| Short Communication | ||
Open Vet. J.. 2025; 15(1): 465-470 Open Veterinary Journal, (2025), Vol. 15(1): 465-470 Short Communication Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, ColombiaHorwald A.B. Llano1*, Itzel López Aguilar2, Natalia Granda Orozco1, Laura Gutiérrez Giraldo1, Gloria Sánchez Zapata1 and Daisy Gómez Ruíz11Grupo de Investigación en Medicina Veterinaria (GINVER), Corporación Universitaria Remington, Medellín, Colombia 2Unidad Académica de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Nayarit, Nayarit, México *Corresponding Author: Horwald A.B. Llano. Grupo de Investigación en Medicina Veterinaria (GINVER), Corporación Universitaria Remington, Medellín, Colombia. Email: horwald.bedoya [at] uniremington.edu.co Submitted: 20/08/2024 Accepted: 17/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
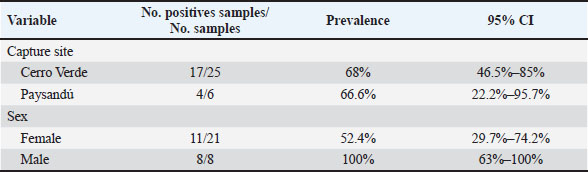
AbstractBackground: Gastrointestinal parasites associated with small wild rodents in Colombia remain poorly understood. Aim: This study aimed to identify the intestinal helminths of Reithrodontomys mexicanus, a common wild rodent species, in rural areas of Medellín, Colombia. Methods: Between June and December 2022, 31 individuals were captured and sex-classified. Fecal samples underwent flotation and sedimentation analysis. Results: The overall helminth prevalence in R. mexicanus was 67.7% (21/31). Identified helminth egg morphotypes included: Ascarid (types 1 and 2), Dicrocoeliidae-like (types 1 and 2), Physaloptera sp., Strongyloides sp., Trichuris sp., and two unidentified nematode eggs. Conclusion: This study reveals that R. mexicanus hosts a diverse range of parasites, including those with significant public health implications such as Ascaris spp., Dicrocoelium spp., and Trichuris spp. Our findings contribute to the understanding of wild rodent helminth fauna in South America and provide the first documentation of parasites in R. mexicanus. Keywords: Endoparasites, Prevalence, Small rodents, Zoonoses. IntroductionRodents are the most successful group of mammals, exhibiting remarkable adaptability and widespread distribution worldwide, except in Antarctica (Marsh et al., 2022). As a result of global changes and habitat conversion, certain rodent species demonstrate adaptability, survival, and population growth in natural areas surrounding human settlements (Dahmana et al., 2020). In these areas, rodents can interact with humans and domestic animals, serving as key vectors for transmitting parasites that cause helminthiasis (Gonzalez-Astudillo et al., 2016). Reithrodontomys mexicanus (Saussure, 1860), known colloquially as the Mexican harvest mouse, is a small rodent species distributed from Mexico to Ecuador, inhabiting elevations from 1000 to 3800 m (Martínez-Borrego et al., 2020). This species is considered locally common and abundant in Colombia and Ecuador (Delgado et al., 2016; Ramírez-Jaramillo et al., 2019), and is typically associated with mature and secondary forests, clearings, and cultivated areas (Reid, 1997). Notably, the endoparasite fauna of this rodent species has not been investigated previously (Martínez-Borrego et al., 2020). Non-invasive sampling through fecal collection offers a valuable alternative for monitoring free-living wild mammals and their gastrointestinal parasites without animal sacrifice (Dib et al., 2020). This approach is particularly relevant for detecting parasites that can potentially infect animals and be transmitted to humans. This study aimed to identify intestinal helminths of R. mexicanus in rural areas of Medellín, Colombia, establishing a baseline for monitoring parasite-rodent interaction in human-modified landscapes, and serving as a tool for public health surveillance. Materials and MethodsThe study area was situated in Santa Elena Corregimiento, 19 km northwest of Medellín city, in Antioquia Department, western Colombia. This region falls within the Low Montane Very Humid Forest (bmh-MB) life zone, characterized by an average temperature of 17°C and elevation of 2,573 m (Castaño-Villa and Patiño-Zabala, 2008). Two sites were selected for rodent capture: Cerro Verde (6°19’93.35’’N, 75°48’50.12’’W), and Paysandú (6°20’60.47’’N, 75°01’98.03’’W), both featuring mixed landscapes of flower crops, grasslands, and native and exotic forest species due to significant human interventions (Alcaldía de Medellín, 2014). Rodent captures occurred between June and December 2022, across four trapping sessions, utilizing 40 Sherman®-type live capture traps (23 × 10 × 11 cm). Traps were placed at each site for three consecutive nights and checked daily. To attract rodents, traps were baited with a mixture of oats, peanut butter, and vanilla essence. Upon capture, sex, and weight data, were recorded for each individual, and ear punches were made to identify recaptures. Fecal samples were collected directly from the traps, and the rodents were released promptly to minimize stress. Fecal samples were preserved in 70% ethanol and analyzed using Sheather’s sugar floatation method, modified by Aguilar et al. (2023) as a coprodiagnostic technique. Eggs were identified based on morphological characteristics (Archer et al., 2017; Dib et al., 2020; Carrera-Játiva et al., 2023). The parasite prevalence was calculated using the following formula: (Number of positive animals/Total population) × 100. Ethical approvalThis study received ethical approval from the Bioethics Committee for Animal Research of the Facultad de Medicina Veterinaria, Corporación Universitaria Remington (CIBA-Acta No. 03–2021). Results and DiscussionA total of 31 mice were captured, consisting of 21 females, 8 males, and 2 individuals of unknown sex. Six individuals were recaptured. Body weight ranged from 8 to 20 g, with a mean weight ± standard error of 14.3 ± 2.49 g for males, and 12.6 ± 2.92 g for females. This weight range is consistent with previous reports for this species (Cervantes and Barrera, 2012). Twenty-one mice (67.7%; 95% CI=48.6–83.3) were infected with at least one helminth species. Prevalence values were similar across capture locations with no significant differences observed (p > 0.05; χ2 test). Notably, all males were infected, whereas only 52.4% of females harbored parasites (Table 1). This disparity may be attributed to endocrinological and behavioral sex differences, as suggested by previous studies (Zuk and McKean, 1996; Hämäläinen et al., 2015). Elevated testosterone levels in males may increase parasite exposure due to increased mobility during mate searching (Raynaud et al., 2012; Mason et al., 2022). Additionally, sex differences in parasite loads are often linked to the immunosuppressive effects of steroid hormones, particularly testosterone (Taneja, 2018). Nine distinct morphotypes helminths eggs were identified (Fig. 1), including two trematode eggs: Dicrocoeliidae-like egg type 1 (34.2 ± 1.3 × 21.7 ± 0.7 µm, n=4), Dicrocoeliidae-like infertile egg type 2 (72.2 ±9.0 × 40.4 ± 0.9 µm, n=2), and seven nematode eggs: Physaloptera spp. (40.6 ± 1.7 × 26.9 ± 1.9 µm, n=21), Strongyloides sp. (48.3 ± 10 × 27.6 ± 2.8 µm, n=2), Trichuris sp. (82.8 ± 3.4 × 38.1 ± 1.8 µm, n=16), Ascarid-like undeveloped egg type 1 egg (35.6 × 20.5 µm, n=1), Ascarid-type 2 egg (108.2 × 65.6 µm, n=1), and two unclassified nematode eggs type 1 (39.2 × 28.5 µm, n=1) and type 2 (121.6 × 70.7 µm, n=1). The most prevalent eggs found were Physaloptera sp. (57.14%), followed by Trichuris sp. (33.3%) and Dicrocoeliidae-like eggs type 1 (12.04%). Other egg types were less common, with Strongyloides sp. and Dicrocoeliidae-like infertile egg type 2 each found in 9.52% of samples, and Ascarid-type 1 and 2 eggs and unidentified nematode egg type 1 and 2 each found in a single sample (4.76%). Physaloptera nematodes are commonly found in rodents, including Cricetidae species (Ederli et al., 2018). Adult Physaloptera worms attach to the stomach wall, causing anemia, catarrhal gastritis, enteritis, and excessive mucous secretion in the host (Lértora et al., 2016). Previous reports have identified this nematode in Chile wild rodents of the families: Cricetidae (Abrothrix olivacea, Akodon longipilis, Oligoryzomys longicaudatus), and Muridae (Phyllotis darwini, Mus musculus) (Carrera-Játiva et al., 2023). Trichuris sp. eggs exhibited bipolar plugs with a thick shell but were larger than those previously reported (Carrera-Játiva et al., 2023). Similar large eggs have been observed in Trichuris trichuria and Trichuris muris (Koyama, 2013). Other eggs detected were similar to Trichuris sp. (Trichurid-type) but slightly smaller, wider, and brownish, potentially belonging to an undescribed species or a spurious parasite. Notably, 29 species within this nematode genus have been recorded in various rodent species in America (Falcón-Ordaz et al., 2020). Table 1. Description of the prevalence of intestinal parasites identified in Reithrodontomys mexicanus mice for the different categories analyzed.
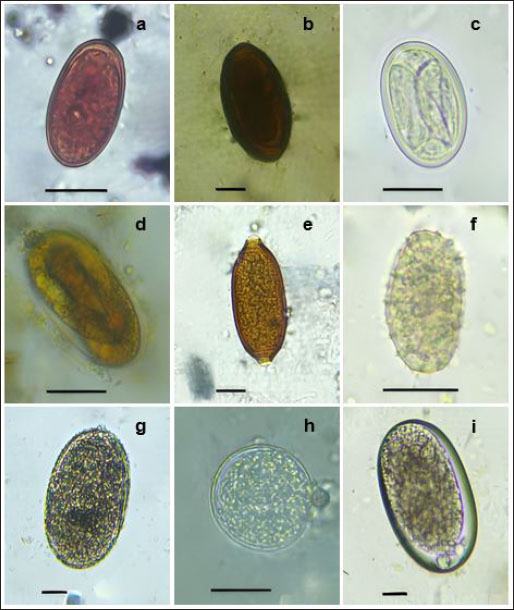
Fig. 1. Morphotypes of helminths eggs 100× (a-b) and 400× (c-i) detected in fecal samples of Reithrodontomys mexicanus from a Suburban Area in Medellin, Colombia. (a) Dicrocoeliidae-like egg type 1, (b) Dicrocoeliidae-like infertile egg type 2, (c) Physaloptera sp., (d) Strongyloides sp., (e) Trichuris sp., (f) Ascaris-like undeveloped egg type 1, (g) Ascarid-like egg type 2, (h) Unclassified nematode egg type 1, (i) Unclassified nematode egg type 2. Scale bar 20 μm. Dicrocoeliidae trematodes are common in rodents, comprising 11 genera that parasitize 14 species across the families Cricetidae, Echimyidae, Hetromyidae, Myocastoridae, and Muridae in the Americas (Martins et al., 2022). These parasites can cause pathological changes in the biliary system, and certain invertebrates such as mollusks and arthropods, may serve as intermediate hosts (Gardner and Pérez-Ponce de Léon, 2002). Within this family, Dicrocoelium dendriticum can affect livestock and other animals, and there is a potential for humans to become incidental hosts (Kumar, 1999). D. dentricum has been reported in interaction with cricetid rodents as a suitable host (Martins et al., 2022). This parasite causes lesions in the liver of affected animals, often leading to economic losses in livestock industries due to reduced production and fertility (Piegari et al., 2021). Strongyloides nematodes, specifically Strongyloides venezuelensis and Strongyloides ratti, are pathogenic to commensal rodents’ species, including Rattus norvegicus and Mus musculus domesticus (Viney and Kikuchi, 2017). However, data on their prevalence and intensity in wild rodent populations remain limited (Viney and Kikuchi, 2017). Rodents serve as reservoir hosts for at least 60 zoonotic diseases, playing a crucial role in their transmission and spread through various means (Dahmana et al., 2020). Notably, Ascarids, intestinal nematodes commonly found in rodents (Lee et al., 2018), include Toxocara apodeme, closely related to Toxocara canis and Toxocara cati, which has been identified in small wild rodents (Kim et al., 2020). The presence of R. mexicanus in peri-urban areas of Medellín represents a potential public health risk due to its ability to transmit pathogens. Notably, rodents can serve as mechanical vectors for helminth eggs of concern, such as Ascaris spp. and Trichuris spp. (Archer et al., 2017; Islam et al., 2020), facilitating their spread to humans and other animals. This risk is particularly significant in the context of rapid urbanization and increasing tourism in the study area. Urban expansion can lead to an increase in the abundance of certain rodent species and alter their interactions with parasites, thereby amplifying the risk of zoonotic diseases at the human-animal interface (Islam et al., 2024; Mendoza et al., 2024). Our study revealed five distinct parasite taxa and four unidentified egg morphotypes in the sampled area of Santa Elena corregimiento. Although parasite identification was not possible at the species level, these findings hold significant epidemiological implications and will contribute to future research. Our results highlight the diverse parasite range in a single wild rodent species. This study provides the first report of helminths parasitizing R. mexicanus in Colombia, underscoring the need for further investigation. To confirm parasite species, future studies should employ a combination of molecular and morphological analysis of adult parasites. AcknowledgmentsThe researchers express their gratitude to the Clinic of the Veterinary Medicine Faculty of Corporación Universitaria Remington. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research was funded by Corporación Universitaria Remington through project No. 4000000320. Authors’ contributionsH.A.B.L.: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Supervision; I.L.A.: Methodology, Investigation; N.G.O.: Methodology, Investigation; L.G.G.: Methodology, Investigation; G.Z.S.: Methodology, data analysis, D.G.R.: Investigation, Writing - original draft & editing, Supervision, Project administration. Data availabilityAll information and data related to this study are explicitly detailed in the manuscript. ReferencesAguilar, I.T.L., Cadena, M.P.E., López, B.C.T. and Llano, H.B. 2023. Presence of Cryptosporidium spp. in calves from dairy herds in Northern Antioquia, Colombia. Arq. Bras. Med. Vet. Zootec. 75(5), 800–806; doi:10.1590/1678-4162-13043. Alcaldía de Medellín. 2014. Departamento Administrativo de Planeación. Plan de Desarrollo Local Corregimiento de Santa Elena-Documento Estratégico Contrato No. 4600056021 de 2014. Available via https://www.medellin.gov.co/ndesarrollo/wp-content/uploads/Archivos_ND/CD_PDLS/CORREGIMIENTO_90/PDL/COMUNA%2090%20SANTA%20ELENA.pdf Archer, C.E., Appleton, C.C., Mukaratirwa, S., Lamb, J. and Schoeman, M.C. 2017. Endo-parasites of public-health importance recovered from rodents in the Durban metropolitan area, South Africa. S. Afr. J. Infect. Dis. 32(2), 57–66; doi:10.4102/sajid.v32i2.55. Castaño-Villa, G. and Patiño-Zabala, J. 2008. Local extinctions of birds in forest fragments in the Santa Elena region, central Andes, Colombia. El hornero 23(1), 23–34. Available via http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0073-34072008000100004&lng=es&tlng=en. Carrera-Játiva, P.D., Torres, C., Figueroa-Sandoval, F., Beltrami, E., Verdugo, C., Landaeta-Aqueveque, C. and Acosta-Jamett, G. 2023. Gastrointestinal parasites in wild rodents in Chiloé Island-Chile. Rev. Bras. Parasitol. Vet. 32(1), e017022; doi:10.1590/S1984-29612023002. Cervantes, F.A. and Barrera, C.B. (Eds.). 2012. Estudios sobre la biología de roedores silvestres mexicanos (pp. XI-XII). Ciudad de México, México: Universidad Nacional Autónoma de México. Dahmana, H., Granjon, L., Diagne, C., Davoust, B., Fenollar, F. and Mediannikov, O. 2020. Rodents as hosts of pathogens and related zoonotic disease risk. Pathogens 9(3), 202; doi:10.3390/pathogens9030202. Delgado, C., Tirira, D., Gómez-Laverde, M., Matson, J. and Samudio, R. 2016. Reithrodontomys mexicanus (errata version published in 2017). The IUCN Red List of Threatened Species 2016: e.T19411A115151358. Available via https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T19411A22386636.en Dib, L.V., Palmer, J.P.S., de Souza Carvalho Class, C., Pinheiro, J.L., Ramos, R.C.F., Dos Santos, C.R., Fonseca, A.B.M., Rodríguez-Castro, K.G., Gonçalves, C.F., Galetti, P.M. Jr, Bastos, O.M.P., Uchôa, C.M.A., Corrêa, L.L. and da Silva Barbosa, A. 2020. Non-invasive sampling in Itatiaia National Park, Brazil: wild mammal parasite detection. BMC Vet. 16, 1–21; doi:10.1186/s12917-020-02490-5. Ederli, N.B., Gallo, S.S.M., Oliveira, L.C. and de Oliveira, F.C.R. 2018. Description of a new species Physaloptera goytaca n. sp. (Nematoda, Physalopteridae) from Cerradomys goytaca Tavares, Pessôa & Gonçalves, 2011 (Rodentia, Cricetidae) from Brazil. Parasitol. Res. 117, 2757–2766. doi:10.1007/s00436-018-5964-x. Falcón-Ordaz, J., Monzalvo-López, R.J. and García-Prieto, L. 2020. New species of Trichuris (Nematoda: Trichuridae) parasitizing Heteromys salvini (Rodentia: Heteromyidae) from Costa Rica, with a key to Trichuris species described from Heteromyidae. Rev. Bras. Parasitol. Vet. 29(2), e022019; doi:10.1590/S1984-29612020028. Gardner, S.L. and de Léon, G.P.P. 2002. Yungasicola travassosi gen. n., sp. n. (Digenea: Dicrocoeliidae: Eurytrematinae) from two species of grass mice of the genus Akodon Meyen (Rodentia: Muridae) from the Yungas of Bolivia. Comp. Parasitol. 69(1), 51–57; doi:10.1654/1525-2647(2002)069[0051:YTGNSN]2.0.CO;2. Gonzalez-Astudillo, V., Ramírez-Chaves, H.E., Henning, J. and Gillespie, T.R. 2016. Current knowledge of studies of pathogens in Colombian mammals. MANTER: J. Parasite Biodiversity 4:1–13; doi:10.13014/K2057CV6. Hämäläinen, A., Raharivololona, B., Ravoniarimbinina, P. and Kraus, C. 2015. Host sex and age influence endoparasite burdens in the gray mouse lemur. Front. Zool. 12, 1–14; doi:10.1186/s12983-015-0118-9. Islam, M.M., Farag, E., Hassan, M.M., Bansal, D., Awaidy, S.A., Abubakar, A., Al-Romaihi, H. and Mkhize-Kwitshana, Z. 2020. Reply to Hamzavi et al. Comment on “Islam et al. Helminth parasites among rodents in the middle east countries: a systematic review and meta-analysis. Animals 2020, 10, 2342”. Animals 10(12), 2342; doi:10.3390/ani13223467. Islam, M.M., Farag, E., Hassan, M.M., Enan, K.A., Mohammadi, A., Aldiqs, A.H., Mulsamani, E.A., Al-Zeyara, A.A., Al-Romaihi, H., Yassine, H.M., Sultan, A.A., Bansal, D. and Mkhize-Kwitshana, Z. 2024. Rodent-borne parasites in Qatar: a possible risk at the human-animal-ecosystem interface. One Health 18, 100708; doi:10.1016/j.onehlt.2024.100708. Kim, H.C., Hong, E.J., Ryu, S.Y., Park, J., Cho, J.G., Chae, J.S., Choi, K.S. and Park, B.K. 2020. Morphological and mlecular characterization of Toxocara apodemi (Nematoda: Ascarididae) from striped field mice, Apodemus agrarius, in Korea. Korean J. Parasitol. 58(4), 403; doi:10.3347/kjp.2020.58.4.403. Koyama, K. 2013. Characteristics and incidence of large eggs in Trichuris muris. Parasitol. Res. 112, 1925–1928; doi:10.1007/s00436-013-3348-9. Kumar, V. 1999 Dicrocoeliasis and eurytremiasis. In Trematode infections and diseases of man and animals. Ed., Kumar, V. Springer: Dordrecht, pp: 215–240; doi:10.1007/978-94-017-3594-0_5. Lee, J.H., Gong, S., Park, Y.C., Kim, H.J., Choi, I.W. and Lee, Y.H. 2018. Infections of intestinal helminth at two species of field mice, Apodemus agrarius and A. Peninsulae, in Gangwondo and Chungcheongnam-do, Korea. Korean J. Parasitol. 56(3), 301; doi:10.3347/kjp.2018.56.3.301. Lértora, W.J., Maria, M., Mussart, N.B., Villordo, G.I. and Sanchez Negrette, M. 2016. Anemia and hyperplastic gastritis in a Giant Anteater (Myrmecophaga tridactyla) due to Physaloptera magnipapilla parasitism. Braz. J. Vet. Pathol. 9(1), 20–26. Marsh, C.J., Sica, Y.V., Burgin, C.J., Dorman, W.A., Anderson, R.C., del Toro Mijares, I., Vigneron, J.G., Barve, V., Dombrowik, V.L., Duong, M. and Guralnick, R. 2022. Expert range maps of global mammal distributions harmonised to three taxonomic authorities. J. Biogeogr. 49(5), 979–992; doi:10.1111/jbi.14330. Martins, N.B.G., Panisse, G., Robles, M.D.R., Diaz, J.I. and Navone, G.T. 2022. Update of Dicrocoeliidae (Digenea) parasitizing rodents from the Americas with a description of a new Platynosomoides species from Argentina. Anais Acad. Brasil. Ci. 94(suppl 3), e20211430; doi:10.1590/0001-3765202220211430. Martínez-Borrego, D., Arellano, E., González-Cózatl, F.X. and Rogers, D.S. 2020. Reithrodontomys mexicanus (Rodentia: Cricetidae). Mammalian Species 52(996), 114–124; doi:10.1093/mspecies/seaa009. Mason, B., Piel, A.K., Modrý, D., Petrželková, K.J., Stewart, F.A. and Pafčo, B. 2022. Association of human disturbance and gastrointestinal parasite infection of yellow baboons in western Tanzania. PLos One 17(1), e0262481; doi:10.1371/journal.pone.0262481. Mendoza, H., López-Pérez, A.M., Rubio, A.V., Barrón-Rodríguez, J.J., Mazari-Hiriart, M., Pontifes, P.A., Dirzo, R. and Suzán, G. 2024. Association between anthropization and rodent reservoirs of zoonotic pathogens in Northwestern Mexico. PLos One 19(2), e0298976; doi:10.1371/journal.pone.0298976. Piegari, G., Pepe, P., De Biase, D., d’Aquino, I., Bosco, A., Cringoli, G., Papparella, S., Rinaldi, L. and Paciello, O. 2021. Immunopathological response, histological changes, parasitic burden, and egg output in sheep naturally infected by Dicrocoelium dendriticum. Animals 11(2), 546; doi:10.3390/ani11020546. Ramírez-Jaramillo, S.M., Bejarano-Muñoz, P., Caiza, A., Novillo, M. and Moreno-Cárdenas, P. 2019. Leucismo en Reithrodontomys Mexicanus Soederstroemi (Rodentia: Cricetidae), Quito, Ecuador. Acta Zool. Mex. 35, 1–4; doi:10.21829/azm.2019.3502078. Raynaud, J., Müller, K. and Schradin, C. 2012. Experimental increase of testosterone levels in free-ranging juvenile male African striped mice (Rhabdomys pumilio) induces physiological, morphological, and behavioral changes. Gen. Comp. Endocrinol. 178(1), 108–115; doi:10.1016/j.ygcen.2012.04.028. Reid, F. 1997. A field guide to the mammals of Central America and Southeast Mexico. New York, NY: Oxford University Press. de Saussure, H. 1860. Note sur quelques mammifères du Mexique. Revue et Magasin de Zoologie Serie 2 12:97–110. Taneja, V. 2018. Sex hormones determine immune response. Front. Immunol. 9, 1931; doi:10.3389/fimmu.2018.01931. Viney, M. and Kikuchi, T. 2017. Strongyloides ratti and S. venezuelensis–rodent models of Strongyloides infection. Parasitol. 144(3), 285–294; doi:10.1017/S0031182016000020. Zuk, M. and McKean, K.A. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26(10), 1009–1023. | ||
| How to Cite this Article |
| Pubmed Style Llano HA, Aguilar IL, Orozco NG, Giraldo LG, Zapata GS, Ruíz DG. Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia. Open Vet. J.. 2025; 15(1): 465-470. doi:10.5455/OVJ.2025.v15.i1.42 Web Style Llano HA, Aguilar IL, Orozco NG, Giraldo LG, Zapata GS, Ruíz DG. Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia. https://www.openveterinaryjournal.com/?mno=215904 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.42 AMA (American Medical Association) Style Llano HA, Aguilar IL, Orozco NG, Giraldo LG, Zapata GS, Ruíz DG. Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia. Open Vet. J.. 2025; 15(1): 465-470. doi:10.5455/OVJ.2025.v15.i1.42 Vancouver/ICMJE Style Llano HA, Aguilar IL, Orozco NG, Giraldo LG, Zapata GS, Ruíz DG. Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 465-470. doi:10.5455/OVJ.2025.v15.i1.42 Harvard Style Llano, H. A., Aguilar, . I. L., Orozco, . N. G., Giraldo, . L. G., Zapata, . G. S. & Ruíz, . D. G. (2025) Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia. Open Vet. J., 15 (1), 465-470. doi:10.5455/OVJ.2025.v15.i1.42 Turabian Style Llano, Horwald A.b., Itzel López Aguilar, Natalia Granda Orozco, Laura Gutiérrez Giraldo, Gloria Sánchez Zapata, and Daisy Gómez Ruíz. 2025. Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia. Open Veterinary Journal, 15 (1), 465-470. doi:10.5455/OVJ.2025.v15.i1.42 Chicago Style Llano, Horwald A.b., Itzel López Aguilar, Natalia Granda Orozco, Laura Gutiérrez Giraldo, Gloria Sánchez Zapata, and Daisy Gómez Ruíz. "Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia." Open Veterinary Journal 15 (2025), 465-470. doi:10.5455/OVJ.2025.v15.i1.42 MLA (The Modern Language Association) Style Llano, Horwald A.b., Itzel López Aguilar, Natalia Granda Orozco, Laura Gutiérrez Giraldo, Gloria Sánchez Zapata, and Daisy Gómez Ruíz. "Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia." Open Veterinary Journal 15.1 (2025), 465-470. Print. doi:10.5455/OVJ.2025.v15.i1.42 APA (American Psychological Association) Style Llano, H. A., Aguilar, . I. L., Orozco, . N. G., Giraldo, . L. G., Zapata, . G. S. & Ruíz, . D. G. (2025) Intestinal helminths infection of Reithrodontomys mexicanus (Rodentia: Cricetidae) from a Suburban Area in Medellín, Colombia. Open Veterinary Journal, 15 (1), 465-470. doi:10.5455/OVJ.2025.v15.i1.42 |