| Research Article | ||
Open Vet. J.. 2023; 13(12): 1543-1553 Open Veterinary Journal, (2023), Vol. 13(12): 1543–1553 Original Research Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, BrazilCarolina Konkel Barbosa1*, Valéria Natasha Teixeira2 and Cláudia Turra Pimpão1,21Postgraduate Program in Animal Science of Pontifícia Universidade Católica do Paraná, Curitiba, Paraná, Brazil 2Veterinary Medicine, School of Life Sciences of Pontifícia Universidade Católica do Paraná, Curitiba, Paraná, Brazil *Corresponding Author: Carolina Konkel Barbosa. Postgraduate Program in Animal Science of Pontifícia Universidade Católica do Paraná, Curitiba, Paraná, Brazil. Email: carolinakonkel [at] gmail.com Submitted: 25/04/2023 Accepted: 16/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
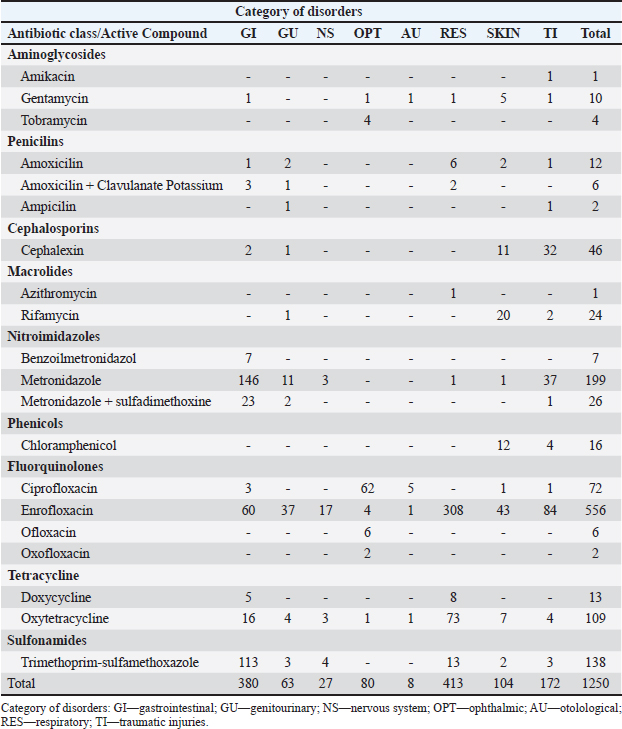
AbstractBackground: The link between the reckless use of antimicrobials with the increasing development of multidrug-resistant strains of antibiotics is well established. To control the spread of antimicrobial resistance (AMR), action plans, recommendations and guidelines on the prudent use of antibiotics have been developed for food-producing animals and companion animals but appear to be lacking in exotic pets due to the scarcity of data and information on the use of antibiotics in these species. Aim: The purpose of this study is to bring qualitative and quantitative data regarding the use of antimicrobials in exotic pets in a veterinary center in southern Brazil, seeking to measure the consumption of these animals to determine and guide future actions to combat AMR. Methods: A retrospective observational study was conducted by surveying 2,190 open care records in a specialized clinic of wildlife medicine in 2018. Data evaluation was performed in a descriptive, qualitative, and quantitative way, and the analysis of antimicrobial consumption was performed by calculating the daily dose/g of animal ml/day/kg by animal order, condition and active principle, adaptation to DDDVet. For statistical analysis, Levene´s, ANOVA followed by Tukey´s tests were used. Results: Approximately 57% (1250/2190) of the visits made use of one or more antimicrobials in the treatment of animals referred to the clinic. Of these, 67% (n=839) were birds, 26% (n=327) were mammals, and 7% (n=84) were reptiles. In 2018, the total consumption of antimicrobials prescribed was 2.21 l for a total biomass of 129.24 kg. The relation between the dosages of the main antimicrobials used and the conditions treated showed that there is no variation between the treatments by disease and by animal class, due to the great variation within each group. Conclusion: The antimicrobial prescription in exotic pets requires a multifaceted and dynamic approach applied to safeguard the efficacy of the antimicrobials, optimizing consumption, minimizing the emergence of AMR and other possible adverse effects, and considering the physiological differences present within each species. The doses between the different classes and conditions do not obtain a significant difference, reflected in a possible nonstandardization of the dosages used, requiring further investigation of the recommended dosages for each animal species, avoiding the over or underdosing of these drugs. It is worth highlighting that professionals must always make rational use of antimicrobials in these species. Keywords: Antibiotics, Antimicrobial management, Antimicrobial resistance, Non-native species, One Health. IntroductionAntimicrobial resistance (AMR) is an increasing threat to global health and the use of antimicrobial agents is a risk factor in its development (Singleton et al., 2017). The abuse of antimicrobials used in human and animal medicine, as well as in agriculture has been reducing the effectiveness of antimicrobials, mainly due to the rapid rise of multidrug-resistant bacteria worldwide, concomitantly with the lack of development of new antibiotics by the pharmaceutical industry (Kummerer, 2009; Ventola, 2015). Evidence of transmission of bacterial resistance between humans, cattle (Economou and Gousia, 2015), and companion animals indicates the need for a one-health approach to preserve the effectiveness of treatments for various infections (Collington and McEwen, 2019). Furthermore, almost all antimicrobials used in veterinary medicine are structurally related to human therapy and can select genes for co-resistance or cross-resistance (Marshall and Levy, 2011). In view of this, the World Health Organization (WHO) has compiled a list of antimicrobials of critical importance to human medicine for managing AMR risks due to nonhuman use. In response to WHO publications, the European Commission (2017) and other international organizations have developed recommendations and policies on the prudent use of antimicrobials, not only for farm animals, but also for pets and horses (The Danish Integrated Antimicrobial Resistance Monitoring and Research Programme, 2015). Recommendations on preventive measures to be taken include surveillance of AMR and monitoring the use of antimicrobials in food animals (Jensen et al., 2004). However, these policies and recommendations do not include wild and exotic animals. The evaluation of the excessive or incorrect use of antimicrobials, prolonged treatments, underdosing, or low compliance of the client, can result in failure of the therapy and, therefore, in the trivial application of antimicrobials, mainly in exotic animals, in which it has little or no information (Broens and van Geijlswijk, 2018). Bacterial zoonoses associated with pets represent a relatively neglected field compared to zoonoses transmitted by food-producing animals (Damborg et al., 2016). Animals, particularly wildlife ones, are believed to be the source of 70% of all emerging infections (Ahmed et al., 2007). Although in recent years special attention has been paid to the role of animals as sentinels and spreaders of AMR, few studies have evaluated the occurrence of AMR bacteria in wild and exotic pets (Chiacchio et al., 2016; Dias et al., 2018; Cabral et al., 2020; Marques et al., 2021). Another factor to be considered is that close contact between pets and people offers favorable conditions for bacterial transmission. The number of pets kept in homes is increasing and the range of species kept for this purpose has extended from traditional domestic animals, such as dogs and cats, to include rodents, rabbits, ferrets, birds, amphibians, reptiles, ornamental fish, among others (Damborg et al., 2016). Therefore, there is a growing need to monitor the consumption of veterinary antimicrobials to identify possible risk factors that can lead to the development and spread of AMR in these animals (Broens and van Geijlswijk, 2018). The collection of data on the use of these drugs is an essential first step in the development and monitoring of responsible use policies. In addition, consumption data for antimicrobials used in veterinary medicine are often incomplete and refer to sales by drug manufacturers (European Medicines Agency, 2015; Collineau et al., 2016; Carmo et al., 2017; Hardefeldt et al., 2018; Hopman et al., 2019a, 2019b). Although sales data provide a rough estimate of the magnitude of antimicrobial consumption in treatment, data on the use of antimicrobials in different species are lacking, especially regarding wild animals kept as pets. The purpose of this study is to bring qualitative and quantitative data regarding the use of antimicrobials in exotic pets in a veterinary center in southern Brazil, seeking to measure the consumption of these animals to determine and guide future actions to combat AMR. Materials and MethodsA retrospective observational study was carried out by collecting attendance files for everyone opened in a clinic specialized in wildlife medicine, in the period of 2018. In total, data were collected from 2,190 clinical care records. The data collected were clinical record number; entry date; taxonomic class, order, and family; common name, genus and species; sex, weight (g), and age (months); diagnostic hypothesis/clinical history: disorders by system; class of antimicrobial, active compound, trade name of the compound, dose (ml), pharmaceutical form, route of drug administration, duration of treatment (days), frequency and completion of care. Diagnostic test results were also collected when available. All data were organized into spreadsheets using Microsoft Excel 365 software. The data evaluation was performed in a descriptive, qualitative, and quantitative way, in which they were grouped according to the taxonomic order of the species due to the amplitude of existing in each group. The analysis of antimicrobial consumption was performed by calculating the daily dose/g of animal (ml/day/kg) by animal order, condition, and active principle, adaptation to DDDVet (European Medicines Agency, 2015). For statistical analysis of the data, Levene´s was used for homogeneity of variances, followed by ANOVA (one way) and Tukey´s test. Ethical approvalNot needed for this study. ResultsOf the total of 2,190 clinical records opened in 2018, 1,250 veterinary records were analyzed and accounted for. The study included all visits where antimicrobials were prescribed or used. Approximately in 57.08% of the visits one or more antimicrobials were used in the treatment of animals referred to the clinic. Of these, 839 (67%) were birds, 327 (26%) mammals and 84 (7%) reptiles. The main taxonomic orders of birds attended during 2018 were Psittaciformes 75.62% (580); Passeriformes 15.24% (117); Columbiformes 2.48% (19); Galliformes 2.35% (18); Anseriformes 1.30% (10). Regarding mammals, the main taxonomic orders were Rodentia 69.42% (193) and Lagomorpha 27.34% (76). Only two orders of the reptile class had antimicrobial prescriptions: Testudines 92.86% (78) and Squamata 7.14% (6). The visits that made prescription or use of antimicrobials were categorized as disorders by systems, the list of antimicrobial drugs by disorders is shown in Table 1. The main cases were by respiratory diseases with (33.12%, n=414), followed by gastrointestinal diseases (30.40%, n=380) and traumatic injuries (13.84%, n=173). Regarding the antimicrobials used, the class of Fluoroquinolones was observed in most records (50.88%, n=636), followed by Nitroimidazoles (16.48%, n=206), Sulfonamides (11.04%, n=138), Tetracyclines (9.76%, n=122), Beta-lactam (5.28%, n=66), the association of Sulfonamides and Nitroimidazoles (2.08%, n=26), Ansamycins (1.92%, n=24), Amphenicols (1.28%, n=16), Aminoglycosides (1.20%, n=16), and Azalides (0.08%, n=1). Table 1. List of antimicrobial drugs by affections used in the clinic, during 2018.
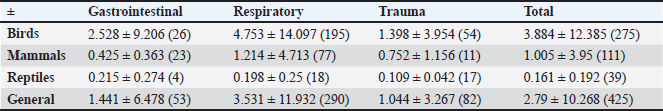
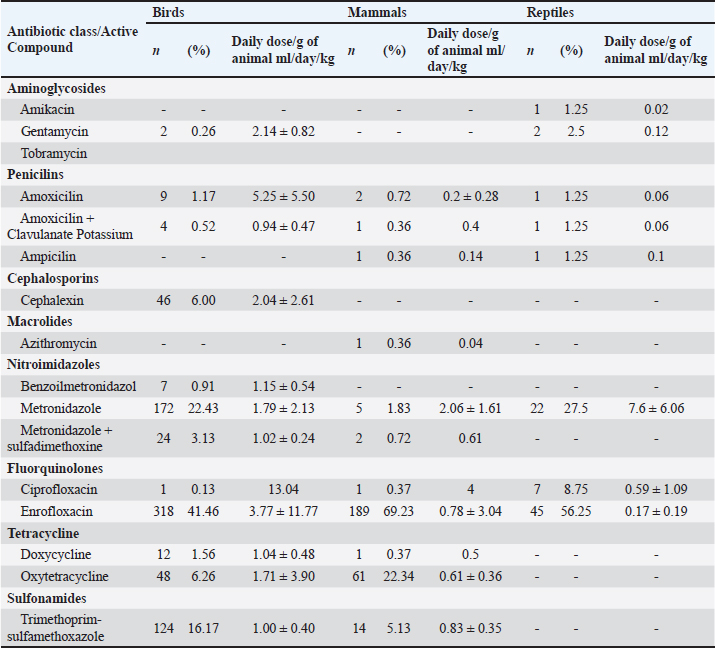
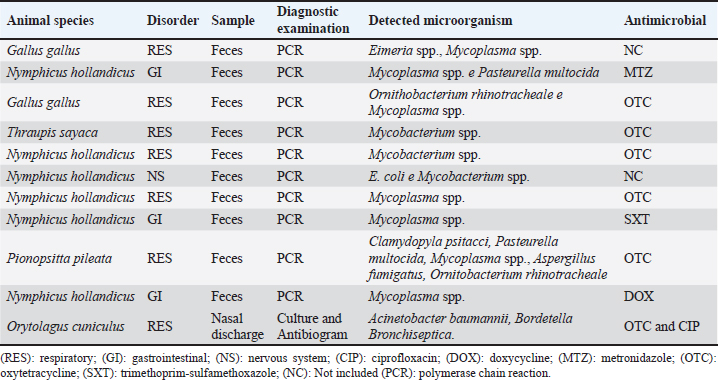
The relation between enrofloxacin dosages and disorders and species served, demonstrated that there was no significant difference between disorders, species, or disorder in each species and species in each disorder due to wide variation within each parameter (Table 2). During 2018, the total consumption of antimicrobials prescribed for birds, mammals, and reptiles in the clinic was 2.21 l for the total biomass of 129.24 kg, the ratio of daily dose per g of animal per ml/day/kg of antimicrobials by animal class is described in Table 3. Of the total of 839 antimicrobials performed in birds, only 10 (0.83%) individuals confirmed the etiological agent, all of which were carried out through molecular assays using the polymerase chain reaction (PCR). In mammals, among the 327 records analyzed in only one of them (0.3%), culture and antibiogram of the patient were performed (Table 4). No complementary examinations were carried out to detect the etiological agent of the disorders in reptiles. Table 2. Dosages of enrofloxacin (daily dose/g of animal ml/day/kg) for the main disorders found in birds, mammals, and reptiles. ( ) indicate the number of animals measured.
Table 3. Relation of daily dose per g of animal per ml/day/kg of antimicrobials in birds, reptiles, and mammals in the year 2018.
The most widely used routes of drug administration in birds and mammals were oral, followed by subcutaneous, nebulization, and intramuscular. In reptiles, the most used route of drug administration for antimicrobials was subcutaneous, followed by intramuscular, and oral administration was performed in only one attendance. Regarding systemic therapy (oral, intramuscular, subcutaneous, and nebulization), the animals treated with antimicrobials were mostly discharged (54.89%, n=595), followed by death (29%, n=334), treatment discontinued (12%, n=143), treatment without clinical improvement (3%, n=31), released without medical discharge (0.89%, n=10), and stopping treatment during the collection and euthanasia period represented (0. 71%, n=8). Table 4. Diagnostic tests were performed to identify the etiologic agent of the disorders in wild animals attended at the clinic in 2018.
DiscussionThe present study was subdivided by taxonomic classes, seeking to cover the particularities of the groups of animals and to correlate them with the therapy adopted in each group. The antimicrobial crisis arising from the rise of AMR is recognized as a global threat to public health. Optimizing the use of these drugs is essential to ensure effectiveness in treating infections, preventing harm caused by unnecessary use of antimicrobials, and combating AMR in both human and veterinary medicine (CDC, 2019; Hopman et al., 2019a). In human medicine, antimicrobial administration programs have been implemented worldwide with the aim of promoting the conscious use of these drugs (World Health Organization, 2018). From a One Health perspective, it is critical that veterinarians and physicians work together to optimize, rationalize, and prudently use antimicrobial therapies in domestic, companion, and exotic animals and humans, as most bacterial pathogens and their resistance mechanisms can be shared between animals and humans (Muñoz-Ibarra et al., 2022). To control the spread of AMR in the animal health arena, action plans, recommendations, and guidelines on the responsible and prudent use of antibiotics have been developed for food-producing animals and companion animals but appear to be lacking in exotic pets. This is likely due to the scarcity and/or lack of data and information on the use, overuse, or misuse of antibiotics in these species (Broens and Geijlswijk, 2018). Drug consumption can be expressed in terms of cost, number of units, number of prescriptions, or physical quantity of drugs. However, these variables can vary between regions and countries over time (Hopman et al., 2019a, 2019b, 2019c). These limits comparisons of medication use at the international level. To address this, a technical unit of measurement, the defined daily dose (DDD), was created for humans and later adapted for animals, DDDVet (European Medicines Agency, 2015; World Health Organization, 2018). However, the bacterial species monitored, and the antimicrobials tested, as well as the methodology used, differ between these countries, making comparisons or conclusive results difficult. A study by Garcia-Migura et al. (2014) established the amounts in mg of each class of antimicrobials (penicillin, tretracycline, cephalosporins, fluoroquinolones, and macrolides) used to produce 1 kg of meat during a period of seven years in seven European countries, although it is not possible to differentiate the species sampled and a number of individuals counted. In Denmark, data can be obtained down to the individual herd level and are reported each year by drug classes and animal species, while in other countries, for example, France only provides total consumption for all animal species (Garcia-Migura et al., 2014). Regarding the consumption of antimicrobials in exotic pets, few studies are available. According to Broens and Geijlswijk (2018), most antimicrobial prescriptions are unnecessary or inappropriate, being most often adopted empirically and not necessarily based on an established diagnosis. However, data regarding the amount or type of antibiotics are lacking. A survey carried out by Muñoz-Ibarra et al. (2022) investigated the laboratory records of the Department of Veterinary Medicine of a large private diagnostic laboratory in Barcelona (Spain), carried out between 2016 and 2020, where the results of 3,156 specimens of exotic animals, microbiological diagnoses and results of sensitivity tests were evaluated. To antibiotics, which, although it does not address prescription and consumption data, provides an overview of potential pathogens and resistance profiles of different classes of animals (birds, reptiles, and mammals). Considering the scarcity of data reporting the consumption of antimicrobials in wild animals, the data found will be compared with domestic species and humans. In this study, approximately 57.08% of consultations were prescribed one or more antimicrobials in the treatment of animals sent to the outpatient clinic, contrary to the findings of Singleton et al. (2017), where the percentage of consultations in which at least one antimicrobial was prescribed was 18.8% for dogs and 17.5% for cats. In birds, the main case series in this period was due to gastrointestinal disorders (40.29%, n=309), such as suspected stomatitis, enteritis, and parasitism. Treatment was instituted by the presentation of clinical signs such as diarrhea, episodes of vomiting, presence of lesions in the oral cavity, presence of flagellates or other microorganisms in the stool, and hematochezia in association with other clinical findings. Later, respiratory disorders (35.33%, n=271), with suspected airsacculitis, pneumonia, and sinusitis, presenting clinical signs such as respiratory difficulty, presence of crackles in alveoli and lungs, presence of nasal secretion, sneezing in association with other symptoms clinical findings. A survey carried out by Muñoz-Ibarra et al. (2022) reports that the most prevalent bacterial agents in birds and mammals were Staphylococcus spp. and Pseudomonas spp. in reptiles. Regarding the most prevalent pathogens by system, Pseudomonas spp. was, in general, the most important agent found in most categories, except in the urinary and musculoskeletal systems, where the most prevalent bacteria were Escherichia coli and Staphylococcus spp., and in the lymphoreticular system, with Enterococcus spp. as the main agent. Staphylococcus spp. and Enterobacteria such as E. coli, Klebsiella spp., and Enterobacter spp. were also frequently isolated from the digestive, respiratory, and external skin/mucosa systems. The zoonotic potential associated with wild animals or exotic pets reinforces the notion that veterinarians should not only be aware of zoonoses but also be ready to educate pet owners about the risks associated with exotic pet ownership (Souza, 2009). For example, Pseudomonas aeruginosa is one of the most clinically and epidemiologically relevant bacteria, being responsible for the major cause of opportunistic infections in immunocompromised patients in hospital environments (Santos et al., 2015). In addition, this pathogen has a special condition that gives it intrinsic resistance to β-lactams through the production of a chromosomally encoded enzyme, AmpC, of Ambler's C class, responsible for resistance to third-generation cephalosporins, in addition to other mechanisms such as pumps that remove beta-lactams, chloramphenicol, macrolides, novobiocin, sulfonamides, fluoroquinolones, tetracycline, and trimethoprim, as well as various dyes and detergents (Santos et al., 2015). The study of antimicrobial susceptibility patterns by Muñoz-Ibarra et al. (2022) showed that 97% of bacteria were resistant to at least one agent from the total antimicrobial categories: 99.5% in birds, 96% in mammals, and 98.5% in reptiles. Furthermore, 16% of the microorganisms showed a multidrug-resistant pattern. These data reinforce the need for diagnostic tests for a more assertive treatment. In mammals, the most common condition of routine care was related to respiratory diseases (41.73%, n=116). Some bacteria such as Streptococcus spp., Mycoplasma spp., Pseudomonas spp., and Pasteurella spp. are normal inhabitants of the respiratory tract of rodents, but in situations of stress, there can be great proliferation, which makes these agents pathogenic (Teixeira, 2014). This data collaborates with the analysis of the veterinary records surveyed in this study. A study by Singleton et al. (2017), with dogs and cats, observed a higher prevalence of gastrointestinal, dermatological, and genitourinary disorders and other causes such as vaccination and postoperative period in dogs, while antimicrobials were prescribed for cats for respiratory and traumatic injuries. These data reinforce the differences found within each species, requiring individual monitoring of each animal group. In contrast, the possibilities of choosing among the available antibiotics for different species are limited and make it difficult to provide antibacterial treatment in exotic animals. Issues such as concentration, palatability, dosage, pharmacological bioavailability, and potential species-specific toxic effects are generally not available for exotic pets and therefore represent a major therapeutic challenge. For example, according to Petritz et al. (2018), antimicrobial-associated enteritis or enterotoxemia can occur from any antimicrobial, but it is uncommon in the sulfa and fluoroquinolone classes, which may justify the empirical use in our research. Enrofloxacin in birds is responsible for reducing the microbiota of the intestinal tract and making them more susceptible to secondary infections by yeasts and anaerobes, in addition to reports of resistance to E. coli, Klebsiella spp., Acinetobacter spp., and Pseudomonas aeruginosa in minimal inhibitory concentrations, the main pathogens responsible for respiratory diseases in these individuals (Flammer, 2006). In the present study, enrofloxacin was the most frequently administered antimicrobial, which may explain the high incidence of gastrointestinal disorders reported in birds. Furthermore, enrofloxacin is partially metabolized by the liver to ciprofloxacin, an equipotent metabolite. The amount of ciprofloxacin produced differs by species and may contribute slightly to antimicrobial activity (Flammer, 2006). The results of the present study diverge from antimicrobial prescription data for companion animals in the United Kingdom, which observed the frequent use of b-lactam antimicrobials (Mateus et al., 2014; Radford et al., 2011; Buckland et al., 2016). In addition, they oppose international recommendations, where the class of fluoroquinolones is classified as antimicrobials of critical importance for human medicine according to the World Health Organization—WHO, restricted to use only in individual animals and after culture and susceptibility tests by legislation Dutch (Hopman et al., 2019c). Regarding consumption data, it is important to note that, due to the pharmacokinetic and pharmacodynamic differences attributed to each animal species, the dose administered to birds is higher than that applied to wild mammals and reptiles. As many wildlife pharmacokinetic parameters are not available for most drugs in use, comparative approaches are made using rodent or human data on absorption, distribution, metabolism, and excretion, however, understanding the physiological similarities and differences between animals and mammals used in the pharmaceutical industry, efficacy/safety trials are improved, so that the wealth of available data can be more effectively applied in ecological risk assessments (Bean et al., 2017). Oxytetracyclines are broad-spectrum bacteriostatic antimicrobials that are very effective against several Gram-positive and Gram-negative bacteria, being the second most used antimicrobial in the clinic. Tetracyclines are low-cost antimicrobials, widely used in the prophylaxis and therapy of human and animal infections and at subtherapeutic levels in animal feeds as growth promoters (Chopra and Roberts, 2001; Grossman, 2016). In the present study, oxytetracycline was used mainly for respiratory disorders, orally and by nebulization. In some cases, it was used when enrofloxacin did not reverse the animal's condition. In research carried out by Merle et al. (2014) in piglets, the class of b-lactams and tetracyclines was the most used, followed by sulfonamides and trimethoprim, and in 48% of the records and 50%, respectively, they were above the recommended dose, only 19% of the records were within of the recommended dose. Metronidazole also represented an important antimicrobial of choice in our survey, being used mainly in gastrointestinal and genitourinary disorders and trauma. Reptiles were the animal class that most consumed this antimicrobial, obtaining a daily average of 7.6 ± 6.06 grams per ml/kg/day, compared to birds and mammals (1.79 ± 2.13 and 2.06 ± 1.61, respectively). Metronidazole is commonly used to combat Clostridium difficile infections in dogs and to treat different types of exotic animal infections, and strains resistant to this drug are being increasingly observed in both humans and animals (Lasheras et al., 2018). In chinchillas, metronidazole has been recommended for the treatment of Giardia infections and Trichomonas infections in birds, as well as for the treatment of anaerobic bacterial infections such as periodontal infections (Mans et al., 2020; Tabari et al., 2021). However, according to the same study, there are several side effects reported after the administration of metronidazole in different animal species. These effects include hyporexia, liver disease, neurological disorders, depression, anorexia, stomatitis, glossitis, vomiting, and diarrhea, and are usually related to prolonged administration or administration of inappropriately high doses of metronidazole (Mans et al., 2020). Despite this, it is indicated as a first-line drug to be used in the absence or pending the results of culture and sensitivity tests, to prevent infection in severely compromised animals (Hedley et al., 2021). Some countries have adopted the metric to measure DDDs of antimicrobials for animals, especially in food-producing animals (Jensen et al., 2004; Hopman et al., 2019a, 2019b; European Medicines Agency—EMA, 2019; Lardé et al., 2020; Redding et al., 2020). However, a major obstacle has been the lack of coordination and standardization, both over time and across countries, which complicates comparisons. This obstacle is even greater when it comes to wild animals, as there is no official approval of the antimicrobial principles authorized for these animals, and in most cases, off-label use is used (Broens and van Geijlswijk, 2018). According to Hopman et al. (2019b) when applying the animal-DDA (DDDA) measure, dosage differences between antimicrobials due to, for example, relative potency and differences in pharmacokinetics, as well as dosage differences between species, are considered. However, in wild animals, the authorized doses for this calculation are lacking. Another point would be the representativeness of the clinic for all wild animal clinics, requiring future studies with a larger number of clinics to explore temporal and cultural trends, seasonality, and determinants of influence of the use of antimicrobials, as well as exploring data from clinical medicine specialists of wild animals at regional, state and national levels. Menéndez González et al. (2010), in the survey, carried out on dairy farms, of the 6,137 records, 52% ( n=3179) of the animals were treated with antimicrobials, being more frequently in adult cows for udder disorders and reproductive disorders, later in calves for respiratory (39%), digestive (39%), events related to childbirth (13%), and prophylactic measures (5%), and less frequent in heifers, mainly due to respiratory problems and locomotor disorders. In a study carried out on 32 broiler farms, in 48 of the 64 (75%) monitored production cycles, antimicrobials were provided through the administration of drinking water (Persoons et al., 2012). These data reinforce the differences found within each species and age group, requiring individual monitoring of each animal group. According to Hedley et al. (2021), the choice of antibiotics should always be carefully considered based on knowledge of the most likely pathogens if culture and sensitivity results are not available. Based on the bibliographic research carried out, practical guidelines should be ideally formulated for each species to optimize therapy and minimize the inappropriate use of antibiotics. Thus, the existence of a septic condition must be confirmed, preferably through culture, antibiogram, and PCR methods, so that appropriate treatment can be instituted and avoid therapeutic failure, potentially promoting the development of multiresistant bacterial strains to antimicrobials (Giacopello et al., 2015). When it is not possible to carry out diagnostic methods for identifying the pathogen, cytology followed by Gram staining should be performed at least before starting any antimicrobial treatment. Exceptions to this would be the use of antibiotics to prevent infection in immunocompromised animals, animals with severe trauma or wounds, and perioperative use (Hedley et al., 2021). In the present data collection, among the treated birds, only 1% (n=11) of the individuals identified the agent causing the lesion through PCR tests, however, without identifying the antimicrobial susceptibility profile for the specific treatment of the conditions. These data reinforce the low adherence to microbiological tests, requiring further investigations regarding the sociocultural and economic factors that surround those responsible for the animals. In addition, although most consultations result in the reversal of the clinical condition for medical discharge, a significant number of patients died or had their treatment interrupted, mainly because the owner did not return, therefore, the conclusion of the procedure is not known frame. In 2.75% of the cases, there was no improvement in the clinical condition, or they were discharged without medical discharge (0.89%) and, in 0.71%, euthanasia was performed. Since, for an adequate therapeutic response, the selection of the drug must consider the susceptibility to the pathogen, the severity of the disease, the conditions at the site of infection, the pharmacokinetics and pharmacodynamics of the selected drugs, the potential toxicity of the drug, the cost of treatment and the owner's commitment to comply with the recommended treatment (Flammer, 2006). Therefore, it is very likely that resistance in wild animals kept in captivity is much greater, because, despite the market, there is still a lack of research that brings answers on the subject. In general, the authors suggested that these variations may be due to differences between the availability of veterinary antibacterial products on the market from country to country, prices, veterinary prescribing behavior, general infectious disease situation in each country, as well as defined policies for managing risk (Sharma et al., 2018). Therefore, it is essential to monitor the consumption of antimicrobials to identify possible risk factors that could lead to the development and spread of AMR in these animals. Collecting data on the use of these medicines is an essential first step in developing and monitoring responsible use policies. ConclusionData like this provide a unique opportunity to monitor the prescription of antimicrobials in veterinary medicine, which is a critical component for risk management and prevention of AMR. Furthermore, these data are the first to report exposure to antimicrobials in a population of wild animals kept in captivity and provide a comprehensive overview highlighting the areas where further research and awareness-raising measures are needed to preserve the therapeutic efficacy of antimicrobial drugs. Despite the present survey registering the clinical discharge in most of the consultations carried out, the antimicrobial prescription behavior in wild animals requires a multifaceted and dynamic approach applied to safeguard the clinical efficacy of the antimicrobials, optimizing consumption, minimizing the emergence of AMR and other possible adverse effects, considering the physiological differences present within each animal class and order. The fact that the doses between the different classes and conditions do not obtain a significant difference, reflects in a possible nonstandardization of the dosages used, requiring further investigation regarding the recommended dosages for each animal species, avoiding the over or underdosing of these drugs. Another point is the absence of commitment from the owners and financial restrictions, as important barriers to the performance of additional diagnoses and a high rate of discontinued treatments. Due to the lack of control and tracking of the use of antimicrobials in veterinary medicine in Brazil, the creation and implementation of current guidelines and legislation must be considered, to encourage the conscious use of these drugs in different species. It is worth highlighting that professionals must always make rational use of antimicrobials in these species. AcknowledgementsThe authors would like to express their sincere gratitude to PUCPR and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing the opportunity and resources that made this study possible. The authors extend their sincere thanks to Vida Livre Clinic for providing the data used in this study. Their collaboration and commitment were crucial for the success of this research. Conflict of interestThe authors declare that there are no conflicts of interest. FundingThis study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. Authors’ contributionCarolina Konkel Barbosa: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review and Editing, Visualization. Valéria Natasha Teixeira: Conceptualization, Methodology, Validation, Investigation, Resources, Data Curation, Visualization. Cláudia Turra Pimpão: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Review and Editing, Visualization, Supervision, Project administration, Funding acquisition. Data availabilityAll data are available in the published manuscript. ReferencesAhmed, A.M., Motoi, Y., Sato, M., Maruyama, A., Watanabe, H., Fukumoto, Y. and Shimamoto, T. 2007. Zoo animals as reservoirs of gram-negative bacteria harboring integrons and antimicrobial resistance genes. Appl. Environ. Microbiol. 73(20), 6686–6690. Andrés-Lasheras, S., Martín-Burriel, I., Mainar-Jaime, R.C., Morales, M., Kuijper, E., Blanco, J.L., Chirino-Trejo, M. and Bolea, R. 2018. Preliminary studies on isolates of Clostridium difficile from dogs and exotic pets. BMC Vet. Res. 14, 77. Bean, T.G., Arnold, K.E., Lane, J.M., Bergström, E., Thomas-Oates, J., Rattner, B.A. and Boxall, A.B.A. 2017. Predictive framework for estimating exposure of birds to pharmaceuticals. Environ. Toxicol. Chem. 36(9), 2335–2344. Broens, E.M. and van Geijlswijk, I.M. 2018. Prudent use of antimicrobials in exotic animal medicine. Vet. Clin. North Am. Exot. Anim. Pract. 21(2), 341–353. Buckland, E.L., O’Neill, D., Summers, J., Mateus, A., Church, D., Redmond, L. and Brodbelt, D. 2016. Characterisation of antimicrobial usage in cats and dogs attending UK primary care companion animal veterinary practices. Vet. Rec. 179(19), 489–489. Cabral, B.G., Davies, Y.M., Menão, M.C., Saidenberg, A.B.S., Gomes, V.T.M., Moreno, L.Z., Sato, M.I.Z., Moreno, A.M. and Knöbl, T. 2020. Companion psittacine birds as reservoir of gentamicin and vancomycin-resistant Enterococcus spp. Pesq. Vet. Bras. 40(2), 129–133. Carmo, L.P., Schüpbach-Regula, G., Müntener, C., Chevance, A., Moulin, G. and Magouras, I. 2017. Approaches for quantifying antimicrobial consumption per animal species based on national sales data: a Swiss example, 2006 to 2013. E.S. 22(6), 1–11. CDC (Centers for Disease Control and Prevention). 2019. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services; https://doi.org/10.15620/cdc:82532. Chiacchio, R.M.G.D., Cunha, M.P.V., Sturn, R.M., Moreno, L.Z., Moreno, A.M., Pereira, C.B.P. and Knöbl, T. 2016. Shiga toxin-producing Escherichia coli (STEC): Zoonotic risks associated with psittacine pet birds in home environments. Vet. Microbiol. 184, 27–30. Chopra, I. and Roberts, M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65(2), 232–260. Collineau, L., Belloc, C., Stärk, K.D.C., Hémonic, A., Postma, M., Dewulf, J. and Chauvin, C. 2016. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in Humans and Animals. Zoo. Public Health 64(3), 165–184. Collington, P.J. and McEwen, A.S. 2019. One health—its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 4(1), 22. Damborg, P., Broens, E.M., Chomel, B.B., Guenther, S., Pasmans, F., Wagenaar, J.A., Weese, J.S., Wieler, L.H., Windahl, U., Vanrompay, D. and Guardabassi, L. 2016. Bacterial zoonoses transmitted by household pets: state of the art and future perspectives for targeted research and policy actions. J. Comp. Pathol. 155(1 Suppl. 1), S27–S40. DANMAP (The Danish Integrated Antimicrobial Resistance Monitoring and Research Programme). 2015. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Retrieved from https://www.whocc.no/ddd/definition_and_general_considera/ (Accessed 1 March 2023). Dias, C., Borges, A., Oliveira, D., Martinez-Murcia, A., Saavedra, M.J. and Simões, M. 2018. Biofilms and antibiotic susceptibility of multidrug-resistant bacteria from wild animals. Peer J. 6, e4974. EC (European Commission). 2017. A European one health action plan against antimicrobial resistance (AMR). Retrieved from https://ec.europa.eu/health/system/files/2020-01/amr_2017_action-plan_0.pdf, 2017 (Accessed 1 March 2023). Economou, V. and Gousia, P. 2015. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 8, 49–61. EMA (European Medicines Agency) 2015. Principles on assignment of defined daily dose for animals (DDDvet) and defined course dose for animals (DCDvet). 015; EMA/710019/2014. EMA (European Medicines Agency). 2019. European surveillance of veterinary antimicrobial consumption. Sales of veterinary antimicrobial agents in 31 European Countries in 2017. EMA/294674/2019. Flammer, K. 2006. Antibiotic drug selection in companion Birds. J. Exot. Pet. Med. 15(3), 166–176. Garcia-Migura, L., Hendriksen, R.S., Fraile, L. and Aarestrup, F.M. 2014. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: the missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 170, 1–9. Giacopello, C., Foti, M., Fisichella, V. and Lo Piccolo, F. 2015. Antibiotic-resistance patterns of gram-negative bacterial isolates from Breeder Canaries (Serinus canaria domestica) with Clinical Disease. J. Exot. Pet. Med. 24(1), 84–91. Grossman, T.H. 2016. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med. 6(4), a025387. Hardefeldt, L.Y., Selinger, J., Stevenson, M.A., Gilkerson, J.R., Crabb, H., Billman-Jacobe, H. and Browning, G.F. 2018. Population wide assessment of antimicrobial use in dogs and cats using a novel data source—a cohort study using pet insurance data. Vet. Microbiol. 225, 34–39. Hedley, J., Whitehead, M.L., Munns, C., Pellett, S., Abou-Zahr, T., Calvo Carrasco, D. and Wissink-Argilaga, N. 2021. Antibiotic stewardship for reptiles. J. Small Anim. Pract. 62(10), 829–839. Hopman, N.E.M., Portengen, L., Heederik, D.J.J., Wagenaar, J.A., van Geijlswijk, I.M. and Broens, E.M. 2019a. Time trends, seasonal differences and determinants of systemic antimicrobial use in companion animal clinics (2012–2015). Vet. Microbiol. 235, 289–294. Hopman, N.E.M., Portengen, L., Hulscher, M.E.J.L., Heederik, D.J.J., Verheij, T.J.M., Wagenaar, J.A. and Broens, E.M. 2019b. Implementation and evaluation of an antimicrobial stewardship programme in companion animal clinics: a stepped-wedge design intervention study. PLoS One 14(11), e0225124. Hopman, N.E.M., van Dijk, M.A.M., Broens, E.M., Wagenaar, J.A., Heederik, D.J.J. and van Geijlswijk, I.M. 2019c. Quantifying antimicrobial use in dutch companion animals. Front Vet. Sci. 6, 158. Jensen, V.F., Jacobsen, E. and Bager, F. 2004. Veterinary antimicrobial-usage statistics based on standardized measures of dosage. Prev. Vet. Med. 64(2–4), 201–215. Kummerer, K. 2009. Antibiotics in the aquatic environment—a review—part I. Chemosphere 75, 417–434. Lardé, H., Dufour, S., Archambault, M., Léger, D., Loest, D., Roy, J.P. and Francoz, D. 2020. Assignment of Canadian defined daily doses and Canadian defined course doses for quantification of antimicrobial usage in cattle. Front Vet. Sci. 7, 10. Mans, C., Fink, D.M., Giammarco, H.E. and Ciarrocchi, C. 2020. Effects of compounded metronidazole and metronidazole benzoate oral suspensions on food intake in healthy chinchillas (Chinchilla lanigera). J. Exot. Pet Med. 36, 75–79. Marques, A.R., Lima, B.P., Teixeira, R.S.C., Albuquerque, A.H., Lopes, E.S., Maciel, W.C., Beleza, A.J.F. and Alencar, T.R. 2021. Zoonotic bacteria research and analysis of antimicrobial resistance levels in parrot isolates from pet shops in the city of Fortaleza. Brazil. Pesq. Vet. Bras. 41, e06837. Marshall, B.M. and Levy, S.B. 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24 (4), 718–733. Mateus, A.L., Brodbelt, D.C., Barber, N. and Stark, K.D. 2014. Qualitative study of factors associated with antimicrobial usage in seven small animal veterinary practices in the UK. Prev. Vet. Med. 117(1), 68–78. Menéndez González, S., Steiner, A., Gassner, B. and Regula, G. 2010. Antimicrobial use in Swiss dairy farms: quantification and evaluation of data quality. Prev. Vet. Med. 95, 50–63. Merle, R., Robanus, M., Hegger-Gravenhorst, C., Mollenhauer, Y., Hajek, P., Käsbohrer, A. and Kreienbrock, L. 2014. Feasibility study of veterinary antibiotic consumption in Germany—comparison of ADDs and UDDs by animal production type, antimicrobial class and indication. BMC Vet. Res. 10(1), 7. Muñoz-Ibarra, E., Molina-López, R.A., Durán, I., Garcias, B., Martín, M. and Darwich, L. 2022. Antimicrobial resistance in bacteria isolated from exotic pets: the situation in the Iberian Peninsula. Animals (Basel). 12(15), 1912. Persoons, D., Dewulf, J., Smet, A., Herman, L., Heyndrickx, M., Martel, A., Catry, B., Butaye. P. and Haesebrouck, F. 2012. Antimicrobial use in Belgian broiler production. Prev. Vet. Med. 105(4), 320–325. Petritz, O.A. and Chen, S. 2018. Therapeutic contraindications in exotic pets. Vet. Clin. North Am. Exot. Anim. Pract. 21(2), 327–340. Radford, A.D., Noble, P.J., Coyne, K.P., Gaskell, R.M., Jones, P.H., Bryan, J.G., Setzkorn, C., Tierney, A. and Dawson, S. 2011. Antibacterial prescribing patterns in small animal veterinary practice identified via SAVSNET: the small animal veterinary surveillance network. Vet. Rec. 169(12), 310. Redding, L.E., Grunwald, H., Melofchik, C., Meily, P., Henry, A. and Stefanovski, D. 2020. Comparison of animal daily doses and days of therapy for antimicrobials in species of veterinary importance. Prev. Vet. Med. 176, 104942. Santos, I.A.L., Nogueira, J.M.R. and Mendonça, F.C.R. 2015. Mecanismos de resistência antimicrobiana em Pseudomonas aeruginosa. Rev. Bras. Anal. Clin. 47(1–2), 5–12. Sharma, C., Rokana, N., Chandra, M., Singh, B.P., Gulhane, R.D., Gill, J.P.S., Ray, P., Puniya, A.K. and Panwar, H. 2018. Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front. Vet. Sci. 4, 237. Souza, M.J. 2009. Bacterial and parasitic zoonoses of exotic pets. Vet. Clin. North Am. Exot. Anim. Pract. 12(3), 401–415. Singleton, D.A., Sánchez-Vizcaíno, F., Dawson, S., Jones, P.H., Noble, P.J.M., Pinchbeck, G.L. and Radford, A.D. 2017. Patterns of antimicrobial agent prescription in a sentinel population of canine and feline veterinary practices in the United Kingdom. Vet. J. 224, 18–24. Tabari, M.A., Poźniak, B., Youssefi, M.R., Roudaki Sarvandani, M.R. and Giorgi, M. 2021. Comparative pharmacokinetics of metronidazole in healthy and Trichomonas gallinae infected pigeons (Columba livia, var. domestica). Br. Poult. Sci. 62(4), 485–491. Teixeira, N.V. 2014. Rodentia—Roedores exóticos (Rato, Camundongo, Hamster, Gerbilo, Porquinho-da-Índia). In Tratado de animais selvagens 2nd edition. Eds., Cubas, Z.S., Silva, J.C.R., Catão-Dias, J.L. São Paulo: Ed. GEN/Roca, pp: 1295–1334. Ventola, L. 2015. The antibiotic resistance crises. Part1: causes and threats. P&T 40(4). PMID: 25859123; PMCID: PMC4378521. WHO (World Health Organization). 2018. Collaborating Centre for Drug Statistics Methodology. DDD: definition and general considerations. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology. Retrieved from https://www.whocc.no/ddd/definition_and_general_considera/ (Accessed 1 March 2023). | ||
| How to Cite this Article |
| Pubmed Style Barbosa CKK, Teixeira VN, Pimpão CT. Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Vet. J.. 2023; 13(12): 1543-1553. doi:10.5455/OVJ.2023.v13.i12.4 Web Style Barbosa CKK, Teixeira VN, Pimpão CT. Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. https://www.openveterinaryjournal.com/?mno=150844 [Access: December 24, 2025]. doi:10.5455/OVJ.2023.v13.i12.4 AMA (American Medical Association) Style Barbosa CKK, Teixeira VN, Pimpão CT. Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Vet. J.. 2023; 13(12): 1543-1553. doi:10.5455/OVJ.2023.v13.i12.4 Vancouver/ICMJE Style Barbosa CKK, Teixeira VN, Pimpão CT. Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Vet. J.. (2023), [cited December 24, 2025]; 13(12): 1543-1553. doi:10.5455/OVJ.2023.v13.i12.4 Harvard Style Barbosa, C. K. K., Teixeira, . V. N. & Pimpão, . C. T. (2023) Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Vet. J., 13 (12), 1543-1553. doi:10.5455/OVJ.2023.v13.i12.4 Turabian Style Barbosa, Carolina Konkel Konkel, Valéria Natascha Teixeira, and Cláudia Turra Pimpão. 2023. Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Veterinary Journal, 13 (12), 1543-1553. doi:10.5455/OVJ.2023.v13.i12.4 Chicago Style Barbosa, Carolina Konkel Konkel, Valéria Natascha Teixeira, and Cláudia Turra Pimpão. "Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil." Open Veterinary Journal 13 (2023), 1543-1553. doi:10.5455/OVJ.2023.v13.i12.4 MLA (The Modern Language Association) Style Barbosa, Carolina Konkel Konkel, Valéria Natascha Teixeira, and Cláudia Turra Pimpão. "Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil." Open Veterinary Journal 13.12 (2023), 1543-1553. Print. doi:10.5455/OVJ.2023.v13.i12.4 APA (American Psychological Association) Style Barbosa, C. K. K., Teixeira, . V. N. & Pimpão, . C. T. (2023) Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Veterinary Journal, 13 (12), 1543-1553. doi:10.5455/OVJ.2023.v13.i12.4 |