| Case Report | ||
Open Vet. J.. 2024; 14(4): 1076-1080 Open Veterinary Journal, (2024), Vol. 14(4): 1076–1080 Case Report CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus)Antón Costas1*, Pierantonio Battiato2, Carolina Magro3, and Vicente Cervera11Diagnostic Imaging Department, Anicura Valencia Sur Hospital Veterinario, Silla, Spain 2Diagnostic Imaging Department, Veterinary Teaching Hospital, University of Milan, Lodi (LO), Italy 3VetOeiras Veterinary Hospital, Oeiras, Portugal *Corresponding Author: Antón Costas. Diagnostic Imaging Department, Anicura Valencia Sur Hospital Veterinario, Silla, Spain. Email: antoncostaspereiro [at] gmail.com Submitted: 06/12/2023 Accepted: 09/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
AbstractBackground: Segmental aplasia of the caudal vena cava (CVC) with azygos continuation is a congenital malformation macroscopically described in mammals including humans, dogs, and rodents. It is usually detected as an incidental finding and the final diagnosis is reached by computed tomography (CT), fluoroscopy, or post-mortem dissection. Case Description: A 3-year-old guinea pig (Cavia porcellus) presented with subacute dyspnea. A computed tomographic examination was performed for the evaluation of subtle pulmonary changes previously suspected on conventional radiography, and a segmental aplasia of the CVC with azygos continuation was identified as an incidental finding. Conclusion: According to database negative results, this is the first report describing a segmental aplasia of the CVC and azygos continuation in a guinea pig by CT. Keywords: Small mammal, Interruption caudal vena cava, Vascular malformation, Congenital malformation. IntroductionThe normal anatomy of the caudal vena cava (CVC), termed the inferior vena cava (IVC) in humans, consists of a single vessel, right-sided to the aorta, formed by the confluence of bilateral common iliac veins at the level of the aortic trifurcation. There are five distinct segments along its pathway to the right atrium: prerenal, renal, prehepatic, hepatic, and posthepatic. The embryogenesis of the CVC is a complex process involving the development, regression, anastomosis, and replacement of paired and symmetric embryonic veins, and during this process, different congenital variants may develop (Chuang et al., 1974; Hunt et al., 1998; Bass et al., 2000; Cornillie and Simoens, 2005; Li et al., 2021). Segmental aplasia of IVC is infrequently reported in human medicine with a prevalence of 0.6% (Bass et al., 2000; Li et al., 2021). In this condition, there is no communication between the prehepatic and hepatic segments of the IVC. As a result, the renal segment of the IVC continues with the azygos (right-sided) or hemiazygos (left-sided) vein. In dogs, segmental aplasia of the CVC with azygos/hemiazygos continuation has also been described, usually detected as an incidental finding in imaging studies performed for diagnosing other vascular anomalies such as portosystemic shunts, or during laparotomy or anatomic dissection (Barthez et al., 1996; Hunt et al., 1998; Harder et al., 2002; Fischetti and Kovak, 2008; Schwarz et al., 2009). Although no prevalence has been documented in canine patients, the incidence of this anomaly is thought to be higher than expected and it is now more frequently reported because of the increased use of advanced diagnostic imaging techniques (Schwarz et al., 2009). Congenital CVC anomalies in Cavia porcellus are poorly described. The necropsy findings of an absent CVC in a Brazilian guinea pig (Cavia aperea), were published (Phisalix, 1898). However, database searches (PubMed, CAB, and Scopus) covering the years 1950–2023 were negative for segmental aplasia of the CVC in Cavia porcellus. In this case report, we present the computed tomography (CT) findings of a segmental aplasia of the CVC and azygos continuation in a guinea pig. Case DetailsA 3-year-old, male castrated, 0.980-kg, American guinea pig (Cavia porcellus) was referred to the exotic animal service of VetOeiras Veterinary Hospital (Oeiras, Portugal) for progressive respiratory sounds and dyspnea for 1-week duration. Previous medical history was unremarkable. These were experienced owners and provided excellent husbandry and care. The animal was fed a high-quality timothy hay diet and fresh water ad libitum. In addition, 150 g of fresh mixed vegetables daily and a tablespoon of guinea pig pellet diet. The cage was cleaned biweekly and the bedding was fleece cage liners. On physical examination, the patient was active, alert, normothermic (39°C), and maintained an ideal body score. Pulmonary auscultation revealed mild tachypnea (134 breaths/minute) with marked respiratory sounds including rhonchi and bilateral crackles, worse on the right side, while the cardiac auscultation was normal with a heart rate of 260 beats/minute. At this time, differentials were made for respiratory distress and included pneumonia, pulmonary edema, upper respiratory tract infection, allergic respiratory disease, or mass-like lesions. Hematology and a biochemistry panel along with thoracic radiographs were proposed to and accepted by the owners. The patient was administered intramuscular butorphanol 0.5 mg/kg (Dolorex 10 mg/ml; Intervet International GmbH, Unterschleissheim, Germany) for peripheral blood collection, in the right vena saphena lateralis, and diagnostic imaging of the thorax. Hematology revealed elevated white blood cell count (14.70 × 103/μl; reference range 1.9–11.70 × 103/μl) with eosinophilia (1.36 × 103/μl; reference range 0–0.71 × 103/μl), heterophilia (7.22 × 103/μl; reference range 0.44–6.80 × 103/μl) and lymphocytosis (6.95 × 103/μl; reference range 0.48–4.82 × 103/μl). The RBC count, hemoglobin, and hematocrit were within normal limits while the biochemistry was unremarkable. The reference values were taken from the previously published literature (Spittler et al., 2021). Thoracic radiographs were performed and revealed a mixed alveolar-interstitial opacity at the region of the right caudal lung lobe. Due to the age of the patient, clinical presentation, and radiographic findings, pneumonia was speculated as the main differential. A primary lung neoplasia, such as a pulmonary adenoma, was considered less likely. Antibiotic treatment was administered (enrofloxacin 5 mg/kg PO BID for 2 weeks) with no relevant improvement of the clinical signs nor resolution of the radiographic findings at the 2-week follow-up. A CT evaluation of the thorax was performed to rule out other concurrent conditions such as the presence of lung mass-like lesions. The patient was pre-anesthetized with a combination of intramuscular 0.5 mg/kg butorphanol and 1 mg/kg midazolam (Midazolam 15 mg/5 ml, Labesfal, Santiago de Besteiros, Portugal), followed by induction with alfaxalone (1 mg/kg diluted injected slowly in the cephalic vein; Alfaxan 10 mg/ml; Jurox Limited; Dublin; Ireland) and maintained with inhaled 2% of isoflurane (Isoflurin 1,000 mg/g; VETPHARMA ANIMAL HEALTH, S.L.; Barcelona, Spain) in a 1.5% flow rate of Oxygen with a facemask. A whole-body CT (Somatom, Siemens) examination was performed with the following technical parameters: 110 Kvp, 70 mA, 0.8 pitch, 1 mm slice thickness, and 0.9 mm slice interval. Pre-contrast images were reconstructed using soft tissue and lung algorithms and post-contrast images were reconstructed using a soft tissue algorithm. The contrast used was Omnipaque (300 mgI/ml; GE Healthcare, Oslo, Norway) administered at a 300 mgI/kg IV dose through a 26G catheter (Kruuse Venocan Mini IV) placed in the right cephalic vein. The CT revealed multifocal areas of increased attenuation affecting the right cranial, middle, and caudal lung lobes, as well as the left cranial lung lobe, mainly with a ventral distribution except for the right caudal lobe which was affected caudodorsally. In addition, a vascular anomaly affecting the CVC and azygos veins was detected. Multiplanar maximum intensity projections (MIPs) and 3D volume renderings reconstructions were evaluated for a better depiction of the vascular structures (Fig. 1). Immediately cranial to the renal segment, the CVC extended craniodorsal and right-sided, medial to the right kidney and dorsal to the right adrenal gland. It crossed into the hiatus aorticus and shared its anatomical intrathoracic pathway abnormally with the azygos vein, which was overtly dilated. A post-hepatic component of the thoracic portion of the CVC was normally detected draining into the right atrium. Communication between the pre-hepatic and hepatic segments of the CVC could not be identified, as reported by CT description in other species. These findings were consistent with a segmental aplasia of the CVC with azygos continuation (Chuang et al., 1974; Bass et al., 2000; Li et al., 2021). Although this vascular anomaly can predispose to thrombus formation and pulmonary thromboembolism (Newman et al., 2020), this was not considered in our case as no aneurysmal dilation of the CVC or filling defects in the pulmonary arteries were identified. Hence, it was deemed an incidental finding unrelated to the clinical signs, whereas the pulmonary changes were probably attributed to an infectious pulmonary process. Tracheo-bronchial lavage was proposed after a CT examination but refused by the owners due to financial restrictions. The patient recovered uneventfully and was discharged the same day. A new antibiotic treatment was started (trimethoprim + sulfamethoxazole 30 mg/kg BID for 30 days), with complete resolution of the clinical signs and radiographic findings at 1-month follow-up. DiscussionDescriptions of vascular anomalies implying the CVC in guinea pigs (Cavia porcellus) are limited to a solitary scientific publication in which the abdominal cavity of euthanized laboratory guinea pigs was observed macroscopically, and duplication of the CVC was frequently recognized, with a 30% prevalence in males and 24% in females (Nakamura et al., 2019). Multiple cardiovascular anomalies were described in another case report of a guinea pig, none of them affecting the CVC (Haris et al., 1976). An absence of the CVC was described in a Cavia aperea, however no CT findings were reported (Phisalix, 1898).
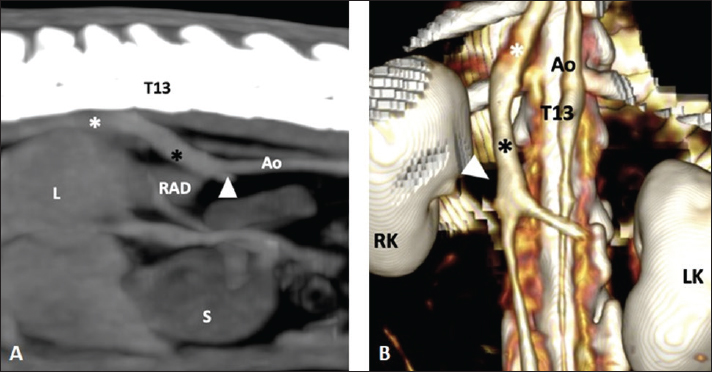
Fig. 1. (A) Parasagittal post-contrast MIP image (WL 40 HU, WW 300 HU) of the abdomen of a 3-year-old, male castrated, 0.980-kg, American guinea pig in which an abnormal communication (black asterisk) is detected between the post-renal segment of the CVC (white arrow) and the right azygous vein (white asterisk). Cranial is to the left of the image. (B) Dorsal 3D view highlighting the anatomy of the CVC and showing the abnormal communication between both vessels. Cranial is at the top of the image. T13: 13th thoracic vertebra, Ao: aorta, RK: right kidney, LK: left kidney, L: liver, S: stomach, RAD: right adrenal gland. The embryogenesis of the CVC (IVC in humans) is widely illustrated in the literature (Chuang et al., 1974; Barthez et al., 1996; Bass et al., 2000; Cornillie and Simoens, 2005; Li et al., 2021) and related to the paired posterior cardinal, subcardinal, and supracardinal embryonic veins. If failure occurs at the time of fusion between the right subcardinal and hepatic (formerly vitelline) veins, then the communication between the postrenal CVC and the cranial portion of the supracardinal vein (precursor of the right azygos or left hemiazygos vein) will be patent (Hunt et al., 1998; Bass et al., 2000; Fischetti and Kovak, 2008; Li et al., 2021). Other explanations have been proposed according to different embryologic models of the CVC genesis (Barthez et al., 1996). It results in the venous blood flow from the renal and post-renal segments being shunted into the azygous or hemiazygos (less frequently) vein with no communication between the pre-hepatic and hepatic segments of the CVC. Although right and left azygos veins are present in the embryo, the pattern is later commonly simplified: in the horse, dog, and cat the right vein persists while in the pig the left or occasionally both persist; in ruminants, both veins are usually present (König and Liebich, 2020). This could lead to variants in this vascular anomaly depending on the species-specific azygos system. In guinea pigs, it is derived from the right postcardinal vein (Potter et al., 1958) which is consistent with the right-sided azygos identified in our case. In human and canine patients, most of the cases with this condition are detected incidentally (Harder et al., 2002). Nevertheless, it has also been reported in symptomatic patients associated with thrombosis of the CVC and/or renal veins, and pulmonary thromboembolism (Harder et al., 2002; Lambert et al., 2010; Lockwood et al., 2018; Newman et al., 2020), as well as with other congenital malformations such as polysplenia, situs anomalies or portosystemic shunt (Bass et al., 2000; Fischetti and Kovak, 2008; Li et al., 2021). In the case described herein, the patient had a CT examination as a part of a diagnostic investigation of clinical signs related to the respiratory system, and the abdomen was included in the protocol to rule out clinically relevant comorbidities. The clinical signs and thoracic CT findings did not appear to be related to the vascular anomaly, which was considered an incidental finding. Consequences of this type of congenital defect, other than the previously mentioned thromboembolic events, include implications for renal surgery and cardiac catheterization in humans, leading to potential hemorrhagic events or vascular injury when not detected in the presurgical planning (González et al., 2017). Another consideration is described in dogs with right adrenal tumors in which a CVC venectomy was required, even with no vascular invasion, when the adrenal gland was displaced ventrally relative to the CVC (Takagi et al., 2021). While CT imaging is not as commonly employed in small mammals like guinea pigs compared to dogs and cats, its utility extends to various clinical scenarios due to its inherent cross-sectional nature, which mitigates the superimposition of anatomical structures. Indications include evaluation of dental disease and other bony structures of the head (Krautwald-Junghanns et al., 2011) or assessment of the thoracic and abdominal cavities for anatomical and medical purposes (Parkinson et al., 2017; Zehtabvar et al., 2023). Furthermore, the injection of intravenous contrast medium enhances the vascular structures of the body and allows the evaluation of potential vascular malformations. ConclusionIn conclusion, this is the first case report describing the tomographic features of a congenital segmental aplasia of the CVC with azygos continuation in a C. porcellus. This finding is usually asymptomatic, but potential clinical or surgical implications emphasize the importance of its identification. Acknowledgments None. Conflict of interestThe authors have no conflict of interest to declare. FundingThis research received no specific grant. Author contributionsAntón Costas: Main author of the manuscript and literature review. Carolina Magro and Pierantonio Battiato: Co-authors of the paper. Vicente Cervera: review of the process and manuscript. Data availabilityThe data supporting the findings of this study are available within the article. ReferencesBarthez, P.Y., Siemens, L.M. and Koblik, P.D. 1996. Azygos continuation of the caudal vena cava in a dog: radiographic and ultrasonographic diagnosis. Vet. Radiol. Ultrasound. 37(5), 354–356. Bass, J.E., Redwine, M.D., Kramer, L.A., Huynh, P.T. and Harris, J.H. Jr. 2000. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics 20(3), 639–652. Chuang, V.P., Mena, C.E. and Hoskins, P.A. 1974. Congenital anomalies of the inferior vena cava. Review of embryogenesis and presentation of a simplified classification. Br. J. Radiol. 47(556), 206–213. Cornillie, P. and Simoens, P. 2005. Prenatal development of the caudal vena cava in mammals: review of the different theories with special reference to the dog. Anat. Histol. Embryol. 34(6), 364–372. Fischetti, A.J. and Kovak, J. 2008. Imaging diagnosis: azygous continuation of the caudal vena cava with and without portocaval shunting. Vet. Radiol. Ultrasound. 49(6), 573–576. González, J., Gaynor, J.J., Albéniz, L.F. and Ciancio, G. 2017. Inferior vena cava system anomalies: surgical implications. Curr. Urol. Rep. 18(2), 10. Harder, M.A., Fowler, D., Pharr, J.W., Tryon, K.A. and Shmon, C. 2002. Segmental aplasia of the caudal vena cava in a dog. Can. Vet. J. 43(5), 365–368. Haris, A.M., Horton, M.L., Terpolilli, R.N., Van Stee, E.W. and Back, K.C. 1976. Anomalous cardiovascular structures in a guinea pig. Vet. Pathol. 13(2), 157–158. Hunt, G.B., Bellenger, C.R., Borg, R., Youmans, K.R., Tisdall, P.L. and Malik, R. 1998. Congenital interruption of the portal vein and caudal vena cava in dogs: six case reports and a review of the literature. Vet. Surg. 27(3), 203–215. König, H.E. and Liebich, H.G. 2020. Veterinary anatomy of domestic animals. Thieme, Sttutgart, Germany, pp: 495. Krautwald-Junghanns, M.E., Pees, M., Reese, S. and Tully, T. 2011. Diagnostic imaging of exotic pets. Hannover, Germany: Schlütersche, p: 242. Lambert, M., Marboeuf, P., Midulla, M., Trillot, N., Beregi, J.P., Mounier-Vehier, C., Hatron, P.Y. and Jude, B. 2010. Inferior vena cava agenesis and deep vein thrombosis: 10 patients and review of the literature. Vasc. Med. 15(6), 451–459. Li, S.J., Lee, J., Hall, J. and Sutherland, T.R. 2021. The inferior vena cava: anatomical variants and acquired pathologies. Insights Imaging. 12(1), 123. Lockwood, A.J., Sinnott-Stutzman, V.B., Mouser, P.J. and Tsai, S.L. 2018. Azygos continuation of the caudal vena cava with segmental aneurysm, lung lobe torsion and pulmonary thromboembolism in a dog. Clin. Case Rep. 6(2), 363–369. Nakamura, T., Norimura, M., Sumi, K., Ichii, O., Elewa, Y.H.A., Kon, Y., Tatsumi, O., Hattori, H., Yoshiyasu, T. and Nagasaki, K.I. 2019. Slc:Hartley guinea pigs frequently possess duplication of the caudal vena cava. Exp. Anim. 68(4), 465–470. Newman, R.J., Egeler, K.M., Conkling, A.L. and Savage, M.Y. 2020. Multimodality imaging of an azygous continuation of the caudal vena cava in a dog with pulmonary thromboembolic disease. Vet. Radiol. Ultrasound. 61(5), E40–E44. Parkinson, L.A.B., Hausmann, J.C., Hardie, R.J., Mickelson, M.A. and Sladky, K.K. 2017. Urethral diverticulum and urolithiasis in a female guinea pig (Cavia porcellus). J. Am. Vet. Med. Assoc. 251(11), 1313–1317. Phisalix, M.C. 1898. Absence of vena cava in Cavia aperea. Comptes; Rend. Soc. Biol. 10, 230–231. Potter, G., Jones, W. and Hermann, C.L. 1958. The circulatory system of the guinea pig. Bios 29(1), 3–13. Schwarz, T., Rossi, F., Wray, J.D., Ablad, B., Beal, M.W., Kinns, J., Seiler, G.S., Dennis, R., McConnell, J.F. and Costello, M. 2009. Computed tomographic and magnetic resonance imaging features of canine segmental caudal vena cava aplasia. J. Small Anim. Pract. 50(7), 341–349. Spittler, A.P., Afzali, M.F., Bork, S.B., Burton, L.H., Radakovich, L.B., Seebart, C.A., Moore, A.R. and Santangelo, K.S. 2021. Age- and sex-associated differences in hematology and biochemistry parameters of Dunkin Hartley guinea pigs (Cavia porcellus). PLoS One 16(7), e0253794. Takagi, S., Kanai, E., Morishita, K., Ogawa, H. and Ogawa, J. 2021. Surgical treatment of an abnormally positioned right adrenal tumor on segmental caudal vena cava aplasia in two dogs. J. Vet. Med. Sci. 83(1), 69–72. Zehtabvar, O., Masoudifard, M., Rostami, A., Akbarein, H., Sereshke, A.H.A., Khanamooeiashi, M. and Borgheie, F. 2023. CT anatomy of the lungs, bronchi and trachea in the mature guinea pig (Cavia porcellus). Vet. Med. Sci. 9(3), 1179–1193. | ||
| How to Cite this Article |
| Pubmed Style Costas A, Battiato P, Magro C, Cervera V. CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus). Open Vet. J.. 2024; 14(4): 1076-1080. doi:10.5455/OVJ.2024.v14.i4.15 Web Style Costas A, Battiato P, Magro C, Cervera V. CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus). https://www.openveterinaryjournal.com/?mno=179932 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i4.15 AMA (American Medical Association) Style Costas A, Battiato P, Magro C, Cervera V. CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus). Open Vet. J.. 2024; 14(4): 1076-1080. doi:10.5455/OVJ.2024.v14.i4.15 Vancouver/ICMJE Style Costas A, Battiato P, Magro C, Cervera V. CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus). Open Vet. J.. (2024), [cited January 24, 2026]; 14(4): 1076-1080. doi:10.5455/OVJ.2024.v14.i4.15 Harvard Style Costas, A., Battiato, . P., Magro, . C. & Cervera, . V. (2024) CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus). Open Vet. J., 14 (4), 1076-1080. doi:10.5455/OVJ.2024.v14.i4.15 Turabian Style Costas, Anton, Pierantonio Battiato, Carolina Magro, and Vicente Cervera. 2024. CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus). Open Veterinary Journal, 14 (4), 1076-1080. doi:10.5455/OVJ.2024.v14.i4.15 Chicago Style Costas, Anton, Pierantonio Battiato, Carolina Magro, and Vicente Cervera. "CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus)." Open Veterinary Journal 14 (2024), 1076-1080. doi:10.5455/OVJ.2024.v14.i4.15 MLA (The Modern Language Association) Style Costas, Anton, Pierantonio Battiato, Carolina Magro, and Vicente Cervera. "CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus)." Open Veterinary Journal 14.4 (2024), 1076-1080. Print. doi:10.5455/OVJ.2024.v14.i4.15 APA (American Psychological Association) Style Costas, A., Battiato, . P., Magro, . C. & Cervera, . V. (2024) CT findings of an incidental segmental aplasia of the caudal vena cava with azygos continuation in a guinea pig (Cavia porcellus). Open Veterinary Journal, 14 (4), 1076-1080. doi:10.5455/OVJ.2024.v14.i4.15 |