| Research Article | ||
Open Vet. J.. 2023; 13(12): 1631-1644 Open Veterinary Journal, (2023), Vol. 13(12): 1631–1644 Original Research Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in EgyptHend Adel Zineldar1, Nasser Zeidan Abouzeid1, Mohamed Ibrahim Eisa1, Emad Mohamed Bennour2 and Wafaa Mohamed El Neshwy1*1Department of Animal Infectious Diseases, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Internal Medicine, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Wafaa Mohamed El Neshwy. Department of Animal Infectious Diseases, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: wafaa892011 [at] gmail.com Submitted: 18/09/2023 Accepted: 21/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
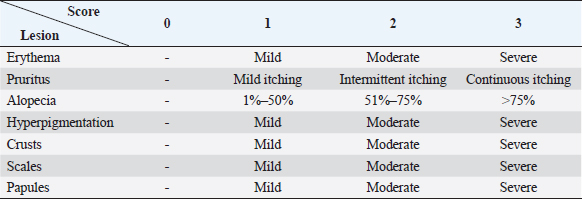
AbstractBackground: Skin diseases are usually chronic in nature but not life-threatening. They affect the well-being and pose a threat to the general health of the affected animals. Aim: This study aimed to investigate epidemiological, clinical, and therapeutic aspects of ectoparasitic infestations in dogs in a number of Egyptian governorates. Methods: Ninety dogs (58 males and 32 females) aged from 1 month to 11 years from 6 Egyptian governorates were clinically examined during the years 2022 and 2023. Skin scraping samples were taken from all examined dogs, and deep ear swab specimens from five dogs suspected to have ear mites were obtained and parasitologically examined. Different ectoparasites were classified according to their morphological features. Twenty dogs were treated in four different patterns of administration of local, systemic, and supportive medications. Results: The prevalence of ectoparasite infestation in examined dogs was 64% (58/90). The majority of ectoparasitic infestations (50/58) were single, while the rest (8/58) were mixed. Nine species of ectoparasites of fourtaxa were identified: a tick species (Rhipicephalus sanguineus); which had the highest prevalence among isolated ectoparasites from dogs (29%, 26/90), three flea species (Ctenocephalides canis, Ctenocephalides orientis, and Ctenocephalides felis) isolated from 18 out of 90 cases (20%), two types of dog chewing louse species (Trichodectes canis and Heterodoxus spiniger) isolated from 2/90 (2.2%) and three mite species: Demodex canis (18/90, 20%), Sarcoptes scabei var. canis (5/90, 6%) and Otodectes cynotis (2/90, 2.2%). The logistic regression analysis of the potential risk factors associated with the prevalence of ectoparasites in dogs revealed that age, breed, housing environment, habitat, and season were the significant factors affecting the prevalence of ectoparasites (p < 0.05) in contrast dog gender did not have a significant effect. Treated dogs showed variations in recovery times and dogs that received ancillary treatment showed rapid skin improvement and hair regrowth. Doramectin was effective against ticks and fleas, but fluralaner was more effective against Demodex mites. Conclusion: The prevalence of ectoparasites in dogs in Egypt could be considered high and necessitates efforts toward accurate diagnosis, treatment, and control to reduce their impact on animal and public health. Keywords: Dogs, Ectoparasites, Egypt, Prevalence, Treatment. IntroductionEctoparasites have an impact on animal wellbeing, cause skin irritation, pruritus, skin lesions (dermatitis and eosinophilic aggregation), and lead to secondary bacterial infections (Abscess formation, pyoderma, and pyemia), anemia due to blood-sucking ectoparasites which may be life-threatening in young and debilitated animals or even toxicosis ‘Tick paralysis’ which is caused by toxins in tick saliva. In addition, they constitute a zoonotic hazard and transmit pathogens such as bacteria (Rickettsia spp., Borrelia spp.), protozoa (Babesia spp., Hepatozoon spp.) and helminths (Acanthocheilonema reconditum, Dipylidium caninum, and Hymenolepis diminuta) (De La Fuente et al., 2008; Beugnet et al., 2014; Rafiqi et al., 2016 and Diakou et al., 2022). Fleas (genus Ctenocephalides) are the most common ectoparasites of veterinary and public health importance. A flea infestation can also cause flea allergic dermatitis (FAD) which is a hypersensitivity reaction to flea saliva (Xhaxhiu et al., 2009; Azrizal et al., 2019 and Diakou et al., 2022). Ticks are the second most common ectoparasite in dogs. Rhipicephalus sanguineus is a three-host brown dog tick distributed worldwide, affecting mainly dogs and occasionally other hosts, including humans (Dantas-Torres, 2008 and Rafiqi et al., 2016). Lice spend their entire lives on the host feeding on epidermal tissue debris, sebaceous fluids, and blood. Lice infestations (pediculosis) in dogs are caused by two different lice species, Trichodectes canis (biting lice) and Linognathus setosus (sucking lice), causing intense irritation, pruritus, excoriations, and anemia. Their predilection sites include the ears, neck, and back (Arther, 2009). Mange in dogs is a common skin disease, that can be caused by burrowing mites: sarcoptic mites (Sarcoptes scabei), non-burrowing mites, and Demodex mites (including Demodex canis, Demodex injai, and Demodex cornei). Burrowing mites produce channels in the skin, in which eggs are deposited. They are highly contagious and could be transmitted by direct contact and indirectly by fomites. Non-burrowing mites pierce the skin, causing dermatitis, hyperkeratosis, and skin inflammation leading to pruritus (Arther, 2009; Arlian and Morgan, 2017). Demodex mites are noncontagious, where transmission from animal to animal is restricted from mother to offspring immediately in the post-natal period (Mueller et al., 2012). Otodectes cynotis mites (non-burrowing mites) cause otocariosis by infesting the external ear canal of dog and feeding on epidermal debris and body fluids. They are characterized by large amounts of dry, dark brown, waxy debris with variable amounts of inflammation (Otitis externa) (Ghubash, 2006). Usually, skin diseases are not life-threatening but can be chronic and affect the animal’s welfare. Their treatment is usually topical or systemic. In-contact animals must also be treated to prevent reinfection of affected animals (Feather et al., 2010). Both local and systemic treatments require several administrations until recovery, and treatment should be continued for 2–4 weeks following recovery, particularly in the case of generalized demodicosis, to prevent recurrence (Mueller et al., 2020). Many preparations have been proven to be effective against many ectoparasites, among them benzyl benzoate at 25%–33%. It is a cheap, effective medication against sarcoptic mange (Hay et al., 2012). Treatment with Amitraz rinses weekly and subcutaneous or oral doramectin administration once or twice weekly has given evidence for their efficacy (Hugnet et al., 2001). Additionally, fluralaner was reported to be highly effective against sarcoptic mange, fleas, and ticks, with an extended period of protection of up to 3 months (Pfister and Armstrong, 2016; Chiummo et al., 2020. Considering the impact of the ectoparasitic infestation on the animal life quality and public health, the present study was carried out in Egypt to investigate the prevalence of ectoparasites and the potential risk factors influencing their occurrence in dogs with an assessment of treatment regimens using recent local and/or systemic preparations. Materials and MethodsAnimalsThis study was conducted on 90 dogs during the period from May 2022 to 2023 in 6 Egyptian governorates (Sharkia, Cairo, Giza, Kafr-Elsheikh, Alexandria, and Ismailia). Complete data about age, gender, breed, habitat, diet, management, living stresses, and administration of acaricides were obtained by interviewing the owners and clinically examining the animals. The subjects (58 males and 32 females) aged approximately 1 month to 11 years old (34 dogs <1 year old, 44 dogs aged 1–5 years old and 12 dogs >5 years) and included 70 large breed dogs (18 German Shepherds,15 Local breed dogs, 12 Golden Retrievers, 10 Pitbulls, 4 Husky dogs, 3 Chow Chow dogs, 3 Labrador Retrievers, 2 Rottweilers, 1 Dogo Argentino, 1 Mastiff, and 1 Cane Corso) and 20 small breed dogs (9 Griffons, 4 Yorkshire Terriers, 3 Cocker Spaniels, 1 Pikinwah, and 1 Japanese Spitz). Clinical examination and scoringThe entire skin coat including the ears of each dog was thoroughly examined for the presence of ectoparasites and any skin lesions such as alopecia, crusting, scaling, and/or others. The sites of lesions were identified and recorded. Each ectoparasite-affected case was assessed by scoring skin lesions on a 0–3 scale (Table 1). The scoring was done by the same investigator. The scoring of each examined body region (e.g., the head, neck, shoulder, or back) was estimated according to the following equations:
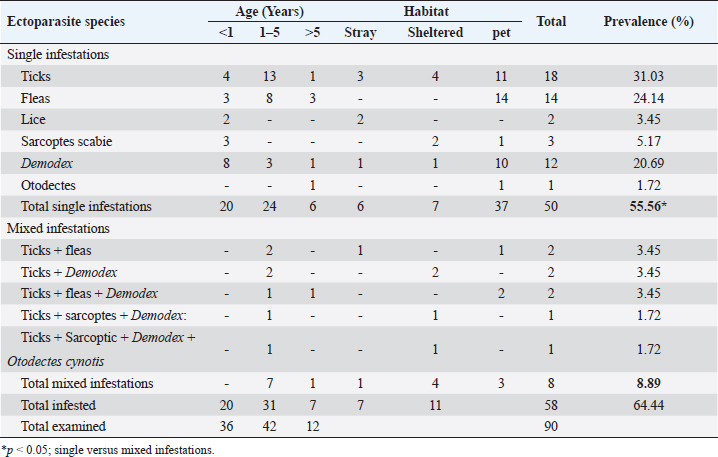
Sample collectionFor the collection of fleas and lice, forceps were used, while ticks were collected manually and placed in sterile containers containing 70% alcohol. Up to five specimens were collected from each dog for identification of ticks, fleas, and lice. For skin mite sampling (Demodex spp. and S. scabei), scrapings were gathered from any skin lesions suspected to be mite-infested (scaling, cutaneous encrustations, and alopecia). Initially, the hair around the affected area was clipped, and scrapings from several body areas of the most recent lesions, till the oozing of superficial blood, were made with a 10-grade scalpel blade, moistened with a bit of mineral oil. Table 1. Clinical index scores for evaluation of skin lesions.
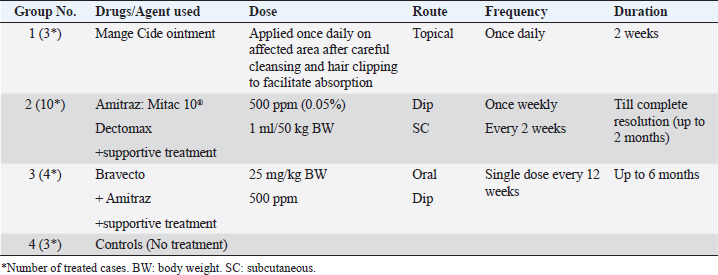
For ear mites detection (O. cynotis), ear swab samples were taken using long sterile cotton swabs from five dogs that showed an abnormal black color on the ear. Laboratory examinationThe samples obtained were either clarified with a lactophenol solution or brought to a boil in 10% KOH to clear the mites and eliminate host cellular debris, and examined under a stereomicroscope. Then, permanent slides were created using polyvol and examined under a light microscope. Ectoparasites were identified based on the descriptions previously provided by Colella et al. (2020); Taylor et al. (2015). Treatment trials under field conditionsTo assess the effectiveness of a selected group of drugs against ectoparasite species isolated in this study, 20 dogs with different ages, gender, breed, locality, and clinical scores were randomly selected and treated with various commonly used acaricides, as shown in Table 2. Briefly, the drugs and treatment protocols used included: Mange Cide ointment (Mega Pharma) acaricidal formulation for the mange treatment composed of benzyl benzoate, salicylic acid, sulfur sublimate, phenol, and tar. The ointment was topically applied on the skin lesion once daily after clipping the hair and removing debris and scales. Dectomax®; injectable doramectin (Zoetis Inc.) given S/C parentally at the dose of 0.2–0.6 mg/kg BW) once weekly (K och et al., 2012). Bravecto®; fluralaner chewable tablets (MSD Animal Health) administered orally as a single dose every 12 weeks to provide a minimum dose of 11.4 mg/lb (25 mg/kg) body weight (Ramsey, 2017). Mitac 10 (AgroEvo®); 250–500 ppm amitraz dips (Papich, 2015). Clinical efficacy was evaluated before, during, and after therapy by scoring skin lesions on a 0–3 scale. The effectiveness of treatment was noticed once the skin lesion score improved, and the hair started regrowth. Ectoparasite-negative skin scraping in the microscopic field during the treatment period assessment was considered an indication of clinical recovery. Treatment was extended two more weeks after the complete resolution of lesions to avoid relapses (Mueller et al., 2012). Statistical analysis and modelPotential risk factors including age, sex, breed, housing environment, habitat, and season were included as independent variables in the model. The odds ratio was used as an approximate measure of relative risk. A multivariate logistic regression model was run using PROC logistics (SAS Institute Inc., 2012) to examine the relationships with potential risk factors. The model was: Log [(Π PRE/(1—ΠPRE))=β0 + β 1AG + β 2 SEX + β 3BR + β 4 HE + β 5HA + β 6SE], where Π PRE is the probability of ectoparasites prevalence, AG is the dog ages, BR is the breed of dog, HE is the housing environment, HA is the habitat, and SE is the examination season. Ethical approvalThe protocol of this study was reviewed and approved (approval number ZU-IACUC/2/F/415/2022) by Zagazig University Institutional Animal Care and Use Committee (ZU-IACUC). ResultsPrevalence of ectoparasites in the examined dogsFollowing the clinical and parasitological examination of the 90 dogs involved in this study from different Egyptian governorates, it was found that 58 dogs (64.44%) were infested with at least 1 species of ectoparasites, of which 50 dogs were infested with a single species of ectoparasites (55.56%) having a significantly higher prevalence than the rest 8 dogs (8.88%) infested concurrently with more than 1 ectoparasite species (p ˂ 0.05) (Table 3). In this study, nine species of ectoparasites of four taxa were identified: one tick species (R. sanguineus) with the highest prevalence among isolated ectoparasites (28.89%, 26/90 dogs), three flea species (Ctenocephalides canis, Ctenocephalides orientis, and Ctenocephalides felis) isolated from 18 subjects (20.00%, 18/90), two types of dog chewing lice species (Trichodectes canis and Heterodoxus spiniger) isolated from 2 cases (2.22%, 2/90) in addition to three mite species, D. canis (20.00%, 18/90), S. scabei var canis (5.56%, 5/90) and O. cynotis (2.22%, 2/90) (Table 3). Table 2. Treatment protocols in 20 dogs with different ectoparasitic infestations and different skin lesions.
Table 3. Prevalence of different ectoparasitic infestations among involved dogs.
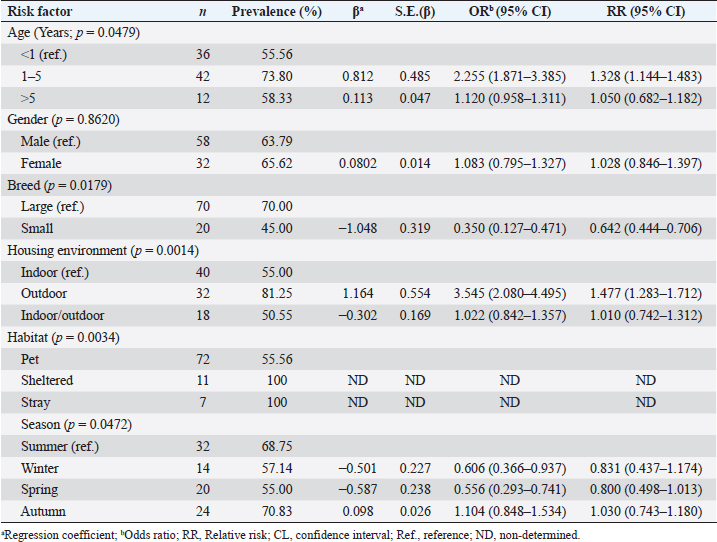
Potential risk factors affecting ectoparasite infestation in dogsResults in Table 4 summarize the logistic regression analysis results of the potential risk factors associated with the prevalence of ectoparasites in dogs. Herein, the age, breed, housing environment, habitat, and season were the significant factors affecting the prevalence of ectoparasites (p < 0.05), while dog gender did not have a significant effect. The likelihood of ectoparasites being present in dogs increases by 2.255 times for dogs between the ages of 1–5 years and 12.0% (OR=1.120) for dogs over 5 years old, compared to their counterparts under 1 year of age. Considering the breed of dogs, small breeds of dogs have a 65% lower likelihood (OR=0.350) of being affected by ectoparasites compared to the larger breeds. Dogs housed outdoors had a 3.545 times higher likelihood of being affected by ectoparasites than those housed indoors. With respect to season, the probability of ectoparasites prevalence decreased by 39.40 (OR=0.606), and 44.4 (OR=0.556), during the winter, spring season respectively, and increased by 10.4% (OR=1.104) during the autumn season compared to summer season. Table 4. Logistic regression model of potential risk factors associated with ectoparasite infestation in dogs.
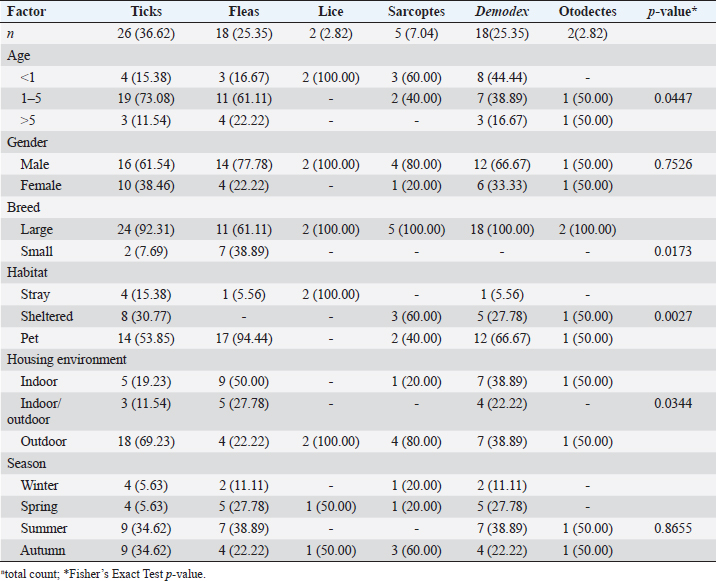
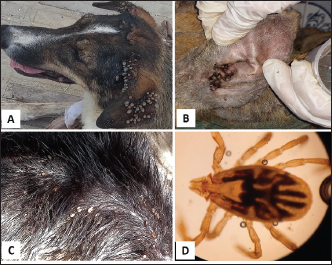
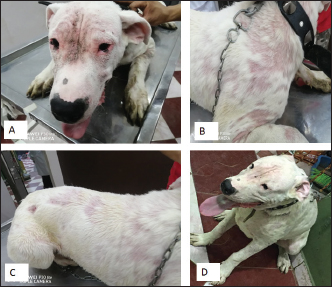
Ticks and fleas were predominantly observed on large pet dog breeds aged between 1 and 5 years. Regarding the housing environment, 69.23% and 50.00% of ticks and fleas’ prevalence were observed in dogs housed outdoors and dogs housed indoors, respectively. Lice were predominantly observed in stray large breeds and dogs aged less than 1 year housed outdoors. Sarcoptes and Demodex ectoparasites were mainly observed in large, sheltered dogs kept outdoors, with Sarcoptes mites being more prevalent in dogs less than 1 year old and Demodex mites being more prevalent in dogs aged between 1 and 5 years. The prevalence of Otodectes ectoparasites showed a relatively even distribution across the studied factors (Table 5). Regarding the prevalence of ectoparasites infestations in different localities, the highest prevalence of ectoparasitic infestation was recorded at Giza governorate (90.9%, 10/11) followed by Ismailia governorate (66.67%, 4/6), Sharkia governorate (59.26%, 32/45), Cairo governorate (55.56%, 10/18), and Kafrelsheikh governorate (50%, 2/4). No ectoparasites infestations were recorded in the six dogs examined in Alexandria governorate. Clinical signsClinical signs of ectoparasitic infestations varied considerably according to the causative ectoparasite species. Early tick infestation was presented with focal irritation sites on the body. Ticks were mainly observed inside and outside the ears and on the head, back, and body sides. Pyoderma was usually associated with heavy tick infestation (Fig. 1). Dogs aged between 1 and 5 years were most commonly affected. Flea-infested dogs were clinically presented with variable pruritus, variable degrees of allergic reactions, erythema, papules, symmetrical to irregular alopecia, crusts, severe skin excoriation, and pyoderma mostly over the caudal back, flanks, and tail (Fig. 2). Dogs of 1–5 years old were most commonly affected. Table 5. Associations between the prevalence of different ectoparasites and potential risk factors.
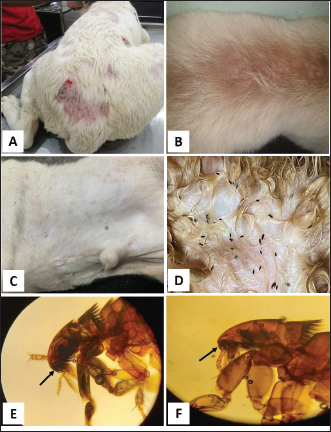
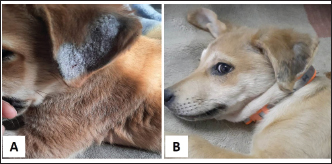
Fig. 1. Tick infestation. A. Heavy tick infestation in a 9-month-old local dog. B. Tick infestation of the external ear canal of sheltered local dog. C. Heavy tick infestation in the back of a German Shepherd kept outdoors. D. Rhipicephalus sanguineus tick in a microscopic field (×10). Lice infestation was observed in two local stray dogs. The clinical appearance was in the form of mild pruritus, rough and dry hair coat, papules, and crusts. Mixed infection with dermatophytes was diagnosed in both dogs (Fig. 3). Sarcoptic mange varied from localized focal to generalized multifocal affections but mainly represented by acute severe pruritus, alopecia, scales, and scab formation. The presence of greyish-yellow erythematous crusted papules was indicative in addition to hyperkeratosis and dermatitis, severe excoriation, lichenification, and patchy alopecia. Predilection sites included the pinnal tips and margins, elbows, hocks, ventral chest, and abdomen (Fig. 4). Demodectic mange was represented by localized or generalized forms. Clinically, areas of scales, alopecia and/or erythema, desquamation, and hyperpigmentation were noticed. The skin was usually presented with follicular dyskeratosis and a musty smell associated with coat greasiness. Juvenile demodicosis affected 8/12 dogs of less than 1 year age. In Juvenile demodicosis, the facial region, back, and limbs were mainly affected. Demodectic mange was observed in cases of poor welfare and management. Some cases suffer from concurrent diseases such as chronic diarrhea, gastrointestinal problems, and a history of corticosteroid treatment. Secondary bacterial infections usually occurred in advanced cases and presented with papules, pustules, and furunculosis (Fig. 5).
Fig. 2. Fleas infestation. A. Erythema and excoriations of the rump with extensive skin allergic reaction in a Dogo Argentino. B. Erythema on the back of a Labrador Retriever. C. Papular dermatitis in a Pitbull dog. D. Fleas on the skin of a Golden Retriever. E. Ctenocephalides felis flea. F. Ctenocephalides orientis (×40).
Fig. 3. Lice infestation in 2-month-old stray local dog with excessive scaling and crusting on the head (A), thighs (B) and back (C). D. Trichodectes canis adult mite with a short thorax, flattened head and quadrangular shape. Each leg has one claw on the tarsus (×10). E. Heterodoxus spiniger adult lice with a subtriangular head, rounded anterior, long thorax and each leg has two claws on the tarsus (×10).
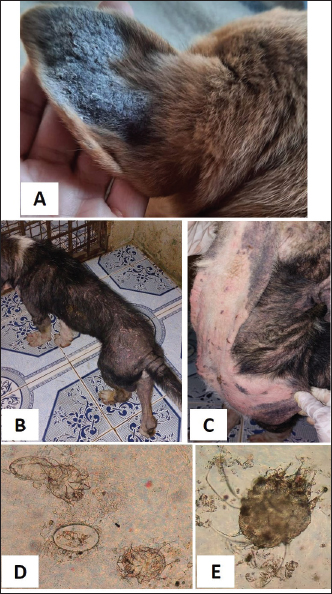
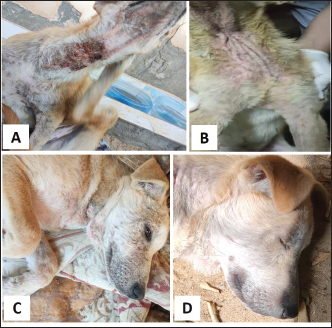
Fig. 4. Sarcoptic mange. A. Localized form on the ear pinnae of a 2-month-old local dog. B and C. Generalized form with alopecia, scales and scab formation in a sheltered local dog. D. Sarcoptes mite egg and larva (×10). E. Adult Sarcoptes mite (×40). Otodectic Mange was characterized by large amounts of dry, dark brown waxy cerumen associated with variable degrees of inflammation (otitis externa) (Fig. 6). Concurrent infestation of different ectoparasites with different etiological agents (dermatophyte and yeast) was found in 16 cases.
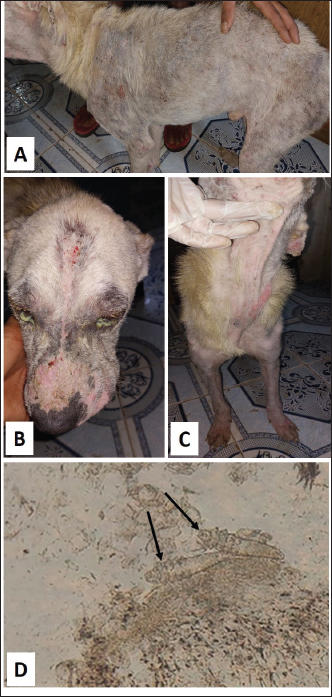
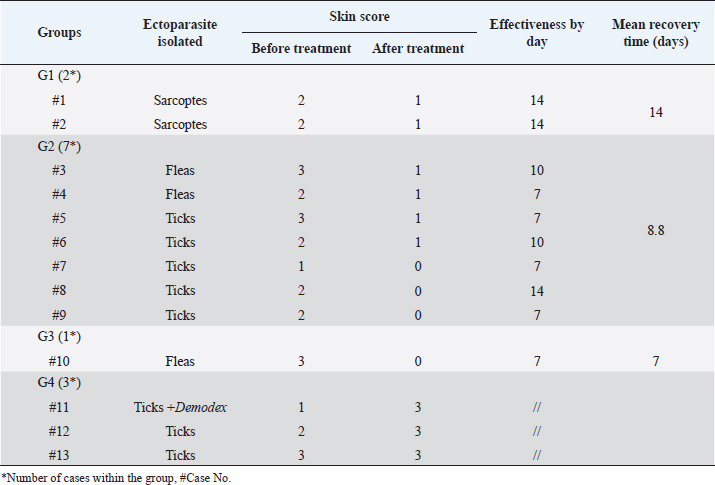
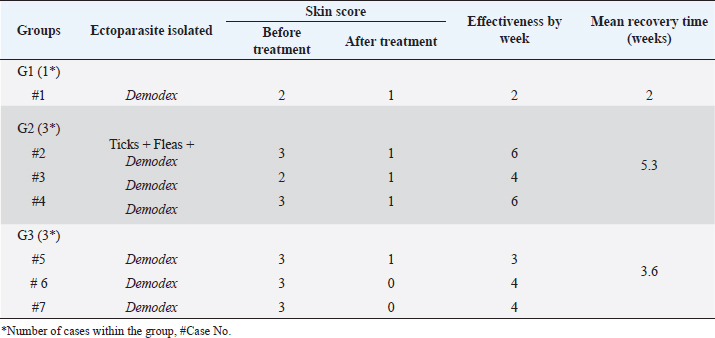
Fig. 5. Demodectic mange. Generalized form in a sheltered local dog on the lateral side (A), the head (B) and the neck (C). D. Adult Demodex mites (arrows) (×10). Treatment outcomeThe follow-up of cases involved in this study and treated with the selected drugs and protocols revealed variable therapeutic effectiveness. Effectiveness of treatment was observed after 2 weeks of Mange Cide ointment application, but alopecia partially improved and hair never started to grow back except after 8 weeks (Fig. 7). Doramectin was effective against ticks (mainly) and fleas. The onset of recovery was seen starting from 4 days post-treatment (Table 6). Cases infested with Demodex mite had the longest recovery time compared with cases infected with the other ectoparasites. The effect of doramectin against Demodex mite infestation (recovery time mean=5.3 weeks) was less than the effect of fluralaner chewable tablets (recovery time mean=3.6 weeks) (Table 7).
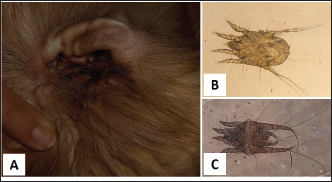
Fig. 6. Otodectic mange. A. Large amounts of dry dark brown waxy cerumen in the ear of 8 years old Golden Retriever. B. Adult female Otodectes mite with an egg (×40). C. Adult Otodectes mite (×40).
Fig. 7. Mange Cide ointment treatment alone in a 2-month-old dog. A. Ear pinna before treatment. B. lesions after 2 months treatment with improved skin but no hair regrowth. The ancillary treatment, along with doramectin and fluralaner increased the effectiveness of the therapy and accelerated the hair regrowth, thus reducing the treatment/recovery time (Figs. 8–11). Fluralaner treatment was highly effective against all isolated ectoparasites with an extended period of effectiveness of up to 3 months. No re-infestation occurred within this period after a single dose administration. DiscussionDomestic animals like dogs represent natural hosts for ectoparasitic infestation. Such affections could have deleterious effects on animal health and productivity as well as on public health due to the pathological role of those parasites either directly, during their survival on the host’s body, or as disease vectors. Therefore, minimizing the impact of those infestations is crucial for a better quality of life for both animals and humans. Table 6. Effectiveness of different drugs and protocols on treatment of 13 dogs infested with different ectoparasites.
Table 7. Effectiveness of different drugs and protocols on treatment of seven dogs infested with Demodex mites.
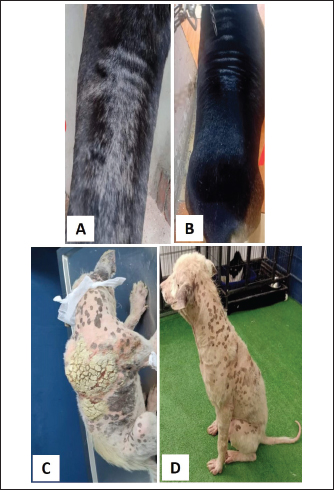
Fig. 8. Trial of Treatment with Dectomax + supportive treatment in a Dogo Argentino with FAD. A–C. Before Treatment. D. After 2-week treatment.
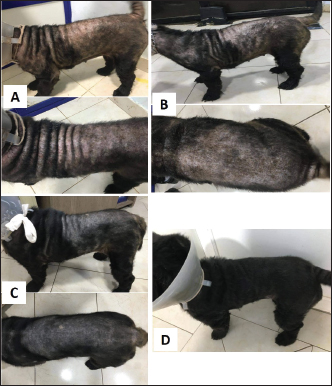
Fig. 9. Local Amitraz dips, Dectomax injections and supportive treatment in a 2-year-oldfemale Rottweiler with generalized demodicosis before treatment (A) and after 1 month of treatment (B). A local dog with severe demodectic mange before treatment (C) and after 1 month of treatment (D).
Fig. 10. Bravecto and supportive treatment in a 9-month-old local dog with generalized demodectic mange. A. Before treatment. B. After 2 weeks of treatment. C. After 3 weeks of treatment. D. After 1 month of treatment.
Fig. 11. Bravecto and supportive treatment in a 6-month-old Caucasian female. A. Before treatment. B. After 2 weeks of treatment. C. After 4 weeks of treatment. D. After 6 weeks of treatment. In the present study, the prevalence of ectoparasitic infestation recorded in the examined dogs of the studied Egyptian governorates was 64.4%. Such a prevalence could be considered high. Tick infestation was the most prevalent followed by fleas, demodectic mites, sarcoptic mites, lice, and then otodectic mites that had the lowest prevalence. A similar high prevalence of ectoparasites infestation was recorded in Egypt (57.45%, 54/94 stray dogs) (Abdel-Mageid, 2010), in India (49.1%) (Krishna murthy et al., 2017) and in Nigeria (81.4%) (Abdulkareem et al., 2018). These three previous studies reported ticks, also, as the most prevalent ectoparasites. On the other hand, a low prevalence of ectoparasites infestation (15.4%) was reported in Albania (Shukullari et al., 2017). This could be attributed to the environmental variations and management practices of dog owners in those countries. Our study focused on the animal habitat and housing environment. These management-related risk factors significantly influence the prevalence of ectoparasite infestation especially for ticks, fleas, and Demodex mites (Cruz-Bacab et al., 2021) and that may interpret the difference in the prevalence of ectoparasites from one study to another. In our study, the highest prevalence of ectoparasite infestations in dogs was recorded in autumn and summer compared to winter and spring. A similar result was reported in Egypt (Aboelela et al., 2022) and in Albania (Shukullari et al., 2017). Other studies in Iran reported the highest prevalence of ectoparasites in dogs in spring and summer (Shoorijeh et al., 2008; Jamshidi et al., 2012). This may be due to the optimum temperature and humidity that provide a favorable condition for ectoparasites life cycle (Wall and Shearer, 2008). Regarding the age of the animal, the prevalence of ectoparasite infestations increased by 2.255 times for dogs between the ages of 1–5 years. This comes in accordance with a previous study in India (Krishna murthy et al., 2017), while Mosallanejad et al. (2012) reported no significant difference in the prevalence of ectoparasites infestation in different age groups. In the present study, there was no significant association between the prevalence of ectoparasite infestation and the gender of dogs. This result agrees with previous study by Mwangengw and Rhanga (2023). The highest prevalence of ectoparasitic infestation was recorded at Giza governorate (90.9%) followed by Ismailia governorate (66.67%). This may be attributed to the large number of dogs living in shelters and stray dogs examined in these governorates. This result is consistent with a previous study in Ismailia governorate, Egypt, where the prevalence of ectoparasite infestation was 100% among 50 examined stray dogs (Abuzeid, 2015). Two types of dog-chewing lice (Trichodectes canis and H. spiniger) were isolated in two examined dogs from two different governorates (Kafr El-Sheikh and Sharkia for each type, respectively). There is little doubt that H. spiniger infests marsupials (e.g., kangaroos and wallabies) (Bermúdez and Miranda, 2011). This species was reported in Nile-delta of Egypt in Kafr El-Sheikh governorate in two stray dogs by Sultan and Khalafalla (2014), in Ismailia by Abuzeid (2015), in Damietta governorate by Aboelela et al. (2022). Now we report the isolation of this species at Sharkia governorate, which may indicate the spread of this species to this governorate and the possible emergence of new geographical locations. In addition, mixed affection of lice infestation and dermatophytosis was observed in this study and this could point to lice infestation as a predisposing factor for dermatophyte infection. In this study, three species of mites were isolated, with a total prevalence of 27.78%. Demodex spp., S. scabei, and O. cynotis were detected at a rate of 20%, 6%, and 2.2%, respectively. These results are in accordance with a study carried out in Mexico on 200 stray dogs with an overall prevalence of mange of 34%. The most frequent mite was D. canis (23.0%) followed by Sarcoptes scabei var. canis (7.0%) and O. cynotis (3.5%) (Bolio, 2003). On the contrary, (Chee et al., 2008) reported that O. cynotis was found to be the most frequent parasite (22.3%), followed by Sarcoptes scabei var canis (19.4%), C. canis (6.8%), D. canis (4.9%), and then T. canis (1%). The variation in mite prevalence may be attributed to environmental and management variation or sampling technique especially in the case of sarcoptic mites diagnosis which is extremely difficult, as it requires several scrapings and specific scraping of greyish-yellow erythematous crusted papules (which are the openings of skin tunnel created by females) to increase the probability of mite detection (Feather et al., 2010). In the current study, the prevalence of demodectic mange was significantly higher in domestic dogs (33.3%, p=0.03) than in stray and sheltered dogs. It was observed in cases of poor welfare and management, cases suffering from chronic diarrhea and in some cases associated with a history of corticosteroids treatment. Demodex mites are common commensals cutaneous microfauna carried by most dogs. Defect in the skin immune system or general immunosuppression allows mites to proliferate in hair follicles. Their transmission does not necessitate direct contact with an infested dog. They are transmitted only from the bitch to the offspring during the first days of life (Mueller et al., 2012). Otodectes cynotis mite was isolated from ear swabs and skin scraping samples from the head. A previous study on 223 dogs revealed that mono-specific and mixed infestations of O. cynotis in dogs were (7.17%) and (4.48%), respectively (Salib and Baraka, 2011), which is greater than that isolated in our study. In both studies, the mixed infestations were found in combination with Sarcoptes and Demodex mites and ticks. The most common clinical manifestations reported in our study were pruritus followed by alopecia and erythema. In the case of tick infestations, mainly the head and the ear were affected. A similar observation was reported in an earlier study where the adult and nymphal stages of ticks were found attached mainly to the ears, neck, and shoulders (Estrada-peña et al., 2003). In the present study, the cases of fleas’ infestations were represented as FAD ranging from wheals and papules to severe hypersensitivity. Infested dogs scratch their skin against hard objects resulting in local or generalized alopecia. The lesions were mainly observed on the rump and caudal half of the body. These clinical signs may be due to the fleas’ feeding mechanism, as fleas are obligate blood-sucking insects with piercing mouthparts and their saliva contain histamine-like compound causing allergic reaction (Iannino et al., 2017). In the present study, the outcomes of treatment of 20 dogs with different drugs and protocols revealed variable therapeutic effectiveness. Doramectin 0.2 mg/kg BW SC was highly effective against ticks and fleas. Doramectin is an avermectin anthelmintic used to treat endoparasites and ectoparasites in dogs. It induces rapid paralysis in arthropods by increasing the activity of trans-membrane chloride ion channels in arthropods’ nerve and muscle cells, causing increased permeability to chloride ions and hyperpolarization of nerve cells (Papich, 2015). The longest recovery time was reported for cases of Demodex mite infestation either single infestation or mixed with other ectoparasites. These results could be interpreted by the difficulty of treatment of generalized demodicosis and the fact that it often requires extended intensive therapy (Elsheikha, 2017). In the current study, fluralaner was found to be more effective than doramectin in cases infested with demodectic mange with a recovery time mean of 3.6 weeks compared to 5.3 weeks in cases treated with doramectin. This may be attributed to the rapid absorption of fluralaner which reaches maximum concentrations within 24 hours after a single oral dosage. In addition, it can be detected in plasma for up to 112 days, providing prolonged activity against ectoparasites (Kilp et al., 2014). Prolonged effectiveness of fluralaner was also confirmed by Allen et al. (2020) in the USA on ticks, and (Duangkaew et al., 2018) in Thailand on demodectic mange. ConclusionIn conclusion, ectoparasite infestation is common in dogs in Egypt, especially in stray and sheltered dogs. Proper application of the commonly used acaricides accompanied with supportive treatment is effective for their treatment and accelerates recovery. Management and owner awareness play an important role in controlling these parasites and consequently, their effects. AcknowledgmentsThe authors would like to express their gratitude to professors Reham Abuela and Asmaa Abueliazed of the department of parasitology, faculty of veterinary medicine, Zagazig University in Egypt, for their assistance. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Data availabilityAll data supporting the findings of this study are available within the manuscript. Authors’ contributionsNasser Abuzeid, Mohamed Eisa and Wafaa El-Neshwy contributed to the study design and the execution of experiments. Hend Zineldar collected and analyzed the samples. Hend Zineldar observed and assessed the skin lesion scores during treatment. Emad Bennour contributed to the manuscript writing. ReferencesAbdel-Mageid, M. 2010. Epidemiological study of ectoparasites in stray dogs in Kalubyia Governorate of Egypt with a special reference to its control in puppies by deltamethrin and ivermectin. Iași, Romania: Lucrări Științifice-Medicină Veterinară, Universitatea de Științe Agricole și Medicină Veterinară” Ion Ionescu de la Brad”, vol. 53, no. 2, pp: 307–317. Abdulkareem, B.O., Christy, A.L. and Samuel, U.U. 2018. Prevalence of ectoparasite infestations in owned dogs in Kwara. Parasite. Epidemiol. Control. 4, e00079. Aboelela, E.M., Sobieh, M.A., Abouelhassan, E.M., Farid, D.S. and Soliman, E.S. 2022. Retrospective seasonal parasitological survey on prevalence and epidemiological determinants of ectoparasitic infestations in dogs and cats of Damietta, Egypt. Adv. Anim. Vet. Sci. 10(8), 1854–1867. Abuzeid, A.M.I. 2015. Studies on ectoparasites of stray dogs in Ismailia city. Egypt. Vet. Med. Soc. Parasitol. J. 11(15), 115–122. Allen, K., Little, S., Petersen, M., Gruntmeir, J., Barrett, A., Herrin, B., Starkey, L., Sun, F. and Guerino, F. 2020. Evaluation of oral fluralaner (Bravecto®) for efficacy against nymphs of Amblyomma americanum and Rhipicephalus sanguineus (sensu lato). Parasit. Vectors. 13(1), 1–8. Arlian, L.G. and Morgan, M.S. 2017. A review of Sarcoptes scabei: past, present and future. Parasit. Vectors. 10(1), 1–22. Arther, R.G. 2009. Mites and lice : biology and control. Vet. Clin. North. Am. Small. Anim. Pract. 39(6), 1159–1171. Azrizal-Wahid, N., Sofian-Azirun, M. and Low, V.L. 2019. Risk factors associated with flea infestation on cats. Trop. Biomed. 36(4), 810–821. Bermúdez, C.S. and Miranda, C.R. 2011. Distribution of ectoparasites of Canis lupus familiaris L. (Carnivora: Canidae) from Panama. Revista MVZ Córdoba 16(1), 2274–2282. Beugnet, F., Labuschagne, M., Fourie, J., Jacques, G., Farkas, R., Cozma, V., Halos, L., Hellmann, K., Knaus, M. and Rehbein, S., 2014. Occurrence of Dipylidium caninum in fleas from client-owned cats and dogs in Europe using a new PCR detection assay. Vet. Parasitol. 205(1-2), 300–306. Bolio, G.M.E. 2003. Factors affecting the prevalence of mange-mite infestations in stray dogs of Yucatán, Mexico. Vet. Parasitol. 115, 61–65. Chee, J., Kwon, J., Cho, H., Cho, K., Lee, Y. and Abd El-Aty, A.M. and Shin, S.S. 2008. A survey of ectoparasite infestations in stray dogs of Gwang-ju city, Republic of Korea. Korean. J. Parasitol. 46(1), 23–27. Chiummo, R., Petersen, I., Plehn, C., Zschiesche, E., Roepke, R. and Thomas, E. 2020. Efficacy of orally and topically administered fluralaner–( Bravecto ® ) for treatment of client—owned dogs with sarcoptic mange under field conditions. Parasit. Vectors. 13, 524. Colella, V., Nguyen, V.L., Tan, D.Y., Lu, N., Fang, F., Zhijuan, Y., Wang, J., Liu, X., Chen, X., Dong, J., Nurcahyo, W., Hadi, U.K., Venturina, V., Tong, K.B.Y., Tsai, Y.L., Taweethavonsawat, P., Tiwananthagorn, S., Le, T.Q., Bui, K.L. and Halos, L. 2020. Zoonotic vectorborne pathogens and ectoparasites of dogs and cats in eastern and Southeast Asia. Emerg. Infect. Dis. 26(6), 1221–1233. Cruz-Bacab, L.E., Perez-De La Cruz, M.C., Zaragoza-Vera, C.V., Zaragoza-Vera, M., Arjona-Jimenez, G., Lesher-Gordillo, J.M., Baak-Baak, C.M., Cigarroa-Toledo, N., Machain-Williams, C.I., Garcia-Rejon, J.E., Gonzalez-Garduño, R. and Torres-Chable, O.M. 2021. Prevalence and factors associated with ectoparasite infestations in dogs from the state of Tabasco, Mexico. J. Parasitol. 107(1), 29–38. Dantas-Torres, F. 2008. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet. Parasitol. 152(3–4), 173–185. Diakou, A., Sofroniou, D., Paoletti, B., Tamvakis, A., Kolencik, S., Dimzas, D., Morelli, S., Grillini, M. and Traversa, D. 2022. Ticks, leas, and harboured pathogens from dogs and cats in Cyprus. Pathogens 11(12), 1–12. De La Fuente, J., Estrada-Pena, A., Venzal, J.M., Kocan, K.M. and Sonenshine, D.E. 2008. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13(18), 6938–6946. Duangkaew, L., Chen, C., Larsuprom, L., Anukkul, P. and Lekcharoensuk, C. 2018. A field trial in Thailand of the efficacy of oral fluralaner for the treatment of dogs with generalized demodicosis.Vet. Dermatol. 29(3), 208–e74. Elsheikha, H. 2017. Ectoparasites: preventive plans and innovations in treatment. Vet. Times. 47(14), 6–12. Estrada-peña, A., Walker, A., Bouattour, A., Camicas, J., Horak, I., Latif, A., Pegram, R. and Preston, P. 2003. Ticks of domestic animals in Africa: a guide to identification of species. In The University of Edinburgh (Issue JANUARY). Feather, L., Gough, K., Flynn, R.J. and Elsheikha, H.M. 2010. A retrospective investigation into risk factors of sarcoptic mange in dogs. Parasitol. Res. 107, 279–283. Ghubash, R. 2006. Parasitic miticidal therapy. Clin. Tech. Small. Anim. Pract. 21(3), 135–144. Hay, R.J., Steer, A.C., Engelman, D. and Walton, S. 2012. Scabies in the developing world-its prevalence, complications, and management. Clin. Microbiol. Infect. 18(4), 313–323. Hugnet, C., Bruchon-Hugnet, C., Royer, H. and Bourdoiseau, G. 2001. Efficacy of 1.25% amitraz solution in the treatment of generalized demodicosis (eight cases) and sarcoptic mange (five cases) in dogs. Vet. Dermatol. 12(2), 89–92. Iannino, F., Sulli, N., Maitino, A., Pascucci, I., Pampiglione, G. and Salucci, S. 2017. Pulci di cane e gatto: specie, biologia e malattie ad esse associate. Vet. Ital. 53(4), 277–288. Jamshidi, S., Maazi, N., Ranjbar-Bahadori, S., Rezaei, M., Morakabsaz, P. and Hosseininejad, M. 2012. A survey of ectoparasite infestation in dogs in Tehran, Iran. Rev. Bras. Parasitol. Vet. 21(3), 326–329. Kilp, S., Ramirez, D., Allan, M.J., Roepke, R.K. and Nuernberger, M.C. 2014. Pharmacokinetics of fluralaner in dogs following a single oral or intravenous administration. Parasit. Vectors. 7, 85. Koch, S.N., Sheila, M.F. and Donald, C.P. 2012. Canine and feline derm atology drug handbook. John Wiley & Sons. Krishna Murthy, C.M., Ananda, K.J. and Adeppa, J. 2017. Prevalence of ectoparasites in dogs of Shimoga, Karnataka. J. Parasit. Dis. 41(1), 167–170. Mosallanejad, B., Alborzi, A.R. and Katvandi, N. 2012. A survey on ectoparasite infestations in companion dogs of ahvaz district, south-west of iran. J. Arthropod-Borne. Dis. 6(1), 70–78 Mueller, R.S., Bensignor, E., Ferrer, L., Holm, B., Lemarie, S., Paradis, M. and Shipstone, M. A. 2012. Treatment of demodicosis in dogs:clinical practice guidelines. Vet. Dermatol. 23(2), 86–96. Mueller, R.S., Rosenkrantz, W., Bensignor, E., Karaś-Tęcza, J., Paterson, T. and Shipstone, M.A. 2020. Diagnosis and treatment of demodicosis in dogs and cats: clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 31(1), 5–27. Mwangengwa, L.M. and Rhanga, S. 2023. Assessment of the prevalence and risk factors of ectoparasite infestation in dogs with regular dipping history in Morogoro Urban and Peri-Urban in Tanzania. World. J. Vet. Sci. 4, 5–9. Papich, M.G., 2015. Saunders handbook of veterinary drugs: small and large animal. Elsevier Health Sciences. Pfister, K. and Armstrong, R. 2016. Systemically and cutaneously distributed ectoparasiticides: a review of the efficacy against ticks and fleas on dogs. Parasit. Vectors. 9(1), 1–15. Rafiqi, S.I., Kumar, S., Chaudhary, R., Farooq, U. and Kirthika, P. 2016. Ectoparasites of companion animals and their control. Int. J. Anim. Vet. Sci. 3, 15–18. Ramsey, I. 2017. BSAVA small animal formulary 9th edition: part A-canine and feline. Quedgeley, UK: British Small Animal Veterinary Association. Salib, F.A. and Baraka, T.A. 2011. Epidemiology, genetic divergence and acaricides of Otodectes cynotis in cats and dogs. Vet. World. 4(3), 109–112. SAS Institute Inc., 2012. SAS/STAT Statistics user’s guide. Statistical analytical system, 5th rev ed. Cary, NC: SAS Institute Inc.,. Shoorijeh, S.J., Ghasrodashti, A.R., Tamadon, A., Moghaddar, N. and Behzadi, M.A. 2008. Seasonal frequency of ectoparasite infestation in dogs from Shiraz, Southern Iran. Turk. J. Vet. Anim. Sci. 32(4), 309–313. Shukullari, E., Rapti, D., Visser, M., Pfister, K. and Rehbein, S. 2017. Parasites and vector-borne diseases in client-owned dogs in Albania: infestation with arthropod ectoparasites. Parasitol. Res. 116, 399–407. Sultan, K. and Khalafalla, R.E. 2014. First record of chewing louse Heterodoxus s piniger (Insecta, Phthiraptera, Boopidae) on stray dogs from northern region of Egypt. Trop. Biomed. 31(2), 378–380. Taylor, M.A., Coop, R.L. and Wall, R.L. 2015. Veterinary parasitology, 4th ed. John Wiley & Sons. Wall, R.L. and Shearer, D. 2008. Veterinary ectoparasites: biology, pathology and control. John Wiley & Sons. Xhaxhiu, D., Kusi, I., Rapti, D., Visser, M., Knaus, M., Lindner, T. and Rehbein, S. 2009. Ectoparasites of dogs and cats in Albania. Parasitol. Res. 105(6), 1577–1587. | ||
| How to Cite this Article |
| Pubmed Style Zineldar HA, Abo-zeid NZ, Eisa MI, Bennour EM, El-neshwey WMI. Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt. Open Vet. J.. 2023; 13(12): 1631-1644. doi:10.5455/OVJ.2023.v13.i12.13 Web Style Zineldar HA, Abo-zeid NZ, Eisa MI, Bennour EM, El-neshwey WMI. Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt. https://www.openveterinaryjournal.com/?mno=169969 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i12.13 AMA (American Medical Association) Style Zineldar HA, Abo-zeid NZ, Eisa MI, Bennour EM, El-neshwey WMI. Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt. Open Vet. J.. 2023; 13(12): 1631-1644. doi:10.5455/OVJ.2023.v13.i12.13 Vancouver/ICMJE Style Zineldar HA, Abo-zeid NZ, Eisa MI, Bennour EM, El-neshwey WMI. Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt. Open Vet. J.. (2023), [cited January 25, 2026]; 13(12): 1631-1644. doi:10.5455/OVJ.2023.v13.i12.13 Harvard Style Zineldar, H. A., Abo-zeid, . N. Z., Eisa, . M. I., Bennour, . E. M. & El-neshwey, . W. M. I. (2023) Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt. Open Vet. J., 13 (12), 1631-1644. doi:10.5455/OVJ.2023.v13.i12.13 Turabian Style Zineldar, Hend Adel, Naser Zeidan Abo-zeid, Mohamed Ibrahim Eisa, Emad Mohamed Bennour, and Wafaa Mohamed Ismail El-neshwey. 2023. Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt. Open Veterinary Journal, 13 (12), 1631-1644. doi:10.5455/OVJ.2023.v13.i12.13 Chicago Style Zineldar, Hend Adel, Naser Zeidan Abo-zeid, Mohamed Ibrahim Eisa, Emad Mohamed Bennour, and Wafaa Mohamed Ismail El-neshwey. "Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt." Open Veterinary Journal 13 (2023), 1631-1644. doi:10.5455/OVJ.2023.v13.i12.13 MLA (The Modern Language Association) Style Zineldar, Hend Adel, Naser Zeidan Abo-zeid, Mohamed Ibrahim Eisa, Emad Mohamed Bennour, and Wafaa Mohamed Ismail El-neshwey. "Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt." Open Veterinary Journal 13.12 (2023), 1631-1644. Print. doi:10.5455/OVJ.2023.v13.i12.13 APA (American Psychological Association) Style Zineldar, H. A., Abo-zeid, . N. Z., Eisa, . M. I., Bennour, . E. M. & El-neshwey, . W. M. I. (2023) Prevalence, clinical presentation, and therapeutic outcome of ectoparasitic infestations in dogs in Egypt. Open Veterinary Journal, 13 (12), 1631-1644. doi:10.5455/OVJ.2023.v13.i12.13 |