| Short Communication | ||
Open Vet. J.. 2024; 14(4): 1072-1075 Open Veterinary Journal, (2024), Vol. 14(4): 1072–1075 Short Communication Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracyAndressa Lorena Silveira Mendes1, Maria Isabel de Azevedo2 and Adriane Pimenta da Costa-Val Bicalho1*1Veterinary Clinics and Surgery, Veterinary School, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil 2Veterinary Preventive Medicine, Veterinary School, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil *Corresponding Author: Adriane Pimenta da Costa-Val Bicalho. Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. Email: adriane [at] ufmg.br Submitted: 12/12/2023 Accepted: 08/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
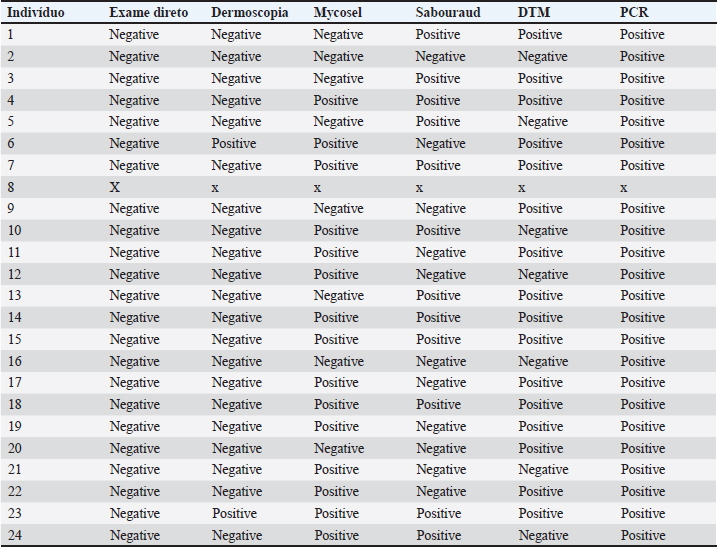
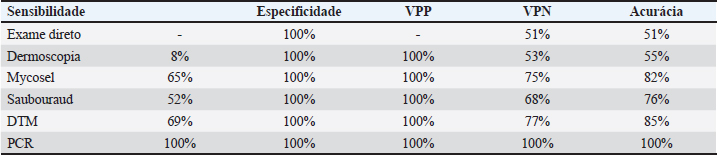
AbstractBackground: Dermatophytosis is a contagious fungal infection that affects mainly cats. It poses significant challenges in veterinary medicine due to its zoonotic potential and impact on animal and public health. Rapid and reliable diagnosis is crucial for preventing the spread of the disease, guiding treatment decisions, and monitoring disease control efforts. Although there are several studies on diagnostic methods in feline dermatophytosis, the comparison between them from the same sample lacks data. The absence of a universally accepted gold standard diagnostic method highlights the need for a multifaceted approach to diagnosing feline dermatophytosis. Aim: This study aims to assess the accuracy and efficacy of different diagnostic techniques comprehensively. Methods: For this, 48 samples of cats were analyzed by dermoscopy, direct hair examination, fungal culture using various media (Mycosel, Sabouraud, and Dermatophyte Test Medium), and polymerase chain reaction (PCR). Results: Direct examination and dermoscopy yielded unsatisfactory results. Mycosel and Sabouraud were suboptimal. DTM demonstrated superior selectivity, making it the most reliable among traditional methods. PCR was the top performer, exhibiting singular sensitivity, specificity, and accuracy. Conclusion: The study suggests that PCR may be the preferred choice for diagnosing feline dermatophytosis in clinical practice, especially when rapid and accurate results are essential. Keywords: Dermoscopy direct examination, Fungal culture, PCR. IntroductionDermatophytosis is a disease due to superficial fungal and zoonotic of great importance for felines. This disease is easily contracted by direct contact with infective arthrospores and may present miscellaneous clinical presentations, making the diagnostic tools important in detection (Moriello, 2014). The absence of a universally accepted gold standard diagnostic highlights the need for a multifaceted approach to diagnosing dermatophytosis in this animal species. Materials and MethodsForty-eight cats were selected based on sample size calculation: 24 cats were needed for both a symptomatic (SF) and asymptomatic (AF) group to ensure reliable results. The cats underwent a dermatological examination, assessed the entire body, and examined the skin by lifting the hair in the opposite direction of its growth. Wood’s lamp (Estek®, Rua Tenente-Coronel Soares Neiva, 412, Vila Aricanduva, São Paulo, SP, Brasil) assessment was utilized to pinpoint lesions and evaluate luminescence, categorizing cats into symptomatic and asymptomatic groups based on lesion presence and luminescence intensity. Dermoscopy was conducted with minimal restraint using an electronic microscope (Imports®, type M 1,000×), with recorded images later subjected to evaluation. The time used to collect images and samples was allowed by the animal, valuing comfort and well-being. Sample collection involved disposable sterile brushes (Kolplast® Estr Municipal Benedito de Souza, 418, Itupeva, SP), with samples obtained from the margins of recent lesions in the SF group and over the entire body in the AF group. The collected hair was subdivided into five sub-samples (A, B, C, D, and E). The direct examination involved transferring Sample A from each cat, in both groups, to a glass slide immersed in 10% Potassium Hydroxide (KOH) for 20 minutes, followed by a 400× examination. Fungal culture samples (B, C, and D) were inoculated onto plates containing Mycosel, Sabouraud (own manufacturing), and dermatophyte test medium (DTM) (Conclue®, Ouro Fino Saúde Animal, Cravinhos, São Paulo) media, respectively, with samples showing growth examined microscopically to identify fungal elements. Samples E underwent DNA Red Extract-N-Amp Plant (SIGMA, Eschenstr. 5 82024 Taufkirchen, Germany) and polymerase chain reaction (PCR) using specific dermatophyte primers (Dąbrowska et al., 2014) and PCR results analyzed via agarose gel electrophoresis. Ethical approvalThe study was approved by the Committee on Ethics in the Use of Animals (CEUA) under protocol number CEUA - 315/2022. All owners of the animals selected for this study signed informed consent forms. Results and DiscussionAs shown in Table 1, no affected hairs were found in the direct examination in the SF group. In dermoscopy, “comma hairs” were found in only 9% of the animals. In fungal cultures, out of the 23 cultures on Mycosel medium, 15 (65%) isolated some dermatophyte. This result was also obtained in 12 (52%) cultures on the Sabouraud medium. In both media, contaminating fungal growth was observed in all plates. All individuals in the SF group would have tested positive for dermatophytosis on the DTM medium if only the color change of the medium was evaluated simultaneously with the growth of a white “cloud”-shaped colony. However, at the end of 28 days, macroconidia of Microsporum canis were observed in only 16 (69%) of the 23 microscopic examinations. Only one DTM tube showed contaminating fungal growth. In the AF group, all plates and tubes of culture media showed contaminating fungal growth. All results from individuals in the SF group were positive in PCR. Considering these results, the values of accuracy, sensitivity, and specificity of the tests were presented in Table 2. In 2017, the World Association for Veterinary Dermatology recognized the absence of a gold standard for feline dermatophytosis (Moriello et al., 2017). This shift in perspective signifies the reconsideration of previously accepted clinical protocols. This study employed the Wood’s Lamp technique to identify lesions and aid in selecting animals for the research. Individuals with ulcerated and exudative dermatological complaints were excluded. Studies have shown that the sensitivity of direct examination varies, and it has been described as unsatisfactory (Moriello et al., 2017). An essential factor highlighted elsewhere (Căpitan et al., 2018) is the examiner’s experience. Due to the lack of positive results, it was not possible to calculate the sensitivity and positive predictive value (PPV) of the technique. Despite 100% specificity, the negative predictive value (NPV) was 51%, and the accuracy was 51%, representing the lowest values among all the tests. Table 1. Results of the methods: direct examination, dermoscopy, cultures, and PCR of the SF Group.
Table 2. Sensitivity and specificity analysis, positive predictive value, negative predictive value, and accuracy.
Dermoscopy was employed in this study as a rapid and cost-effective method (Dong et al., 2016). However, extensive physical restraint would have been required to obtain more precise imaging, contrary to the findings of other researchers (Scarampella et al., 2015). The level of physical restraint needed was time-consuming and substantial compared to alternative techniques, and no literature supported the use of chemical restraint. The significant magnification of the camera, coupled with the subject’s constant movement, posed challenges in image analysis. Areas of alopecia, skin scaling, and damaged hairs were observed in all SF groups, consistent with previous studies (Malvehy, 2007; Scarampella et al., 2015). However, the “comma hairs,” found in only two SF group cats, were considered unequivocally positive (Malvehy, 2007). Veterinary medicine lacks standardization for interpreting “comma hairs.” In human medicine, they are associated with dermatophytosis. This lack of standardization raises concerns about potential false positives when using “comma hairs” as a diagnostic criterion. Nonetheless, the absence of these hairs in the AF group led to a specificity of 100%, resulting in a high PPV (100%). However, the technique’s sensitivity in this study was low at 8% due to its reliance on “comma hairs” as a diagnostic determinant, yielding an NPV of 75%. In this study, all fungal culture media in the SF group exhibited fungal growth, with both Mycosel and Sabouraud media showing multiple colonies on all plates after 4 weeks: contaminating fungi were observed even when dermatophytes were present. This rapid growth of non-pathogenic fungi may compete with dermatophytes on the plate, potentially leading to false negatives. Both media demonstrated similar performance, with the Mycosel medium achieving a sensitivity of 65%, an NPV of 75%, and an accuracy of 82%, surpassing that of the Sabouraud medium. These values aligned with findings from other researchers (Kaufmann et al., 2016). Due to the absence of false positives, both techniques exhibited maximum specificity and PPV of 100%, ensuring the reliability of positive results. DTM media in the SF group exhibited a color change between 3 and 6 days, with whitish cottony colonies growing over the 4-week observation period. However, microscopic confirmation of M. canis presence was possible in only 69%. Therefore, microscopy evaluation remains crucial, as recommended (Kaufmann et al., 2016). The lack of complete fungus growth in seven tubes after 28 days led to their classification as negative. One probable reason for unsatisfactory growth is the limited space for fungal expansion within the culture medium tubes, which have a width of 2.5 cm, significantly smaller than the plates used with Mycosel and Sabouraud media. This space limitation and potential nutrient limitation can impede fungal growth and macroconidia development, as previously observed for dermatophytes in DTM (Moriello et al., 2010). In the AF group, there was some color variation in 66% of the tubes, but the dark red color seen in the SF group was not consistently achieved. This phenomenon indicates that contaminating fungi can induce the pH change responsible for color conversion (Moriello et al., 2017). Historically, the color change in DTM was considered evidence of dermatophyte presence. This was not confirmed microscopically, with no dermatophytes found in these samples. DTM presented the highest values among traditional fungal cultures. The PCR technique for dermatophytosis was developed and published in 2014 (Dąbrowska et al., 2014). Using the “Pan-dermatophyte” primer did not allow the identification of the dermatophyte species. Although a positive diagnosis for dermatophytosis is already sufficient to initiate treatment, it is not specific to each dermatophyte species. Thus, reliability in the result and speed are more valuable factors for the clinical veterinarian than species differentiation. There were no false positives or negatives for PCR in this study, demonstrating 100% sensitivity, PPV, NPV, and accuracy. The test was also one of the fastest, providing results in less than 8 hours, comparable to dermoscopy and direct examination regarding result readiness. ConclusionThis study evaluated various diagnostic techniques for dermatophytosis in feline patients with clinical signs of active infection. While direct examination and dermoscopy performed unsatisfactorily, traditional fungal culture media, including Mycosel and Sabouraud, provided better but still less than ideal results. Notably, the DTM showed superior selectivity, making it the most reliable among the traditional methods. However, the real standout was PCR, which demonstrated singular sensitivity, specificity, and accuracy, outperforming all other techniques. Due to its speed and reliability, these findings suggest that PCR may be the preferred choice for diagnosing feline dermatophytosis in animals with classical signs of the disease, especially when rapid and accurate results are crucial. However, the use of complementary examinations is often needed for a more accurate diagnosis. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Authors contributionsAll authors contributed to this study. All authors read and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesCăpitan, R., Schievano, C. and Noli, C. 2018. Evaluation of the value of staining hair samples with a modified Wright-Giemsa stain and/or showing illustrated guidelines for the microscopic diagnosis of dermatophytosis in cats. Vet. Dermatol. 29, 308–e106. Dąbrowska, I., Dworecka-Kaszak, B. and Brillowska-Dąbrowska, A. 2014. The use of a one-step PCR method for the identification of Microsporum canis and Trichophyton mentagrophytes infection of pets. Acta Biochim. Pol. 61, 375–378. Dong, C., Angus, J., Scarampella, F. and Neradilek, M. 2016. Evaluation of dermoscopy in the diagnosis of naturally occurring dermatophytosis in cats. Vet. Dermatol. 27, 275–e65. Kaufmann, R., Blum, S.E., Elad, D. and Zur, G. 2016. Comparison between point-of-care dermatophyte test medium and mycology laboratory culture for diagnosis of dermatophytosis in dogs and cats. Vet. Dermatol. 27, 284–e68. Malvehy, J., Puig, S., Argenziano, G., Marghoob, A.A. and Soyer, H. 2007. International Dermoscopy Society Board members. Dermoscopy report: proposal for standardization. Results of a consensus meeting of the International Dermoscopy Society. J. Am. Acad. Dermatol. 57, 84–95. Moriello, K. 2014. Feline dermatophytosis: aspects pertinent to disease management in single and multiple cat situations. J. Feline Med. Surg. 16, 419–431. Moriello, K.A., Coyner, K., Paterson, S. and Mignon, B. 2017. Diagnosis and treatment of dermatophytosis in dogs and cats: clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 28, 266–e68. Moriello, K.A., Verbrugge, M.J. and Kesting, R.A. 2010. Effects of temperature variations and light exposure on the time to growth of dermatophytes using six different fungal culture media inoculated with laboratory strains and samples obtained from infected cats. J. Feline Med. Surg. 12, 988–990. Scarampella, F., Zanna, G., Peano, A., Fabbri, E. and Tosti, A. 2015. Dermoscopic features in 12 cats with dermatophytosis and in 12 cats with self-induced alopecia due to other causes: an observational descriptive study. Vet. Dermatol. 26, 282–e63. | ||
| How to Cite this Article |
| Pubmed Style Mendes ALS, Azevedo MID, Bicalho APDC. Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy. Open Vet. J.. 2024; 14(4): 1072-1075. doi:10.5455/OVJ.2024.v14.i4.14 Web Style Mendes ALS, Azevedo MID, Bicalho APDC. Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy. https://www.openveterinaryjournal.com/?mno=180641 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i4.14 AMA (American Medical Association) Style Mendes ALS, Azevedo MID, Bicalho APDC. Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy. Open Vet. J.. 2024; 14(4): 1072-1075. doi:10.5455/OVJ.2024.v14.i4.14 Vancouver/ICMJE Style Mendes ALS, Azevedo MID, Bicalho APDC. Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy. Open Vet. J.. (2024), [cited January 24, 2026]; 14(4): 1072-1075. doi:10.5455/OVJ.2024.v14.i4.14 Harvard Style Mendes, A. L. S., Azevedo, . M. I. D. & Bicalho, . A. P. D. C. (2024) Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy. Open Vet. J., 14 (4), 1072-1075. doi:10.5455/OVJ.2024.v14.i4.14 Turabian Style Mendes, Andressa Lorena Silveira, Maria Isabel De Azevedo, and Adriane Pimenta Da Costa-val Bicalho. 2024. Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy. Open Veterinary Journal, 14 (4), 1072-1075. doi:10.5455/OVJ.2024.v14.i4.14 Chicago Style Mendes, Andressa Lorena Silveira, Maria Isabel De Azevedo, and Adriane Pimenta Da Costa-val Bicalho. "Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy." Open Veterinary Journal 14 (2024), 1072-1075. doi:10.5455/OVJ.2024.v14.i4.14 MLA (The Modern Language Association) Style Mendes, Andressa Lorena Silveira, Maria Isabel De Azevedo, and Adriane Pimenta Da Costa-val Bicalho. "Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy." Open Veterinary Journal 14.4 (2024), 1072-1075. Print. doi:10.5455/OVJ.2024.v14.i4.14 APA (American Psychological Association) Style Mendes, A. L. S., Azevedo, . M. I. D. & Bicalho, . A. P. D. C. (2024) Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy. Open Veterinary Journal, 14 (4), 1072-1075. doi:10.5455/OVJ.2024.v14.i4.14 |