusion method, antibiotic susceptibility testing was conducted on E. coli against specific classes of antibiotics, and the multiple antibiotic resistance index was calculated. The polymerase chain reaction was utilized for the molecular identification of the tetracycline resistance genes, specifically tet A and B. Results: Out of the 75 samples analyzed, 24 of 75 were positive for E. coli with an overall prevalence of 24/75 (32.0%). The small intestine and colon had higher percentages of E. coli isolates 6/15 (40.0%). However, E. coli isolates were resistant to erythromycin, tetracycline, and ampicillin which ranged from 20.0% to 75.0%, and susceptible to gentamycin and ciprofloxacin at a range of 75.0%–100.0%. Exactly, 8 (33.3%) isolates were both multidrug and tetracycline-resistant. The presence of tet A 6/8 (75.0%), tet B 5/8 (62.5%), and tet A and B 4/8 (50.0%) was reported. Conclusion: Multidrug and tetracycline resistance genes have been observed in E. coli isolated from internal organs of swine and are of public health concern. Keywords: Tetracycline resistance genes, E. coli, Internal organs, Swine, Public health. IntroductionThe lower intestine of both humans and animals frequently contains the rod-shaped, Gram-negative bacterium Escherichia coli. In Nigerian pig farms, E. coli illness causes significant productivity losses in both pre- and post-weaned piglets (Egbule et al., 2021). Resistance profiles of E. coli recovered from food animals, such as pigs and poultry, have a strong association with those of isolates from bloodstream infections in people (Vieira et al., 2011; Effendi et al., 2018). Piglets and pigs suffering from livestock illnesses such as E. coli disease are a serious danger to the profitability and long-term viability of the Nigerian pig industry. Nigerians consume a lot of pork, and pigs carry a higher risk of spreading multidrug-resistant E. coli to people through the food chain (Egbule et al., 2021). Highly adapted organisms called E. coli inhabit the gastrointestinal tracts of both people and animals, thriving in conditions such as water, dirt, sediment, and feces. The presence of E. coli is a reliable indicator of environmental fecal contamination and is essential for tracking the spread of antimicrobial resistance (AMR) genes among bacterial communities (Tyasningsih et al., 2022; Mustika et al., 2024). In terms of the transmission of AMR, the relationships between humans, animals, and the environment are complex (Oloso et al., 2018; Yanestria et al., 2022). Nearly similar IncI1 plasmids encoding third-generation cephalosporin resistance determinants are carried by the genetically different E. coli isolates from humans and animals. This plasmid aids in the transmission of multidrug-resistant organisms from food animals (such as chickens and pigs) to humans (de Been et al., 2014). Drug-resistant E. coli can occur via gene transfer in a variety of settings, as a result of contaminating livestock transport vehicles, trading venues, herd expansion through the addition of new animals, and slaughterhouse lairage (Schmithausen et al., 2018). Since surface water, wastewater, and drinking water can all be contaminated with E. coli, there is a chance that MDR will be transferred from the environment to pigs. It is known that holding pens in stables and lairage in abattoirs are important hubs for the spread of Enterobacteriaceae that produce ESBLs throughout the pig and other animal production chain (Schmithausen et al., 2015; Ugbo et al., 2023). Food handlers are in danger of spreading resistant bacteria, and food processing settings are thought to be significant intermediate reservoirs and vectors of AMR bacteria (Oniciuc et al., 2019). Some data suggested the possible transfer of MDR E. coli clones between pigs and piggery workers (de Beenet et al., 2014). Multidrug resistance E. coli were considered as one of the major threats to animal and human health (Kendek et al., 2024). It also causes colibacillosis, a disease that commonly affects pigs from birth to weaning and is characterized by white to yellow diarrhea (Hartadi et al., 2020). Resistant to antibiotics humans can get E. coli from animals through the environment, direct contact, or the food chain (Schwaiger et al., 2012). The discovery of antibiotic resistance genes in E. coli extracted from animals has garnered significant interest, particularly in organisms capable of transmitting resistance genes (Effendi et al., 2021). The use of antibiotics, such as bacitracin, lincomycin, neomycin/ oxytetracycline, and penicillin in promoting animal growth, has contributed to multidrug resistance worldwide. Antibiotic resistance in bacteria may arise as a result of the frequent and inappropriate use of these drugs (Gholami-Ahangaran et al., 2021). Gram-negative bacteria, E. coli, used an efflux pump system as one of the mechanisms of resistance to tetracycline, and tetracycline-resistant genes that can be found in E. coli include tetA, tetB, tetC, tetD, tetE, tetG; thus, tetM, tetO, and tetS encode ribosomal protection systems (Sigirci et al., 2020). Tet genes are responsible for the emergence of resistance to tetracycline antibiotics in E. coli. Studies have demonstrated the presence of the tetA, tetB, and tetM genes in the effluent of pig slaughterhouses in Germany and Portugal where tetA, tetB, tetM, and tetK genes were detected in the waste of a pig farm, and the tetA, tetB, tetK, tetL, tetM, tetO, and tetA(P) genes in the effluent of pig slaughterhouses (Pazra et al., 2023). Additionally, information regarding genes responsible for tetracycline-resistant E. coli in swine is lacking in many parts of Nigeria including Abakaliki; thus, the need for this study on molecular identification of tetracycline resistance genes (tet A&B) in E. coli isolates from internal organs of swine sold in a slaughterhouse at Abakaliki, Ebonyi State, Nigeria. Materials and MethodsSample collection and processingA total of 75 samples were collected from the swine slaughterhouses (15 each from the large intestine, small colon, kidney, liver, and small intestine of swine). The samples were collected from April 2023 to June 2023 and from both male and female pigs. Then, exactly 5 g of each sample was collected using a sterile knife and universal container, and all the samples were labeled with the unique sample number and date; the samples were transported to the laboratory at the Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, for microbiological analysis. The collected samples were analyzed for the presence of E. coli by inoculating 5 g each of well-homogenized swine samples into different separate test tubes containing sterile buffered peptone water, and nutrient broth (Himedia) and incubated at 37°C for 24 hours. A loopful of the murky broth culture was aseptically seeded after being cultured overnight on sterile, solidified MacConkey agar (MAC) (Himedia) and Eosin methylene agar (EMB) (Merck USA). The samples were then incubated for 24 hours at 37°C. The distinctive appearance (color, consistency, and shape) of E. coli from positive cultures was used to identify them on the differential media. For Gram-staining response, hanging drop test (motility test), and biochemical profiling, the pink colonies on MAC and the greenish metallic shiny colonies on EMB were sub-cultured on sterilized solidified nutrient agar and incubated at 37°C for 24 hours to generate pure culture, following standard methods. The following biochemical tests were performed: the citrate utilization test (Simmon citrate agar), the oxidase test, and the indole test (Effendi et al., 2022). Antibiotic susceptibility testingThe Kirby–Bauer disk diffusion method was used to determine the antibiotic susceptibility of the phenotypically determined E. coli isolates from the swine internal organs. The Mueller–Hinton agar medium (Merck USA) was made in compliance with the guidelines provided by the manufacturer. A pure culture of E. coli isolates was cultured for overnight in nutrient broth, with the turbidity adjusted to 0.5 McFarland. After inoculating the plates, the bacterial isolates were left there for ten minutes for proper absorption on the plate. Antibiotics, which include fluoroquinolone (CIP: ciprofloxacin; 5 µg), macrolides (E: erythromycin; 30 µg), tetracycline (TE: tetracycline; 30 µg), beta-lactams (AMP: ampicillin; 30 µg), and aminoglycosides (CN: gentamycin; 500 µg), were placed on the surface of the Mueller–Hinton agar at a distance of 15 mm from the edge of the plate, 30 mm away from the center disk and incubated at 37°C for 18–24 hours. A calibrated transparent ruler was used to measure the inhibition zone diameters surrounding each antibiotic disk, and the results were reported in millimeters. The “resistant” and “sensitive” status of each bacterium was ascertained using a typical Clinical and Laboratory Standards Institute breakpoint table (CLSI, 2019). Multiple antibiotic resistance indexAmong the isolates resistant to three or more antimicrobial drugs, several antibiotic resistances were identified. The number of antibiotics to which an isolate is resistant (a) divided by the total number of antimicrobial agents the isolate was tested against (b) yields the MAR index for that isolate (Ejikeugwu et al., 2018). Molecular identification of Tetracycline-resistant genes using PCRPCR was used to further examine the tetracycline-resistant E. coli isolates for the presence of tet A and B genes. The pure culture of E. coli was inoculated in nutrient broth (TITAN Biotech, India) and cultured overnight at 37°C. The ZR fungal/bacterial DNA MiniPrep kit (manufactured by Zymo Research) was employed for the DNA extraction following the manufacturer’s instructions. Industrial ready-to-use prepared PCR master mix solution (GoTaq Green Master Mix), which contains Taq DNA polymerase, dNTPs, MgCl2, and a reaction buffer at a concentration of 12.5 μl were used. The primers used were acquired from Whitehead Scientific Ltd, Cape Town, South Africa. A C1000 thermo cycler machine (Bio-Red) was used to conduct the PCR reactions for the tet A and B genes. The primers sequences used are tet A–F: GCT ACA TCC TGC TTG CCT TC; tet A–R: CAT AGA TCG CCG TGA AGA GG with 210 base pair; tet B–F: TTG GTT AGG GGC AAG TTT TG; tet B–R: GTA ATG GGC CAA TAA CAC CG with 659 base pair and PCR methods was according to previously described (Kallau et al., 2018; Gholami-Ahangaran et al., 2021). PCR products were visualized using 1.5% agarose gel electrophoresis and photographed under a UV transilluminator using a gel documentation system. Ethical approvalPork samples were acquired from the slaughterhouse; hence, ethical approval was not necessary. Samples were collected from the slaughterhouse, as per the standard collection procedure. ResultsOut of the 75 samples collected to determine the presence of E. coli in swine, 24/75 (32.0%) were positive. However, E. coli isolates identified had a different prevalence; the large intestine harbored 4/15 (26.7%), the small colon had 6/15 (40.0%), the kidney had 5/15 (33.3%), the liver harbored 3/15 (20.0%), and the small intestine had 6/15 (40.0%) (Table 1). Thus, E. coli isolates from the large intestine, kidney, liver, small colon, and small intestine had different resistance patterns to erythromycin, tetracycline, and ampicillin, which ranged from 20.0% to 75.0%. The E. coli isolates studied showed great susceptibility to gentamycin and ciprofloxacin. The susceptibility patterns observed among the isolates ranged from 75.0% to 100.0% (Table 2). In addition, three major multiple antibiotic resistance index/patterns were observed among the E. coli isolates, which are presented as TE-CN-E-AMP, TE-E-AMP-CIP, and TE-E-AMP with an average multiple antibiotic resistance index (MARI) of 0.70 (Table 3). Out of the 24 E. coli isolates studied, 8/24 (33.3%) were resistant to tetracycline antibiotics and were further studied for the presence of tet A and B genes. Exactly, 6/8 (75.0%) E. coli harbored tet A, 5/8 (62.5) had tet B, and 4/8 (50.0%) harbored both tet A and tet B genes (Table 4). The presence of tet A and tet B genes with the molecular size of 210 bp and 659 bp, respectively. Table 1. Frequency of E. coli isolates from internal organs of swine sold in slaughterhouses at Abakaliki.
Table 2. Antimicrobial susceptibility profile of E. coli isolates from internal organs of swine sold in slaughterhouses at Abakaliki.
Table 3. Multiple antibiotic resistance patterns of E. coli isolates from internal organs of swine sold in slaughterhouses at Abakaliki.
Table 4. Occurrence of tetracycline resistance genes in E. coli from internal organs of swine.
DiscussionEscherichia coli is a major bacterium that colonizes the intestine of piglets at birth and in disease conditions; it creates serious problems for the pig farm managers/owners. This research shows that the prevalence of E. coli from swine slaughtered at Abakaliki is 24 (32.0%) out of the 75 swine samples analyzed. A study on E. coli causing diseases in pig farms in Bulgarian has reported 52 (30.4%) of E. coli from fecal samples of swine (Dimitrova et al., 2021) and is by the findings of this study. According to the study, 50.0% of E. coli cases in Southern Nigeria were from retail stores and pig slaughterhouses (Egbule et al., 2021). The presence of E. coli isolates has been reported from livestock, which includes pigs and chickens (Wibisono et al., 2020; Yanestria et al., 2022) and pathogenic E. coli from healthy pigs in Malang district, East Java, Indonesia (Effendi et al., 2022) and pig farms at Bulgarian (Dimitrova et al., 2021). It was confirmed by earlier research that pathogenic E. coli isolates can travel from the natural environment to humans or other livestock if they are excreted by animals (Dohmen et al., 2017). Studies have shown that E. coli can contaminate pigs, regardless of whether meat samples are obtained at the slaughterhouse or after processing (Bassitta et al., 2022). However, E. coli isolates from the large intestine, kidney, liver, small colon, and small intestine were 20.0%–75.0% resistant to erythromycin, tetracycline, and ampicillin and 75.0%–100.0% susceptible to gentamycin and ciprofloxacin. This study also identified the presence of E. coli isolates that were resistant to β-lactam (ampicillin) antibiotics and agrees with the observation that E. coli in food-producing animals conferring resistance to β-lactam antimicrobials (Reich et al., 2013). The reason for this resistance possessed by this organism could be that some of these antimicrobial agents used as growth promoters and treatment of food animals in veterinary medicine range from oxytetracycline, penicillin, and ampicillin have been abused by the farms (Widodo et al., 2020). E. coli that was resistant to tetracycline antibiotics was also discovered in this study and is in line with the observation that E. coli isolates from livestock can resist most antibiotics such as aminoglycosides, tetracyclines, chloramphenicol, trimethoprim, sulphonamides, and quinolones (Wibisono et al., 2021). The E. coli isolates from this study had antibiotic antibiotic-resistant ranging from 20.0% to 75.0%, and this is akin to the report that E. coli isolates from pigs had a resistant range of 53.0%–75.0% (Dimitrova et al., 2021). A study done in Uganda on E. coli isolates from pig slaughterhouses presented antibiotic resistance to amoxicillin (30.4%), erythromycin (34.8%), and ciprofloxacin (100%) susceptibility (Katushabe et al., 2022) and it is by the observation of this research. Another researcher discovered that antimicrobial resistance was dominated by tetracycline (69.1 %) (Ugbo et al., 2023). Regardless of a strain’s virulence characteristics, antimicrobial resistance may pose health problems since carriers may act as a conduit for genetic transfer within the gastrointestinal tract. Research has demonstrated that resistant bacteria, such as E. coli, can survive in the gastrointestinal tract after being consumed orally through pork (Enne et al., 2008). Managers of swine farms frequently deal with the issue of bacterial pathogens causing disease in pigs. Antibiotics are now used to prevent and treat diseases as a result of this (Van et al., 2020). This study has reported the presence of multidrug-resistant E. coli and the isolates presented MDR as follows TE-CN-E-AMP, TE-E-AMP-CIP, and TE-E-AMP with average MARI as 0.70. A retrospective study revealed that 43 E. coli isolates had an 88.4% multidrug resistance rate (Kakooza et al., 2021). There have been reports of multidrug-resistant bacteria in various livestock, particularly in pigs and dairy cattle (Byaruhanga et al., 2022). The occurrence of multidrug resistance in livestock might be increased by the irrational and inappropriate use of antimicrobial drugs (Larb et al., 2021). Antibiotics are still used as growth boosters on farm animals such as pigs, cattle, poultry, and birds in certain countries that have not outlawed their usage. This has significantly accelerated the spread of antibiotic resistance in livestock (Yang et al., 2019). Antibiotic-resistant bacteria have the detrimental effect of making therapy for bacterial illnesses more difficult or perhaps failing. Ineffective therapy affects the duration of treatment, the usage of more costly medications, and, of course, the expenses incurred (Widodo et al., 2020). Exactly, 8 (33.3%) E. coli isolates were multidrug-resistant and tetracycline-resistant and were further studied for the presence of tet A and tetB genes. However, the E. coli was reported to harbor tet A gene 6/8 (75.0%); tet B gene 5/8 (62.5%); and both tet A and B genes 4/8 (50.0%). A high prevalence of the tet A gene was observed when compared with tet B gene and agrees with the observation of Alam et al. (2023) who reported that 84.0% of the tetracycline-resistant E. coli isolates encode tet A gene. Different types of tetracycline genes, which include tet(A), tet(B), tet(C), tet(D), and tet(E), have been previously reported by several studies to cause tetracycline resistance in bacteria such as E. coli (Sigirci et al., 2020; Pazra et al., 2023). Another study carried out on E. coli isolated from a pig farm in Indonesia reported the presence of multidrug and tetracycline resistance E. coli to harbor tet A (23.0%), tet E (46.0), and tet A and B (23.0%) genes (Kallau et al. 2018). The tetA gene was more prevalent (17/18) at 94%, followed by the tetB gene at (10/18) 56% in E. coli (Indrawati et al., 2021), and is by the detection of tet A gene 6/8 (75.0%) and tet B gene 5/8 (62.5%) in this research. A study done in clinical and non-clinical tetracycline resistance E. coli isolates in Nigeria detected tetA (34.8%), tet B (2.2%), and tet A and B (14.8%) (Perewari et al., 2022). The E. coli isolates that were resistant to tetracycline and harboring tetA (42.3%), tetB (46.2%), and tetA and B (11.5%) genes were reported in healthy and diarrheic pet birds (Gholami-Ahangaran et al., 2021). Thus, this research has found that tetracycline resistance E. coli in swine is due to sales, use of tetracycline antibiotics, and cross-resistant antimicrobials in Abakaliki metropolis. This study also shows that there is a correlation between the use of tetracycline and resistance of tetracycline. ConclusionIn conclusion, this study has reported the presence of antimicrobial-resistant E. coli in swine. Multidrug-resistant and tetracycline resistance genes (tet A, tet B, both tet A and B genes) were also reported in E. coli isolates from internal organs of swine for the first time in Abakaliki and is of public health concern. Thus, swine should be considered a potential source of MDR organisms capable of causing public health threats/hazards. This discovery has impacted optimizing the use of antibiotics as growth promoters, and disease treatment and the data obtained are also helpful in reducing the increasing incidence and spread of antimicrobial resistance among food animals, humans, and their environment at the grass root level and even the remote and rural regions of Nigeria that deal with the practice of pig/swine farm and other livestock. We therefore recommend strong stringent antibiotic administering policies for swine and surveillance on the emergence of antimicrobial resistance in livestock. AcknowledgmentsThe authors thank the Universitas Airlangga. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe authors would like to acknowledge Ebonyi State University, Abakaliki Nigeria, and Lembaga Penelitian dan Pengabdian Masyarakat Universitas Airlangga, Indonesia, for their support. This study was partly supported by the International Research Consortium, Lembaga Penelitian dan Pengabdian Masyarakat, Universitas Airlangga, Surabaya, Indonesia Year 2024 with grant number: 171/UN3.LPPM/PT.01.03/2024. Author’s contributionsAIU and ENU: Conceived, designed, and coordinated the study. IBM and RCO: Designed data collection tools, supervised the field sample and data collection, and laboratory work as well as data entry. WT, HMR, and MHE: Validation, supervision, and formal analysis. BNA and SMY: Contributed reagents, materials, and analysis tools. KHPR, FE, and ARK: Carried out the statistical analysis and interpretation and participated in the preparation of the manuscript. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data are available in the manuscript. ReferencesAlam, G.S., Hassan, M.M., Ahaduzzaman, M., Nath, C., Dutta, P., Khanom, H., Khan, S.A., Pasha, M.R., Islam, A. and Magalhaes, R.S. 2023. Molecular detection of tetracycline-resistant genes in multi-drug-resistant Escherichia coli isolated from broiler meat in Bangladesh. Antibiotics 12(2), 418. Bassitta, R., Kronfeld, H., Bauer, J., Schwaiger, K. and Hölzel, C. 2022. Tracking antimicrobial resistant E. coli from pigs on farm to pork at slaughter. Microorganisms 10(8), 1485. Byaruhanga, J., Ssebunya, Y., Vudriko, P. and Rwego, I.B. 2022. Antimicrobial resistance patterns and bovine sub-clinical mastitis burden in low and high tick acaricide resistance regions of Uganda. Open J. Vet. Med. 12(8), 71–87. Clinical and Laboratory Standards Institute. 2018. M100 performance standards for antimicrobial susceptibility testing. 28th ed. Clinical and Laboratory Standards Institute. www.clsi.org. Published in Wayne City, Pennsylvania (PA). De Been, M., Lanza, V.F., de Toro, M., Scharringa, J., Dohmen, W., Du, Y., Hu, J., Lei, Y., Li, N. and Tooming-Klunderud, A. 2014. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet. 10(12), 90–95. Dimitrova, L., Kaleva, M., Zaharieva, M.M., Stoykova, C., Tsvetkova, I., Angelovska, M., Ilieva, Y., Kussovski, V., Naydenska, S. and Najdenski, H. 2021. Prevalence of antibiotic-resistant Escherichia coli isolated from swine faeces and lagoons in Bulgaria. Antibiotics 10(8), 940–946. Dohmen, W., Dorado-García, A., Bonten, M.J.M., Wagenaar, J.A., Mevius, D. and Heederik, D.J. 2017. Risk factors for ESBL-producing Escherichia colion pig farms: a longitudinal study in the context of reduced use of antimicrobials. PLoS One 12(3), 174–184. Effendi, M.H., Harijani, N., Yanestria, S.M. and Hastutiek, P. 2018. Identification of Shiga toxin-producing Escherichia coli in raw milk samples from dairy cows in Surabaya, Indonesia. Philipp. J. Vet. Med. 55(SI), 109–114. Effendi, M.H., Hartadi, E.B., Witaningrum, A.M., Permatasari, D.A. and Ugbo, E.N. 2022. Molecular identification of blaTEM gene of extend¬ed-spectrum beta-lactamase-producing Escherichia coli from healthy pigs in Malang district, East Java, Indonesia. J. Adv. Vet. Anim. Res. 9(3), 447–452. Effendi, M.H., Tyasningsih, W., Yurianti, Y.A., Rahmahani, J., Harijani, N. and Plumeriastuti, H. 2021. Presence of multidrug resistance (MDR) and extended-spectrum beta-lactamase (ESBL) of Escherichia coli isolated from cloacal swab of broilers in several wet markets in Surabaya, Indonesia. Biodiversitas 22(1), 304–309. Egbule, O.S., Iweriebor, B.C. and Odum, E.I. 2021. Beta-lactamase-producing Escherichia coli isolates recovered from pig handlers in retail shops and abattoirs in selected localities in Southern Nigeria: implications for Public Health. Antibiotics 10(1), 9. Ejikeugwu, C., Esimone, C., Iroha, I.R. and Adikwu, M. 2018. First detection of FOX-1 AmpC β-lactamase gene expression among Escherichia coli isolated from abattoir samples in Abakaliki, Nigeria. Oman Med. J. 33(3), 243–249. Enne, V.I., Cassar, C., Sprigings, K., Woodward, M.J. and Bennett, P.M. 2008. A high prevalence of antimicrobial resistant Escherichia coli isolated from pigs and a low prevalence of antimicrobial resistant E. coli from cattle and sheep in Great Britain at slaughter. FEMS Microbol. Lett. 278(2), 193–199. Gholami-Ahangaran, M., Karimi-Dehkordi, M., Miranzadeh-Mahabadi, E. and Ahmadi-Dastgerdi, A. 2021. The frequency of tetracycline resistance genes in Escherichia coli strains isolated from healthy and diarrheic pet birds. Iran J. Vet. Res. 22(4), 337–341. Hartadi, E.B., Effendi, M.H., Plumeriastuti, H., Sofiana, E.D., Wibisono, F.M. and Hidayatullah, A.R. 2020. A review of enterotoxigenic Escherichia coli infection in piglets: public health importance. Syst. Rev. Pharm. 11(9), 687–698. Indrawati, A., Khoirani, K., Setiyaningsih, S., Affif, U., Safika, S. and Ningrum, G. 2021. Detection of tetracycline resistance genes among Escherichia coli isolated from layer and broiler breeders in West Java, Indonesia. Trop. Anim. Sci. J. 44(3), 267–272. Kallau, N.H.G., Wibawan, I.W.T., Lukman, D.W. and Sudarwanto, M.B. 2018. Detection of multi-drug resistant (MDR) Escherichia coli and tet gene prevalence at a pig farm in Kupang, Indonesia. J. Adv. Vet. Anim. Res. 5(4), 388–396. Kakooza, S., Muwonge, A., Nabatta, E., Eneku, W., Ndoboli, D. and Wampande, E. 2021. A Retrospective analysis of antimicrobial resistance in pathogenic Escherichia coli and Salmonella spp. isolates from poultry in Uganda. Int. J. Vet. Sci. Med. 9(1), 11–21. Katushabe, P., Byamukama, B. and Byaruhanga, J. 2022. Burden of multidrug-resistant Escherichia coli in pigs slaughtered in Uganda and its implication on veterinary public health. Open. J. Vet. Med. 2022(12), 187–200. Kendek, I.A., Putri, M.F.R., Wibisono, F.J., Effendi, M.H., Tyasningsih, W., Ugbo, E.N. and Agumah, N.B. 2024. Molecular detection of hlyF gene on multidrug resistance of avian pathogenic Escherichia coli isolated from ducks on wet markets of Surabaya, Indonesia. Biodiversitas 25(3), 1246–1252. Larbi, R.O., Ofori, L.A., Sylverken, A.A., Ayim-Akonor, M. and Obiri-Danso, K. 2021. Antimicrobial resistance of Escherichia coli from broilers, pigs, and cattle in the greater Kumasi Metropolis, Ghana. Int. J.Microbiol. 7(2), 31–39. Mustika, Y.R., Kinasih, K.N., Effendi, M.H., Puspitasari, Y., Kurniawan, S.C., Khairullah, A.R., Samodra, M.E.E., Hasib, A., Agustin, A.L.D., Moses, I.B. and Silaen, O.S.H. 2024. Molecular detection of extended-spectrum β-lactamase-producing Escherichia coli from bat caves on Lombok Island. Open Vet. J. 14(2), 699–706. Oloso, N.O., Fagbo, S., Garbati, M., Olonitola, S.O., Awosanya, E.J., Aworh, M.K., Adamu, H., Odetokun, I.A. and Fasina, F.O. 2018. Antimicrobial resistance in food animals and the environment in Nigeria: a review. Int. J. Environ. Res. Public Health 15(6), 1284. Oniciuc, E.A., Likotrafiti, E., Alvarez-Molina, A., Prieto, M., López, M. and Alvarez-Ordóñez, A. 2019. Food processing as a risk factor for antimicrobial resistance spread along the food chain. Curr.Opin. Food. Sci. 30(12), 21–26. Pazra, D.F., Latif, H., Basri, C., Wibawan, I.W.T. and Rahayu, P. 2023. Distribution analysis of tetracycline resistance genes in Escherichia coli isolated from floor surface and effluent of pig slaughterhouses in Banten Province, Indonesia, Vet. World 16(3), 509–517. Perewari, D.O., Otokunefor, K. and Agbagwa, O.E. 2022. Tetracycline-resistant genes in Escherichia coli from clinical and nonclinical sources in Rivers State, Nigeria. Int. J. Microbiol. 2022(7), ID9192424. Reich, F., Atanassova, V. and Klein, G. 2013. Extended spectrum beta-lactamase and AmpC-producing Enterobacteria in healthy broiler chickens, Germany. Emerg. Infect. Dis. 19(8), 1253–1259. Schmithausen, R.M., Schulze-Geisthoevel, S.V., Heinemann, C., Bierbaum, G., Exner, M., Petersen, B. and Steinhoff-Wagner, J. 2018. Reservoirs and transmission pathways of resistant indicator bacteria in the Biotope pig stable and along the food chain: a review from a One Health Perspect. Sustain. 10(11), 3967–3969. Schmithausen, R.M., Schulze-Geisthoevel, S.V., Stemmer, F., El-Jade, M., Reif, M., Hack, S., Meilaender, A., Montabauer, G., Fimmers, R. and Parcina, M. 2015. Analysis of transmission of MRSA and ESBL-E among pigs and farm personnel. PLoS One 10(9), 76–81. Sigirci, B.D., Celik, B., Halac, B., Adiguzel, M.C., Kekec, I., Metiner, K., Ikiz, S., Bagcigi, A.F., Ozgur, N.Y., Ak, S. and Kahraman, B.B. 2020. Antimicrobial resistance profiles of Escherichia coli isolated from companion birds. J. King Saud Univ. Sci. 32(1), 1069–1073. Tyasningsih, W., Ramandinianto, S.C., Ansharieta, R., Witaningrum, A.M., Permatasari, D.A., Wardhana, D.K., Effendi, M.H. and Ugbo, E.N. 2022. Prevalence and antibiotic resistance of Staphylococcus aureus and Escherichia coli isolated from raw milk in East Java, Indonesia. Vet. World 15(8), 2021–2028. Ugbo, E.N., Jacob, J.I., Effendi, M.H., Witaningrum, A.M., Agumah, B.N., Ugbo, A.I. and Moses, B.I. 2023. Poultry slaughterhouse wastewater as reservoirs for spreading extended-spectrum beta-lactamase-producing Escherichia coliin Abakaliki, Nigeria. Biodiversitas 24(9), 4960–4966. Van, T.T.H., Yidana, Z., Smooker, P.M. and Coloe, P.J. 2020. Antibiotic use in food animals worldwide, with a focus on Africa: pluses and minuses. J. Glob. Antimicrob. Resist. 5(20), 170–173. Vieira, A.R., Collignon, P., Aarestrup, F.M., McEwen, S.A., Hendriksen, R.S., Hald, T. and Wegener, H.C. 2011. Association between antimicrobial resistance in Escherichia coli isolates from food animals and blood stream isolates from humans in Europe: an ecological study. Foodborne Pathog. Dis. 8(12), 1295–1301. Wibisono, F.J., Sumiarto, B., Untari, T., Effendi, M.H., Permatasari, D.A. and Witaningrum, A.M. 2020. Pattern of antibiotic resistance on extended-spectrum beta-lactamases genes producing Escherichia coli on laying hens in Blitar, Indonesia. Biodiversitas 21(10), 4631–4635. Wibisono, F.J., Sumiarto, B., Untari, T., Effendi, M.H., Permatasari, D.A. and Witaningrum, A.M. 2021. Molecular identification of ctx gene of extended spectrum betalactamases (ESBL) producing Escherichia coli on layer chicken in Blitar, indonesia. J. Anim. Plant. Sci. 31(4), 954–959. Widodo, A., Effendi, M.H. and Khairullah, A.R. 2020. Extended-spectrum beta-lactamase (ESBL)-producing Eschericia coli from livestock. Syst. Rev. Pharm. 11(7), 382–392. Yanestria, S.M., Dameanti, F.N.A.E.P., Musayannah, B.G., Pratama, J.W.A., Witaningrum, A.M., Effendi, M.H. and Ugbo, E.N. 2022. Antibiotic resistance pattern of extended-spectrum β-lactamase (ESBL) producing Escherichia coli isolated from broiler farm environment in Pasuruan district, Indonesia. Biodiversitas 23(9), 4460–4465. Yang, H., Paruch, L., Chen, X., van Eerde, A., Skomedal, H., Wang, Y., Liu, D. and Clarke, J.L. 2019. Antibiotic application and resistance in swine production in China: current situation and future perspectives. Front. Vet. Sci. 6(5), 136–137. | ||
| How to Cite this Article |
| Pubmed Style Ugbo EN, Effendi MH, Ugbo AI, Tyasningsih W, Agumah BN, Raharjo HM, Khairullah AR, Ogba RC, Ekawasti F, Yanestria SM, Moses IB, Riwu KHP. Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria. Open Vet. J.. 2025; 15(1): 171-178. doi:10.5455/OVJ.2025.v15.i1.16 Web Style Ugbo EN, Effendi MH, Ugbo AI, Tyasningsih W, Agumah BN, Raharjo HM, Khairullah AR, Ogba RC, Ekawasti F, Yanestria SM, Moses IB, Riwu KHP. Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria. https://www.openveterinaryjournal.com/?mno=217618 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.16 AMA (American Medical Association) Style Ugbo EN, Effendi MH, Ugbo AI, Tyasningsih W, Agumah BN, Raharjo HM, Khairullah AR, Ogba RC, Ekawasti F, Yanestria SM, Moses IB, Riwu KHP. Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria. Open Vet. J.. 2025; 15(1): 171-178. doi:10.5455/OVJ.2025.v15.i1.16 Vancouver/ICMJE Style Ugbo EN, Effendi MH, Ugbo AI, Tyasningsih W, Agumah BN, Raharjo HM, Khairullah AR, Ogba RC, Ekawasti F, Yanestria SM, Moses IB, Riwu KHP. Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 171-178. doi:10.5455/OVJ.2025.v15.i1.16 Harvard Style Ugbo, E. N., Effendi, . M. H., Ugbo, . A. I., Tyasningsih, . W., Agumah, . B. N., Raharjo, . H. M., Khairullah, . A. R., Ogba, . R. C., Ekawasti, . F., Yanestria, . S. M., Moses, . I. B. & Riwu, . K. H. P. (2025) Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria. Open Vet. J., 15 (1), 171-178. doi:10.5455/OVJ.2025.v15.i1.16 Turabian Style Ugbo, Emmanuel Nnabuike, Mustofa Helmi Effendi, Agatha Ifunanya Ugbo, Wiwiek Tyasningsih, Bernard Nnabuife Agumah, Hartanto Mulyo Raharjo, Aswin Rafif Khairullah, Rebecca Chinenye Ogba, Fitrine Ekawasti, Sheila Marty Yanestria, Ikechukwu Benjamin Moses, and Katty Hendriana Priscilia Riwu. 2025. Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria. Open Veterinary Journal, 15 (1), 171-178. doi:10.5455/OVJ.2025.v15.i1.16 Chicago Style Ugbo, Emmanuel Nnabuike, Mustofa Helmi Effendi, Agatha Ifunanya Ugbo, Wiwiek Tyasningsih, Bernard Nnabuife Agumah, Hartanto Mulyo Raharjo, Aswin Rafif Khairullah, Rebecca Chinenye Ogba, Fitrine Ekawasti, Sheila Marty Yanestria, Ikechukwu Benjamin Moses, and Katty Hendriana Priscilia Riwu. "Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria." Open Veterinary Journal 15 (2025), 171-178. doi:10.5455/OVJ.2025.v15.i1.16 MLA (The Modern Language Association) Style Ugbo, Emmanuel Nnabuike, Mustofa Helmi Effendi, Agatha Ifunanya Ugbo, Wiwiek Tyasningsih, Bernard Nnabuife Agumah, Hartanto Mulyo Raharjo, Aswin Rafif Khairullah, Rebecca Chinenye Ogba, Fitrine Ekawasti, Sheila Marty Yanestria, Ikechukwu Benjamin Moses, and Katty Hendriana Priscilia Riwu. "Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria." Open Veterinary Journal 15.1 (2025), 171-178. Print. doi:10.5455/OVJ.2025.v15.i1.16 APA (American Psychological Association) Style Ugbo, E. N., Effendi, . M. H., Ugbo, . A. I., Tyasningsih, . W., Agumah, . B. N., Raharjo, . H. M., Khairullah, . A. R., Ogba, . R. C., Ekawasti, . F., Yanestria, . S. M., Moses, . I. B. & Riwu, . K. H. P. (2025) Molecular identification of tetracycline resistance genes in Escherichia coli isolates from internal organs of swine sold on Abakaliki, Nigeria. Open Veterinary Journal, 15 (1), 171-178. doi:10.5455/OVJ.2025.v15.i1.16 |