| Original Article | ||
Open Vet J. 2022; 12(1): 91-98 Open Veterinary Journal, (2022), Vol. 12(1): 91–98 Original Research Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: Exploratory studyMiguel Ángel Cabezas1*, Javier Benito2, Álvaro Ortega3 and Elena Garcia-Pedraza41Department of Medicine and Surgery, Faculty of Veterinary Medicine, Puerta de Hierro, Universidad Complutense de Madrid, 28040 Madrid, Spain 2Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Montreal (UdeM), Saint-Hyacinthe, Quebec, Canada 3Road Fuencarral 24, Edificio Europa I portal 3, 25. 28108. Alcobendas, Madrid, Spain 4Rue de la Victoire 2 floor (37), 75009, Paris, France *Corresponding Author: Miguel Ángel Cabezas. Department of Medicine and Surgery, Faculty of Veterinary Medicine, Puerta de Hierro, Universidad Complutense de Madrid, 28040 Madrid, Spain. Email: dolorvet [at] gmail.com Submitted: 12/05/2021 Accepted: 16/01/2022 Published: 05/02/2022 © 2022 Open Veterinary Journal
AbstractBackground: Undenatured type-II collagen (UC-II®) has demonstrated its benefits in degenerative joint disease (DJD) management. Aim: Exploratory clinical study to investigate the beneficial effects of FLEXADIN® Advanced in dogs. Methods: Multicentre clinical prospective study, 110 client-owned dogs with a clinical diagnosis of DJD. Dogs received FLEXADIN® Advanced during 6 months. Pain, general condition, appetite, mobility, and lameness were assessed monthly by veterinarians and owners for 6 months. Compliance was assessed at 3 and 6 months. Statistical analysis was performed with non-parametric tests (significance, p < 0.05). Results: Parameters assessed were significantly lower comparing inclusion with all subsequent months (p < 0.0001). Compliance at 3 months showed a good score but was significantly lower (p < 0.0001) at 6 months. Conclusion: FLEXADIN® Advanced, containing UC-II®, can be considered as a good complementary feed to provide joint support in dogs with mild to moderate DJD. Keywords: UC-II, Dog, Degenerative joint disease, Supplement, Compliance. IntroductionDegenerative joint disease (DJD) is a very common condition in canine patients, with different prevalence reported depending on the age, breed, or joint studied. In the United States, with a population of at least 15 million dogs, approximately 20% of the canine population over 1 year of age spontaneously develop DJD (McLaughlin, 2000). The cause of the disease is multifactorial including injury, obesity, aging, or immune disorders (McLaughlin, 2000; Frye et al., 2016). Some breeds are also genetically predisposed to develop arthritis (Gupta et al., 2012). This condition is a chronic, painful, and inflammatory disease that affects any joint in humans and animals, lead to mobility impairment, and decreases quality of life (Hielm-Björkman et al., 2003). A multimodal management of DJD in dogs includes the use of oral joint complementary feed that is thought to help decrease inflammation and protect joint structures (Rychel, 2010). There are several oral joint complementary feed supplements (like chondroitin sulphate and glucosamine, polyunsaturated fatty acids, undenatured type-II collagen among others) available but their efficiency and supporting scientific evidence in patients with DJD remains unclear (Trentham, 1998; Comblain et al., 2016). One of these ingredients is the undenatured type-II collagen (UC-II®), a patented form of collagen extracted from the cartilage of the chicken’s sternum (Comblain et al., 2016). This glycoprotein maintains its three-dimensional structure and glycosylation (Bagchi et al., 2002; Harris et al., 2021). An oral intake of small amounts of glycosylated UC-II® presents active epitopes, with the correct three-dimensional structures, to the Peyer’s patches, this process triggers the signalling required for the development of immune tolerance (Bagchi et al., 2002). The UC-II® has demonstrated the ability to induce this tolerance, effectively reducing joint pain and swelling in rheumatoid arthritis subjects (Bagchi et al., 2002, Stabile et al. 2019). It is believed to engage the immune system and the joint cartilage metabolism: UC-II® can alleviate T-cell response and activate T-regulatory cells via its oral tolerance mechanism, which eventually may reduce cartilage damage (Trentham, 1998; Deparle et al., 2005; Tong et al., 2010; Gencoglu et al., 2020). When collagen is denatured, either by hydrolysis or heating, the three-dimensional structure and glycosylation pattern is lost and therefore no oral tolerance is promoted (Bagchi et al., 2002; Harris et al., 2021). This mechanism limits the deleterious response to type-II collagen present in canine articular cartilage and decreases the action of cells involved in the normal breakdown of collagen and other extracellular matrix proteins. Administration of UC-II® to dogs has shown to reduce joint swelling and pain during DJD improving the mobility as shown with the force plate Peak Vertical Force and Vertical Impulse tests (Gupta et al., 2012). The aim of this exploratory study was to investigate the beneficial effects of FLEXADIN® Advanced, an oral formulation containing UC-II®, in canine patients with mild to moderate DJD. A second objective was to evaluate the compliance of the oral administration of this product to dogs. We hypothesized oral administration of UC-II® in dogs would help to decrease pain and would improve the overall joint health status in dogs. Materials and MethodsThis study was conducted as exploratory, clinical, prospective, non-randomized, non-blinded, non-controlled design. Clinical veterinary general practices were invited to participate in this study during the years 2015 and 2016, involving dogs with signs of DJD related mobility impairment and pain. Dogs diagnosed with mild to moderate DJD by their regular veterinarians were included for a 6-month period of oral supplementation of UC-II®. Owners were asked to report observations for 6 months. FLEXADIN® Advanced included a daily dose of 40 mg of UC-II® as main ingredient. The administered dose of UC-II® was not dependent on the weight of the dog, several studies in different species demonstrating it (Gencoglu et al., 2020). The investigated product was administered by owners once a day for a period of 6 months (1 chew daily per dog) and owners were asked to bring the dog to the clinic once a month to be evaluated. Inclusion criteria included dogs with initial signs of mild to moderate DJD related mobility and impairment pain, confirmed with an orthopaedic exam and preferably confirmed with X-Ray imaging. Dogs were also included if the visual analogue scale (VAS) pain scores from the second to the fourth face values (10 to 40 mm in the veterinary and owner assessments) (Lequesne et al., 1990). Dogs were excluded if any analgesic treatment (for DJD or another systematic disease) was being administered at least 1 month prior to the initiation of the administration of UC-II®. To assess the evolution of the dogs with the investigated product, questionnaires were completed by veterinarians and the owners, monthly, for a period of 6 months. These assessments included:
Statistical analysisThis was an exploratory clinical prospective, non-randomized, non-blinded, non-controlled design, with each dog being repeatedly assessed over time. Continuous numerical data (age and weight) were tested for normality distribution using the Kolmogorov–Smirnov and D’Agostino & Pearson. Qualitative data are described as median and range (25th interquartile value-75th interquartile value) and quantitative data as median [standard deviation (SD)]. The Friedman test for serial values and Dunn’s test for multiple comparisons between the different recording time unit (months) were employed both treatment groups. The Wilcoxon t-test for paired samples was used to compare the ease of Administration. Data were analyzed by means of a commercial software (GraphPad Prism, Version 8.0 for windows; GraphPad Software 2008). A p-value < 0.05 was considered significant for all statistical analyses. Ethical approvalThe study was developed by 28 veterinarians under regular clinical assessment and by owners at home, so the European legislation for research studies in animals (Directive 2010/63/EU) and the local legislation (RD53/2013) were not applicable. Furthermore, Institutional Ethical Review Board for human participants and research (veterinarians and owners clinical and pain subjective score opinions) was not necessary since no personal information about the individuals (veterinarians and owners), opinions, perceptions, choices, decisions regarding themselves were sought. ResultsDemographic dataA total of 110 dogs from 28 different general private veterinary practices in Spain were included in the study during 2015 and 2016. Age of the dogs [Median (range; IQ25–IQ75)] was 9 (7–11) years. The weight [Median (range; IQ25–IQ75)] of the dogs was 30 (16–36) kg. General condition regarding DJD [Median (range; IQ25–IQ75)] at the inclusion time was scored 1 (0–2), mildly altered. A 98% (n=108) of the included dogs had DJD according to the veterinarian’s anamnesis and examination criteria performed by them. However, only 76% (n=84) of the patients had DJD confirmed by the orthopaedic and radiographic examination. Two dogs (1.8%) were included in this study with neither a clear orthopaedic diagnosis of DJD nor radiographic confirmation of the joint impairment, justified by the veterinarian considerations regarding the anamnesis history indicated a suspicion of DJD. All included dogs ended the study and were discharged 6 months after monthly observations by owners and the veterinarians without any post-administration complications.
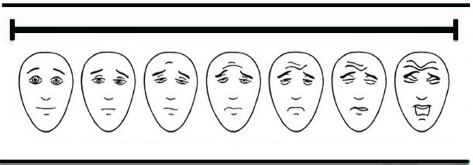
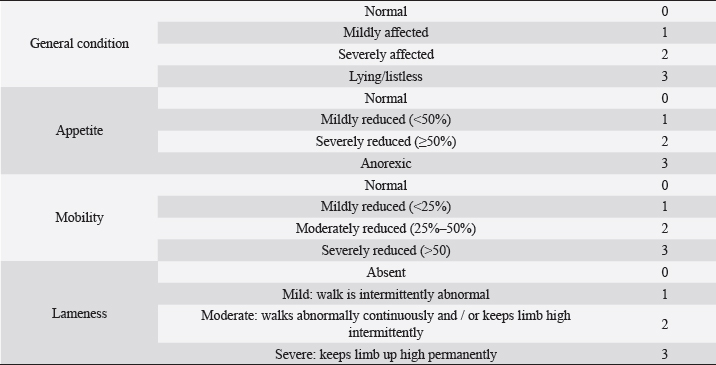
Fig. 1. Visual analogue scale (VAS) facial expressions (Chapman et al., 2008). Table 1. Simple descriptive scale (SDS) domains and scores (Murrell et al., 2014).
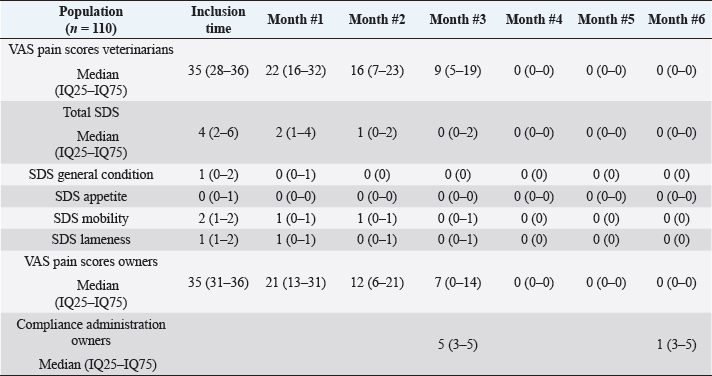
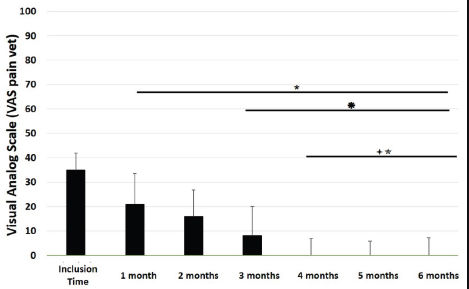
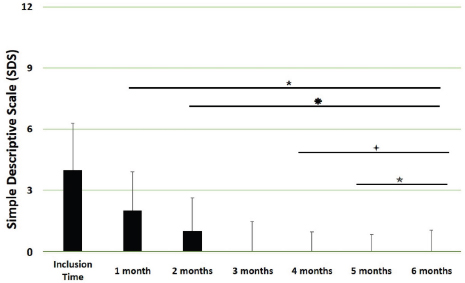
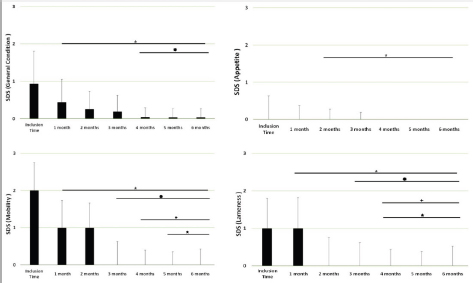
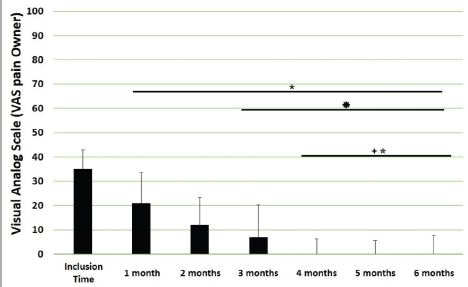
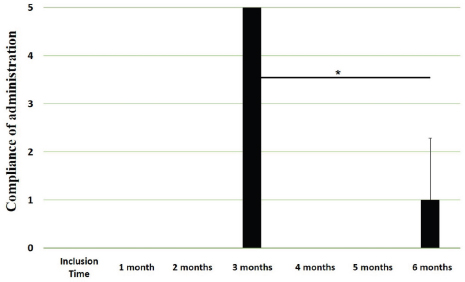
Main outcomesVeterinary assessmentsThe VAS pain scores during the orthopaedic exam recorded by the veterinarians were decreased (p < 0.0001) with time in dogs administered the oral supplementation (Table 3 and Fig. 2). The total SDS score also decreased over time (p < 0.0001) (see Table 3 and Fig. 3). Overall, the VAS pain and the SDS assessment were significantly lower when compared the values at inclusion time at all subsequent months. Scores for months 4, 5 and 6 after inclusion time were significantly lower from the previous month (Figs. 2 and 3). Exploring the different categories for the SDS scores, changes in general condition, appetite, mobility and lameness were significantly improving (less altered) over time (p < 0.0001) respectively (Fig. 4). Owners´ assessmentsThe owner’s VAS pain scores were significantly decreasing (p < 0.0001) over time. Overall, the pain scores were significantly lower compared to the owner’s VAS pain scores at inclusion time at all subsequent months. There were no significant differences between months 1 and 2 post-inclusion, and 2 and 3 post-inclusion, although there was a trend toward less perception of pain by the owner. Months 4, 5 and 6 post-inclusion were significantly different from all previous months in the scores provided by owners, but there were no differences in pain between those months (Fig. 5). The compliance and ease of administration of the product, “Ease of chew administration” scores, assessed at months 3 and 6 post-inclusion were significantly worsening (lower score, higher disagreement) (p < 0.0001) with the passage of months (Fig. 6). Table 2. Compliance/ease of product administration.
Table 3. VAS pain and SDS scores.
Fig. 2. VAS pain scores. Assessments were recorded by veterinarians and values are plotted as median (SD). (★): Significantly different versus Inclusion Time scores; (✹): Significantly different to assessments at month 1; (✦): Significantly different to assessments at month 2; (✯): Significantly different to assessments at month 3. A p value < 0.05 was considered significant.
Fig. 3. SDS overall scores. Assessments were recorded by veterinarians and values are plotted as median (SD). (★): Significantly different versus Inclusion Time scores; (✹): Significantly different to assessments at month 1; (✦): Significantly different to assessments at month 2; (✯): Significantly different to assessments at month 3. A p value < 0.05 was considered significant.
Fig. 4. SDS for the different domains. Assessments were recorded by veterinarians and values are plotted as median (SD) for the four different domains: general condition; appetite; mobility; and lameness. (★): Significantly different versus Inclusion Time scores; (✹): Significantly different to assessments at month 1; (✦): Significantly different to assessments at month 2; (✯): Significantly different to assessments at month 3. A p value < 0.05 was considered significant. DiscussionThe results of this study suggest a clinical improvement of DJD, derived from the oral supplementation of FLEXADIN® Advanced, with a relevant reduction of signs by 4 months. The feed supplement was able to alleviate the clinical signs of pain in dogs with mild to moderate DJD and to improve the four parameters assessed (general condition, mobility, appetite, and lameness) by a SDS from a veterinarian and owner perspectives. This improvement was also demonstrated in the results obtained in the VAS pain scores that were lower and statistically significant after 1 month of supplementation. Such improvement was consistent in time and also measurable over the whole study period. considering a joint supplement was evaluated. This has been already described by Stabile et al. (2019) and Peal et al. (2007). The pain score reduction continued gradually over the 6 months of the study, in line with previous published data regarding UC-II® (Gupta et al., 2012). A greater percentage in the reduction of pain was observed during the first 3 months reaching a stable plateau in the fourth, fifth and sixth months of the study, with average VAS scores in these last 3 months below (VAS < 5/100 mm).
Fig. 5. VAS pain scores by owners. Assessments were recorded by owners and values are plotted as median (SD). (★): Significantly different versus Inclusion Time scores; (✹): Significantly different to assessments at month 1; (✦): Significantly different to assessments at month 2; (✯): Significantly different to assessments at month 3. A p value < 0.05 was considered significant.
Fig. 6. Compliance / ease of administration scores. Assessments were recorded by owners at month #3 and month #6 of oral supplementation. Values are plotted as media (SD). (✯): Significantly different to assessments at month 3. A p value < 0.05 was considered significant. Similar to pain alleviation, general condition, mobility, appetite and lameness also showed a gradual improvement over time. Mobility actually improved in 4 of the 6 months assessed. Regarding the compliance of the feed supplementation and ease of administration of the product, at 3 months assessment point most of the owners agreed that the product was easy to be administered (average score 4) but the score decreased significantly at the 6 months assessment point. This highlights the difficulty on the long-term compliance of any recommended treatment for pets as already communicated in previous study (Talamonti et al., 2015) FLEXADIN® Advanced is usually administered as an adjunct feed supplement in a multimodal plan in cases of canine DJD. Therefore, a treatment based only in this oral supplementation should be considered only in dogs with mild to moderate DJD (VAS between 10 and 40). Then, the true therapeutic benefits of this feed supplement can be assessed. However, this does not mean patients with mild to moderate DJD may benefit also from a multimodal therapeutic approach. Overall, signs of DJD in these patients suggest a clear improvement within 6 months of treatment. Further studies should determine whether such improvement is maintained thereafter, or additional treatment should be provided. It seems to be statistically evident that there is a reduction in the degree of pain in the patients included in the study, as well as an improvement in the parameters described (general condition, mobility, appetite, and lameness). In all the analyzed parameters there is an evident clinical improvement from month 1 to month 6, except for the continuity of administration of the tablets. Considering that animal supplements do not need to demonstrate clinical improvement as a requirement for commercialization, this type of studies provide scientific evidence. Our results further support the use of supplements containing UC-II® in people but also dogs, cats, horses (Comblain et al., 2016, Gencoglu et al., 2020). The study has several limitations and a randomized, blinded, controlled study should confirm our results. Furthermore, this study was performed in 110 dogs under field conditions and it should be representative for the dog-owned population and justifies the interest for the communication of results. Other points for improvement are related to the use of validated pain scales, the inclusion of a control group and monitoring. In addition, weight and exercise daily routines should have been monitored to avoid potential bias. The VAS scale used included human face expressions (Hielm-Björkman et al., 2003) to help the veterinarians and owners with their assessments since a canine facial expressions’ scale is not available, although employed, it has proven to be valid and reliable. More objective assessments of the joint, like gait analysis, ground forces and/or activity monitors could be useful to demonstrate the effectiveness of the treatment. In conclusion, overall results suggest FLEXADIN® Advanced, a formulation containing UC-II®, was able to improve signs associated with mild to moderate DJD in dogs within a 6-month period as the only supplement provided. Further controlled, blinded, studies should confirm these findings. AcknowledgmentsThis study was supported by Vetoquinol Spain. Conflict of interestMiguel Angel Cabezas and Javier Benito had no conflict of interests. Alvaro Ortega and Elena García-Pedraza declare that both work for Vetoquinol, provider of the product for the field study. Javier Benito and Miguel Angel Cabezas have received funding to carry out the study. Author contributionsMiguel Angel Cabezas: Experimental design, results analysis, and preparation of manuscript. Javier Benito: Statistical analysis, discussion of results and preparation of manuscript. Alvaro Ortega: Experimental design, results analysis, and preparation of manuscript. Elena García-Pedraza: Discussion of results and preparation of manuscript. ReferencesBagchi, D., Misner, B., Bagchi, M., Kothari, S.C., Downs, B.W., Fafard, R.D. and Preuss, H.G. 2002. Effects of orally administered undenatured type-II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int. J. Clin. Pharmacol. Res. 22(3–4), 101–110. Chapman, C.R. 2008. Progress in pain assessment: the cognitively compromised patient. Curr. Opin. Anaesthesiol. 21(5), 610–615. Comblain, F., Serisier, S., Barthelemy, N., Balligand, M. and Henrotin, Y. 2016. Review of dietary supplements for the management of osteoarthritis in dogs in studies from 2004 to 2014. J. Vet. Pharmacol. Ther. 39(1), 1–15. Deparle, L.A., Gupta, R.C., Canerdy, T.D., Goad, J.T., D’Altilio, M., Bagchi, M. and Bagchi, D. 2005. Efficacy and safety of glycosylated undenatured type-II collagen (UC-II) in therapy of arthritic dogs. J. Vet. Pharmacol. Ther. 28(4), 385–390. Frye, C.W., Shmalberg, J.W. and Wakshlag, J.J. 2016. Obesity, exercise and orthopedic disease. Vet. Clin. North Am. Small Anim. Pract. 46(5), 831–841. Gencoglu, H., Orhan, C., Sahin, E. and Sahin, K. 2020. Undenatured type-II collagen (UC-II®) in joint health and disease: a review on the current knowledge of companion animals. Anim. (Basel). 10(4), 697. Gupta, R.C., Canerdy, T.D., Lindley, J., Konemann, M., Minniear, J., Carroll, B.A., Hendrick, C., Goad, J.T., Rohde, K., Doss, R., Bagchi, M. and Bagchi, D. 2012. Comparative therapeutic efficacy and safety of type-II collagen (UC-II®), glucosamine and chondroitin in arthritic dogs: pain evaluation by ground force plate. J. Anim. Physiol. Anim. Nutr. 96, 770–777. Harris, R.B., Fonseca, F.L.A., Sharp, M.H. and Ottinger, C.R. 2021. Functional characterization of undenatured type ii collagen supplements: are they interchangeable? J. Diet. Suppl. 1–16; doi:10.1080/19390211.2021.1931621 Hielm-Björkman, A.K., Kuusela, E., Liman, A., Markkola, A., Saarto, E., Huttunen, P., Leppäluoto, J., Tulamo, R.M. and Raekallio, M. 2003. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 222(11), 1552–1558. Lequesne, M., Dougados, M., Abiteboul, M., Bontoux, D., Bouvenot, G., Chicheportiche, V., Dreiser, R.L., Dropsy, R., Maheu, E. and Mazières, B. 1990. Comment évaluer l’évolution de l’arthrose à long terme. Tests pour les essais de traitements de fond (rachis exclu) [How to evaluate the long-term course of osteoarthritis. Tests for trials of fundamental treatments (spine excluded)]. Rev. Rhumat. Malad. Osteo-Articulaires 57(9(Pt 2)), 24S–31S. McLaughlin, R. 2000. Management of chronic osteoarthritic pain. Vet. Clin. North Am. Small Anim. Pract. 30, 933–949. Murrell, J., Grandemange, E., Woehrle, F., Menard, J. and White, K. 2014. Clinical efficacy and tolerability of cimicoxib in dogs with osteoarthritis: a multicentre prospective study. Open J. Vet. Med. 4, 78–90. Peal, A., D’Altilio, M., Simms, C., Alvey, M., Gupta, R.C., Goad, J.T., Canerdy, T.D., Bagchi, M. and Bagchi, D. 2007. Therapeutic efficacy and safety of undenatured type-II collagen (UC-II) alone or in combination with (-)-hydroxycitric acid and chromemate in arthritic dogs. J. Vet. Pharmacol. Ther. 30(3), 275–278. Rychel, J.K. 2010. Diagnosis and treatment of osteoarthritis. Top. Comp. Anim. Med. 25(1), 20–25. Stabile, M., Samarelli, R., Trerotoli, P., Fracassi, L., Lacitignola, L., Crovace, A. and Staffieri, F. 2019. Evaluation of the effects of undenatured type-II collagen (UC-II) as compared to robenacoxib on the mobility impairment induced by osteoarthritis in dogs. Vet. Sci. 6(3), 72. Talamonti, Z., Cassis, C., Brambilla, P.G., Scarpa, P., Stefanello, D., Cannas, S., Minero, M. and Palestrini, C. 2015. Preliminary study of pet owner adherence in behaviour, cardiology, urology, and oncology fields. Vet. Med. Int. 2015, 618216. Trentham, D.E. 1998. Oral tolerization as a treatment of rheumatoid arthritis. Rheum. Dis. Clin. North Am. 24(3), 525-536. Tong, T., Zhao, W., Wu, Y.Q., Chang, Y., Wang, Q.T., Zhang, L.L. and Wei, W. 2010. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 59, 369–377. | ||
| How to Cite this Article |
| Pubmed Style MAC, Benito J, Ortega A, Garcia-Pedraza E. Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study. Open Vet J. 2022; 12(1): 91-98. doi:10.5455/OVJ.2022.v12.i1.11 Web Style MAC, Benito J, Ortega A, Garcia-Pedraza E. Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study. https://www.openveterinaryjournal.com/?mno=80854 [Access: April 18, 2024]. doi:10.5455/OVJ.2022.v12.i1.11 AMA (American Medical Association) Style MAC, Benito J, Ortega A, Garcia-Pedraza E. Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study. Open Vet J. 2022; 12(1): 91-98. doi:10.5455/OVJ.2022.v12.i1.11 Vancouver/ICMJE Style MAC, Benito J, Ortega A, Garcia-Pedraza E. Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study. Open Vet J. (2022), [cited April 18, 2024]; 12(1): 91-98. doi:10.5455/OVJ.2022.v12.i1.11 Harvard Style , M. A. C., Benito, . J., Ortega, . A. & Garcia-Pedraza, . E. (2022) Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study. Open Vet J, 12 (1), 91-98. doi:10.5455/OVJ.2022.v12.i1.11 Turabian Style , Miguel Angel Cabezas, Javier Benito, Alvaro Ortega, and Elena Garcia-Pedraza. 2022. Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study. Open Veterinary Journal, 12 (1), 91-98. doi:10.5455/OVJ.2022.v12.i1.11 Chicago Style , Miguel Angel Cabezas, Javier Benito, Alvaro Ortega, and Elena Garcia-Pedraza. "Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study." Open Veterinary Journal 12 (2022), 91-98. doi:10.5455/OVJ.2022.v12.i1.11 MLA (The Modern Language Association) Style , Miguel Angel Cabezas, Javier Benito, Alvaro Ortega, and Elena Garcia-Pedraza. "Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study." Open Veterinary Journal 12.1 (2022), 91-98. Print. doi:10.5455/OVJ.2022.v12.i1.11 APA (American Psychological Association) Style , M. A. C., Benito, . J., Ortega, . A. & Garcia-Pedraza, . E. (2022) Long-term supplementation with an undenatured type-II collagen (UC-II®) formulation in dogs with degenerative joint disease: exploratory study. Open Veterinary Journal, 12 (1), 91-98. doi:10.5455/OVJ.2022.v12.i1.11 |