| Review Article | ||
Open Vet J. 2021; 11(4): 555-568 Open Veterinary Journal, (2021), Vol. 11(4): 555–568 Review Article An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory diseaseWafaa A. Abd El-Ghany*Poultry Diseases Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt *Corresponding Author: Wafaa A. Abd El-Ghany. Poultry Diseases Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. Email: wafaa.ghany [at] yahoo.com Submitted: 29/04/2021 Accepted: 20/09/2021 Published: 15/10/2021 © 2021 Open Veterinary Journal
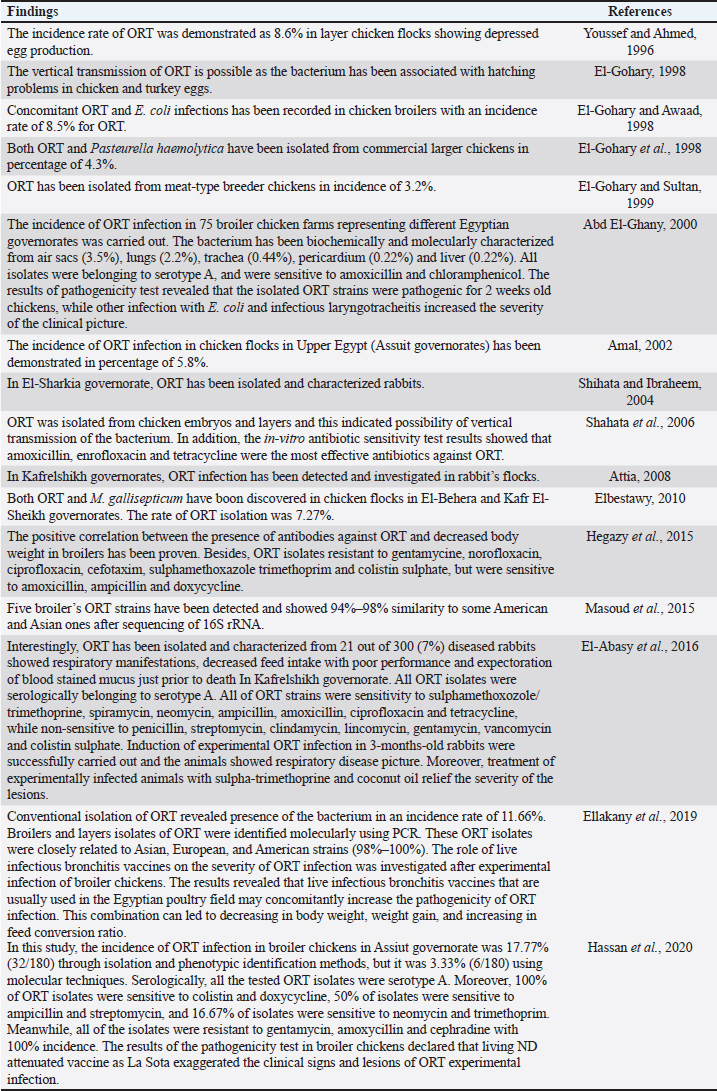
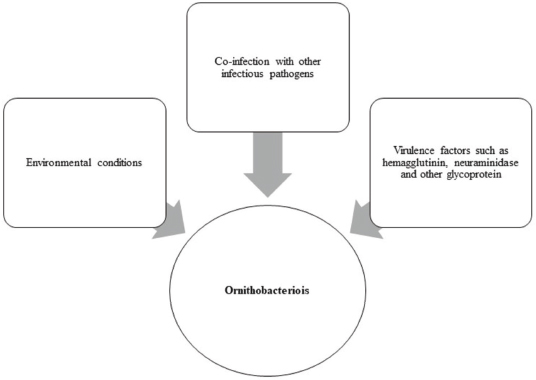
AbstractOrnithobacteriosis is an important emerging respiratory disease of domestic and wild birds caused by Ornithobacterium rhinotracheale (ORT) bacterium. The disease has been detected in some countries since 1980, which rapidly spread worldwide later on. Ornithobacteriosis can transmit either horizontally or even vertically. Infection with ORT is mainly characterized by respiratory distress, poor performance, acute death, and a drop in egg production. However, the most characteristic necropsy lesions of dead turkeys and chickens are yoghurt like airsacculitis and pneumonia, usually unilateral. Unfortunately, infection with ORT was misdiagnosed in most of the poultry flocks due to similarity with other respiratory pathogens and the lack of the ideal protocols for diagnosis. Recently, some molecular and serological techniques have been used to detect the infection. Treatment of ORT with antibiotics is very difficult and variable as a result of acquired resistance. Many vaccines have been developed to counteract such infection in broiler, layers, and breeder chicken and turkey flocks. Inactivated, live, and sub-unit vaccines have been used with satisfactory results. Thus, this review paper aimed to address ornithobacteriosis, emphasizing the distribution, transmission, clinical picture, diagnosis, and disease control. Keywords: Diagnosis, Incidence, ORT, Treatment, Vaccination. IntroductionRespiratory infections of poultry are regarded as very important problems that cause high economic losses in the production system. One of these infections is ornithobacteriosis. It is a relatively novel emerging respiratory contagious disease among turkeys and chickens caused by Ornithobacterium rhinotracheale (ORT) bacterium. The bacterium is a highly polymorphic Gram-negative rod, non-motile or spore former, and belongs to genus nov., species nov. in the rRNA superfamily V and family Flavobacteriaceae (Vandamme et al., 1994). Ornithobacteriosis induces severe adverse negative impact on the poultry industry worldwide. Poor growth rate, acute mortalities, increasing the medication costs, high condemnation rates at processing, and decreasing the quantity and quality of eggs and hatchability are the economic losses of infection (Chin et al., 2013). The disease showed rapid evolution and spread all over the world with increase in the incidence rate. Ornithobacteriosis is mainly characterized by respiratory manifestations with the presence of yogurt-like fibrinous exudates in the airsacs and uni/or bilateral lung consolidation (Hafez, 1996; Banani et al., 2001). However, the severity of the clinical picture is affected by the presence of other complicating infectious agents and non-infectious environmental conditions along with some virulence factors (van Empel and Hafez, 1999; Barbosa et al., 2019). Accordingly, ornithobacteriosis may be regarded as a part of a complex of other respiratory viral and bacterial pathogens that synergize to induce the infection (Welchman et al., 2013; Kursa et al., 2021). Definitive diagnosis of ornithobacteriosis is based on isolation and identification of ORT bacterium using either conventional phenotypic methods (De la Rosa-Ramos et al., 2018; Hassan et al., 2020) and/or molecular techniques (Ellakany et al., 2019; Veiga et al., 2019; Hassan et al., 2020; Karimi-Dehkordi et al., 2021). Despite the fact that ORT infection can be successfully treated with antibiotics, the bacterium can rapidly develop antibiotic resistance (Devriese et al., 2001). Therefore, some trials have been undertaken to produce inactivated, live, and sub-unit vaccines to counteract ORT infection (Lopes et al., 2002; Schuijffel et al., 2006; Ghasemipour et al., 2020). This review paper aimed to address ornithobacteriosis, emphasizing the distribution, transmission, clinical picture, diagnosis, and control of the disease. The worldwide incidence and distributionOrnithobacteriosis has been detected in domestic and wild birds with respiratory conditions in several countries worldwide. Early in 1987 in Hungary, Pasteurella-like organisms were isolated from ducks with respiratory signs. In addition, Riemerella anatipestifer like bacteria was found in turkeys showing respiratory affection in 1991 and 1992 in Germany. However, in 1991 in South Africa, highly pleomorphic Gram-negative rods were isolated from a 28-day-old broiler chicken flock suffering respiratory manifestations, mortalities, bad performance, foamy yoghurt like airsacculitis and pneumonia (van Beek et al., 1994). Moreover, in the Netherlands and Germany in 1993, respiratory problems, poor growth rate, and acute mortalities have been observed in turkey and chicken broiler flocks (Hafez et al., 1993; Hinz et al., 1994; van Beek et al., 1994). Later on, the disease was seen rapidly spreading across many countries like the USA (Charlton et al., 1993), France (LeÂorat et al., 1994), Israel (Bock et al., 1995), Peninsula (Odor et al., 1997; Salem et al., 1997), Canada (Joubert et al., 1999), and South Africa (Vandamme et al., 1994; Buys, 1996; Travers, 1996; Goovaerts et al., 1998). In the different states of America, ORT has been isolated from turkeys and chicken flocks with respiratory signs and variable mortalities (DeRosa et al., 1996, 1997; Tahseen, 1997; Roepke et al., 1998; Heeder et al., 2001; Malik et al., 2003; Walters, 2014; Hauck et al., 2015). However, antibodies to ORT infection have been serologically detected in broiler and breeder’s sera in South America (Arns et al., 1998), Netherlands (van Veen et al., 2001), Southern Brazil (Canal et al., 2003), Taiwan (Tsai and Huang, 2006), Thailand (Chansiripornchai et al., 2007), and Pakistan (Siddique et al., 2008). Besides, the bacterium has been isolated and characterized from broiler chickens in the Rio Grande do Sul state of Brazil (Canal et al., 2005) and Peru (Hung and Alvarado, 2001; Koga and Zavaleta, 2005), along with laying hens in Cuba (Espinosa et al., 2011; Chávez et al., 2017). Recently, Ha et al. (2016) succeeded in isolating ORT from broiler chickens in New Zealand, while Szabó et al. (2017) characterized ORT field isolates in Hungary. In Japan, Umali et al. (2017) detected the possibility for isolation of ORT from the heart, liver, kidney, spleen, and ovaries of broiler chickens after systemic infection. Regarding the Middle East countries, ORT has been characterized from Iranian (Rahimi and Banani, 2007; Asadpour et al., 2008; Ghanbarpour and Salehi, 2009; Hassanzadeh et al., 2010; Asadpour et al., 2011; Mayahi et al., 2016; Ghasemipour et al., 2020), Turkish (Ozbey et al., 2004; Türkyilmaz, 2005), and Jordanian (Roussan et al., 2011) chickens and turkey flocks. In Egypt, ornithobacteriosis was early detected in the 1990s, since ORT organism has been isolated alone or concomitantly with other bacteria from layers and broiler chicken and turkey flocks (Youssef and Ahmed, 1996; El-Gohary, 1998, El-Gohary and Awaad, 1998; El-Gohary et al., 1998; El-Gohary and Sultan, 1999; Abd El-Ghany, 2000). Later, several studies have been carried out to isolate, characterize and treat ORT in different Egyptian governorates (Amal, 2002; Shihata and Ibraheem, 2004; Shahata et al., 2006; Attia, 2008; Elbestawy, 2010; Hegazy et al., 2015; Masoud et al., 2015; El-Abasy et al., 2016; Ellakany et al., 2019; Hassan et al., 2020). The incidence of ornithobacteriosis in Egyptian poultry flocks is summarized in Table 1. The causative agent and the virulence factorsAs a result of the difficulties in isolation and characterization of ORT bacterium, it was early named as Pasteurella like (van Empel and Hafez, 1999), Kingella like (van Beek et al., 1994), polymorphic Gram-negative rode bacterium (Charlton et al., 1993), and TAXON 28 (van Empel and Hafez, 1999). However, ORT was genotypically and taxonomically classified in the early 1990’s as a new genus and species (Hafez et al., 1993; Vandamme et al., 1994). The species rhinotracheale belongs to genus Ornithobacterium. The bacterium was classified as a Gram-negative and highly pleomorphic rod of the rRNA superfamily V, in the taxonomic neighborhood of the genera Cytophaga, Riemerella, and Flavobacterium (Vandamme et al., 1994; Canal et al., 2005). The genus Ornithobacterium belongs to the family Flavobacteriaceae (Hafez et al., 1994), which also includes the genus Riemerella with R. anatipestifer and the genus Coenonia with C. anatina. Besides a new species named Candidatus Ornithobacterium hominis sp. nov., ORT is the only species described within the genus Ornithobacterium. Before the first taxonomic identification of ORT bacteria, misdiagnosis of infection was common, and the causative agent was attributed to some other bacteria such as Pasteurella, Riemerella, Bordetella, or Haemophilus (Hafez et al., 1993; Bragg et al., 1997) as well as other viruses as Pneumovirus (Marien et al., 2005). The Pathogenicity and persistence of ORT organisms in the host are influenced by environmental conditions, biofilm formation, and coinfection with other organisms (Marien et al., 2005; De la Rosa-Ramos et al., 2015, 2018). Serotypes A, B, C, D, and E of ORT showed different virulence factors with variable adherence profiles (Chansiripornchai et al., 2007; De Haro-Cruz et al., 2013). The tissue’s adherence and colonization with ORT are associated with the presence of some virulence factors such as hemagglutinin, neuraminidase, and other glycoprotein (Kastelic et al., 2013; De la Rosa-Ramos et al., 2018). Hemolytic isolates of ORT have been described and differentiated from R. anatipestifer isolates (Walters, 2014). Figure 1 shows the factors that influence the severity of ornithobacteriosis infections. Host susceptibility and transmission of infectionOrnithobacteriosis is incriminated in infection of all commercial avian species and wild birds in many countries of the world. There is a wide range of birds that could be infected with ORT or carry the bacterium in their respiratory tracts. The bacterium is present in the apparent healthy captive and free-ranging non-galliform species. Turkey, chicken, duck, goose, guinea fowl, gull, ostrich, partridge, pheasant, pigeon, quail, and rook showed ornithobacteriosis (Charlton et al., 1993; Anonymous, 1995; Devriese et al., 1995; Buys, 1996; Hafez, 2002). Infection with ORT was first described in turkeys, the main susceptible host (Hafez, 1996; Karimi-Dehkordi et al., 2021, Kursa et al., 2021), as well as in chickens (Roussan et al., 2011; Hassan et al., 2020). It has been reported that the incidence rate of ORT in turkeys was higher (41%) than that in chickens (6.9%) (Hauck et al., 2015). Besides, the disease has been reported in the Egyptian Muscovy and Balady ducks (El-Abasy, 2008), and the bacterium has been molecularly detected in pigeons and birds of prey (Tsai and Huang, 2006; Thieme et al., 2016). Interestingly, ORT has been demonstrated in rabbit farms with respiratory problems in some Egyptian governorates (Shihata and Ibraheem, 2004; Attia, 2008; El-Abasy et al., 2016 Table 1. The incidence of ornithobacteriosis in Egyptian poultry flocks.
Fig. 1. Factors influence the severity of ornithobacteriosis infections. Ornithobacteriosis spreads horizontally through inhalation and direct contact or indirectly through the drinking water (Chin and Charlton, 2008). Provide strong evidence of vertical transmission (from the hen to the egg through the ovary), the entrance of the bacterium via eggshell is different. It is also probable since ORT was isolated from reproductive organs, infertile and hatching eggs, and from dead embryos (Tanyi et al., 1995/1996; El-Gohary, 1998; Shahata et al., 2006). The bacterium was also found on the eggshells and in the yolk sacs of day-old- chicks but at very low incidence (1%) (van Empel, 1997). This type of transmission can occur either trans-ovarian or by cloacal contamination (van Empel, 1997). Experimental studies showed that ornithobacteriosis infected turkey breeder hens showed survival of the bacterium in the ovary and oviduct without signs (Back et al., 1996, 1998a; Nagaraja et al., 1998). Wild birds may also be considered as an important source of infection to the commercial poultry flocks. Ornithobacteriosis is regarded as a threatening but not a zoonotic disease (Cobb and Smith, 2015; World Organization for Animal Health, 2018). Clinical signs and lesionsBirds infected with ORT showed reduced food intake, decreased weight gains, sneezing, nasal discharge, wet eyes with lacrimation, sinusitis, facial edema followed by coughing, dyspnea, prostration, and death (Canal et al., 2005; Rahimi and Banani, 2007; Asadpour et al., 2008). Sudden death with or without respiratory signs has been found in chickens with nervous manifestations (Chin et al., 2008). Certain reported cases showed that ornithobacteriosis might induce sudden death due to meningitis (van Empel and Hafez, 1999). Experimental infection of chickens and turkeys with ORT revealed decrease in body weight and growth retardation (van Empel et al., 1996; Ellakany et al., 2019). The clinical picture of ORT could be vanished within a week or become more complicated in the presence of other pathogens or even non-recognized as an ORT infection anymore (van den Bosch, 2001). The necropsy findings of ornithobacteriosis are associated with sinusitis, tracheitis, pericarditis, airsacculitis, peritonitis, and exudative pneumonia (Amonsin et al., 1997). However, the most characteristic post-mortem lesions are the presence of foamy white, “yoghurt-like” exudate in the airsacs, predominantly in the abdominal airsacs and fibrino-purulent pneumonia (Hinz et al., 1994; Banani et al., 2001). Moreover, in South Africa, subcutaneous oedema over the cranium and severe osteitis without respiratory affections have been detected in 28-day-old broiler chickens (Goovaerts et al., 1998). Actually, the lesions become more severe if other complicating infectious pathogens are associated with ORT infection and often lead to death (Abd El-Ghany, 2000; Chin et al., 2013). Accordingly, the severity of the clinical picture of ornithobaxteriosis, disease duration, and mortality rates are extremely variable and influenced by the virulence of the bacterial strain, the immune status of the host, the environmental conditions like bad management, poor ventilation, overcrowding, poor litter quality, bad hygiene, and high ammonia levels along with the presence of concurrent or secondary infections (Travers, 1996; Bisgaard et al., 2008). For instance, administration of living Newcastle (ND) La Sota vaccine at 5 days before ORT challenge induced a more serious increase in airsacculitis and pneumonia scores compared to both ORT challenge and ND La Sota vaccine administration alone (van Empel et al., 1996). Furthermore, Abd El-Ghany (2000) revealed that ORT strains were pathogenic for 2-week-old chickens, while co-infections with Escherichia coli (E. coli) and infectious laryngeotracheitis increased the severity of the clinical picture. Pan et al. (2012a) demonstrated that the experimental infection of broiler chickens with ORT could induce a mortality rate of around 50%. In comparison, mixed co-infection of ORT with H9N2 avian influenza virus (AIV) led to a higher mortality rate of 70% and 90%, respectively, if ORT inoculation was simultaneously made with H9N2 or if H9N2 AIV was inoculated after 3 days. In the same context, ORT infection alone could induce a disease condition with mortalities, but co-infection with Streptococcus zooepidemicus was more lethal (Pan et al., 2012b). Recently, Ellakany et al. (2019) confirmed that concomitant experimental ORT infection and Mycoplasma gallisepticum increased the severity of clinical respiratory signs and lesions and hurts the performance and growth parameters of broiler chickens. The histopathological lesions of ORT were represented as granulomatous pneumonia, tracheitis, and fibrinous airsacculitis (van Empel and Hafez, 1999; Abd El-Ghany, 2000; Chin et al., 2008; Ellakany et al., 2019). Kilic et al. (2009) described the microscopic lesions after experimental ORT infection in chickens as focal epithelial hyperplasia along with necrosis and inflammatory lesions of the lamina propria in the upper respiratory tract, air sacs, as well as around bronchioles and some lung areas. Laboratory diagnosisSigns and lesions associated with ornithobacteriosis are of little value and not sufficiently specific to diagnose the disease since a similar clinical picture could be seen in other infections (Hafez and Sting, 1996). Hence, diagnosis of ornithobacteriosis mainly relies on phenotypic and molecular detection of the bacterium or immunogenic detection of antibodies (Ellakany et al., 2019). Despite conventional ORT isolation method could be difficult owing to the overgrowth by other opportunistic bacteria (Churria et al., 2011, 2012), it is still necessary for serotyping, determination of in vitro antimicrobial sensitivity test as well as production of autogenous vaccines (Vandamme et al., 1994; Hafez and Sting, 1996; Hegazy et al., 2015). Techniques of isolation and identification may differentiate ORT bacterium from other similar respiratory bacteria as Pasteurella multocida, E. coli, or Avibacterium paragallinarum. For successful isolation of ORT, samples should be from the airsacs, lungs, and trachea after natural and experimental infections (Joubert et al., 1999; Abd El-Ghany, 2000; Welchman et al., 2013; Hauck et al., 2015; Gavrilović et al., 2016). Moreover, ORT bacterium was isolated from the nasal mucosa and orbital sinuses swabs of infected turkeys (Karimi-Dehkordi et al., 2021). Isolation of the bacterium from the heart, liver, kidney, spleen, ovaries, and brain is suggestive after systemic infection (van Beek et al., 1994; Nagaraja et al., 1998; Umali et al., 2017). Samples should be collected from suspected flocks as early as possible. Tissues and swabs could be maintained at 4°C for 2 days or at −20°C for 5 days without adverse effect on the variability of ORT organism or growth of other bacteria (Numee et al., 2012). Samples should be inoculated on 5%–10% sheep blood agar media and incubated under microaerophilic (5%–10% CO2) or anaerobic conditions for 24–48 hours. As ORT is a growing fastidious organism, it needs special media’s supplement and special environmental conditions (Travers, 1996). Most ORT isolates showed resistance to gentamicin or polymyxin. So, adding these antibiotics (5–10 µg/ml) to the media can suppress other contaminating overgrowing bacteria such as Pseudomonas, Portus, and E. coli species (Back et al., 1997; Hassan et al., 2020). Positive cultures of ORT appear as non-hemolytic, pinpoint to pinheaded, gray to grayish white, circular, convex, and reddish glow colonies with a distinct butyric acid odor (Shahata et al., 2006). Some isolates of ORT showed incomplete β hemolysis, especially after 96 hours of incubation. In addition, the bacterium can grow in brain heart infusion broth and on trypticase soya agar but not on MacConkey agar (Roepke et al., 1998; Post et al., 1999; Asadpour et al., 2008; Mayahi et al., 2016; Hassan et al., 2020). Microscopic identification of stained smears from suspected ORT colonies showed Gram-negative, highly pleomorphic, non-motile, or spore-forming rods (van Empel and Hafez, 1999; Chin and Charlton, 2008; Espinosa et al., 2011; Chin et al., 2013). Regarding the biochemical identification, ORT isolates are positive oxidase and negative catalase (van Empel et al., 1996; Chin and Droual, 1997; Hafez, 1998; van Empel, 1998; Ryll et al., 2002). Commercial kits (API-20 NE and API-ZYM) are used for the biochemical characterization of ORT isolates. The bacterium is positive for arginine dihydrolase, β- galactosidase, gelatin liquefaction, and Voges-Praskauer tests, while negative for L-lysine decarboxylase, ornithine decarboxylase, and H2S production tests (Chin and Charlton, 2008; Hassan et al., 2020). Regarding sugar fermentation tests, ORT reveals positive sucrose, arabinose, lactose, fructose, galactose, and maltose, but negative glucose, mannitol, inositol, and sorbitol (Rahimi and Banani, 2007; Mayahi et al., 2016). There is a great possibility to reduce the detection rate of ORT after culturing due to the presence of tiny colonies, slow growth of the bacterium, and the need for enriched media and microphonic conditions (Zahra et al., 2013). Therefore, molecular detection of ORT DNA using polymerase chain reaction (PCR) and gene sequencing of 16S rRNA and rpoB genes are now used for the routine diagnosis (Hafez and Beyer, 1997; Veiga et al., 2019). These recent techniques are regarded as very important and fruitful tools for the definitive detection of infection (Ozbey et al., 2004; Banani et al., 2009; Ellakany et al., 2019). Moreover, they are fast, sensitive, and specific for the characterization of bacterial strains (Hung and Alvarado, 2001; Li and Diao, 2009; Montes De Oca-Jimenez et al., 2018; Veiga et al., 2019). Many types of modified PCR techniques are used for the characterization of ORT. Enterobacterial repetitive intergenic consensus-PCR, repetitive element palindromic-PCR, random amplified polymorphic DNA-PCR, and multilocus sequence typing have been developed (Szabó et al., 2017). Great variations among ORT isolates have been found using phylogenetic analyses of the 16S rRNA gene (Banani et al., 2009; Montes de Oca-Jimenez et al., 2018). Lately, Veiga et al. (2019) suggested using ORT rpoB gene for partial sequencing of isolates from different avian species. The phylogenetic relationship indicated the existence of a greater genetic variability (Montes De Oca-Jimenez et al., 2018; Veiga et al., 2019), particularly between ORT strains from different hosts (Thieme et al., 2016). Serotyping of ORT isolates has been carried out using agar gel precipitation test and enzyme-linked immunosorbent sssay (ELISA) with specific antisera against 18 (A–R) serotypes (van Empel et al., 1997; van Empel, 1998; Türkyilmaz, 2005; Wu et al., 2010; Hassan et al., 2020). Within ORT species, several serotypes and strains with different virulence are present (Ryll et al., 1996). The different ORT serotypes have no direct relationship with virulence. Rapid slide agglutination test was also used to type ORT isolates (Back et al., 1998b). Reports indicated that all the tested ORT isolates belong to serotype A, which is the most prevalent among strains of chickens (94%) and turkeys (57%) (Siddique et al., 2008). Serotypes A, B, D, and E are most common in turkeys, while serotypes F, K, and M are sometimes isolated from chickens and turkeys (van Empel and Hafez, 1999). A cross-reaction between serotypes B and A and between serotypes I and L were observed after using rapid serum plate agglutination test in layers. Besides, cross-reactions have been detected for serotypes A, E, and I, but not with serotype C (Türkyilmaz, 2005). Serological identification of ORT is hampered by limitations, such as cross-reactivity between strains (Szabó et al., 2017). Due to the difficulties in serotyping methods, the presence of un-determined new serotypes of ORT has been suggested (Numee et al., 2012; De la Rosa-Ramos et al., 2018). To overcome these disadvantages, a wide range of techniques have been implemented over the last years. The matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) has become more efficient than biochemical tests for routine laboratory diagnosis of microorganisms as it is a rapid, reliable, and direct technique for the identification (Alispahic et al., 2014). In the recent study of Alispahic et al. (2021), the molecular characterization of 47 ORT field strains derived from Austrian turkey farms was carried out using MALDI-TOF MS and whole genome sequencing techniques. The results of MALDI-TOF MS revealed that most ORT strains were grouped within one cluster although they comprised of different serotypes, except serotypes F, K, and M that formed a different cluster. The whole genome sequencing results confirmed that the previous data indicated that serotypes F, K, and M were clearly different from the other ORT strains and may belong to different Ornithobacter species. High seroprevalence of ORT was demonstrated among broiler and breeders chicken and turkey flocks in several regions worldwide using ELISA (van Empel et al., 1996; Canal et al., 2003; Hegazy et al., 2015). For instance, Hafez and Sting (1996) detected antibodies to ORT in 79% of broiler breeder chicken flocks, and 26% and 55% of broiler chickens and meat turkey flocks, respectively. Furthermore, Ryll et al. (1997) demonstrated the presence of antibodies in 96.6% of the sera of broiler turkeys. In Iran, antibodies of ORT were found in 205 (44.2%) out of 463 broiler chickens and in 340 (72%) out of 472 breeder chicken serum samples (Allymehr, 2006). Another study showed the presence of antibodies in the sera of 289/460 (83%) broiler breeder flocks (Asadpour et al., 2008). Moreover, of the 420 serum samples, 134 (31.9%) were positive for ORT antibodies (Ghanbarpour and Salehi, 2009). High (100%) seroprevalence to ORT was detected in broiler breeder chicken flocks in Brazil (Canal et al., 2003) and layer chicken flocks in the United States (Heeder et al., 2001). It is important to note that antibodies of ORT and other pathogens were detected using ELISA. For example, antibodies of ORT and turkey rhinotracheitis virus (Hafez, 1997a, 1997b) and Chlamydia psittaci were demonstrated (Hafez et al., 1998). Prevention and controlThe prevention of ornithobacteriosis in the poultry production system should be considered since the disease has become endemic worldwide. Adoption of strict biosecurity measures and husbandry practices is critical. All in-all out policy should be applied. Thorough cleaning and disinfection of poultry houses are essential to avoid the possibility of ORT re-infection or spreading, especially in endemic areas. In vitro study of Hafez and Schulze (2003) declared that a concentration of 0.05% aldehydes and organic acids (formic and glyoxyl) disinfectant preparations effectively inactivated ORT bacteria within 15 minutes. The in vitro antibiotic susceptibility pattern of ORT strains is greatly inconsistent. It depends on the locality, the source of strain, the inherent genetic differences between bird breeds, and the routinely used antibiotics in the area (Odor et al., 1997; Malik et al., 2003; Türkyilmaz, 2005; Mayahi et al., 2016). In addition, the mutation of ORT plasmids plays an important role in developing antibiotic resistance (Back et al., 1997). For instance, an increase in the minimal inhibitory concentration of enrofloxacin from 0.03 to 0.25 mg/ml for ORT treatment in turkeys was due to mutations in the gyrA gene (Marien et al., 2006). Different classes of antibiotics, even the recently used ones, became inefficient against ORT strains, maybe due to transfer of the resistance among them (Devriese et al., 2001) or increase in the resistance for different drugs (Cauwerts et al., 2002). As a result of frequently acquired resistance, the treatment of ornithobacteriosis is difficult and cannot be effectively achieved through antibiotics (Devriese et al., 2001). An early study by van Beek et al. (1994) declared that the oral treatment of ORT infected turkeys using enrofloxacin and trimethoprim/sulphonamide was not effective. However, twice injections of tetracyclines and penicillin gave good results. Treatment of ORT-infected birds with 250 ppm amoxicillin and 500 ppm chlortetracycline in the drinking water for 3–7 days was effective in relieving infection (Hafez, 1997b). In Germany, Hafez (1996) observed that 90%–100% of the ORT strains were sensitive to tetracycline, chloramphenicol, and amoxicillin, while they were resistant to enrofloxacin, gentamycin, neomycin, and trimethoprim/sulphonamide. Although 90% of the abovementioned isolates were resistant to enrofloxacin in Germany, they were sensitive to the same antibiotic in Belgium and France (Devriese et al., 1995; Dudouyt et al., 1995; Roger and LeÂorat, 1997). Moreover, Chin and Droual (1997) demonstrated that water treatment with amoxicillin, tetracycline, and chloramphenicol was satisfactory. Isolates of ORT in France showed resistance to gentamicin and colistin but sensitivity to amoxicillin, spectinomycin, and tylosin (Roger and LeÂorat, 1997). In USA, 100% of ORT revealed susceptibility to ampicillin, penicillin, spectinomycin, erythromycin, and tylosin, 79.4% were susceptible to neomycin, tetracycline, and sarafloxacin, and the rest of the isolates were susceptible to streptomycin, gentamicin, and trimethoprim (Nagaraja et al., 1998). Strains of ORT strains in the Netherlands showed susceptibility to amoxicillin, tetracycline, enrofloxacin, and trimethoprim/sulphonamid (van Veen et al., 2001). However, later on, the sensitivity of these ORT strains to amoxycillin and tetracycline decreased from 62% to 14%. Even four out of the strains were non-susceptible to enrofloxacin the combination of trimethoprim-sulphonamide. In Mexico, Soriano et al. (2003) declared that ORT strains were sensitive to amoxicillin, enrofloxacin, and oxytetracycline, while resistant to gentamicin and fosfomycin. In addition, Mohd-Zain et al. (2008) demonstrated that 100% of ORT strains were resistant to ampicillin, enrofloxacin, and sulfanomide/trimethoprim, while they were sensitive to chloramphenicol. In the study by Asadpour et al. (2011), the authors found that all ORT strains were non-sensitive to enrofloxacin, ciprofloxacin, erythromycin, tetracycline, while all of them were sensitive to ceftriaxone. Moreover, two strains (66.70%) showed moderate susceptibility to amoxicillin and florfenicol. Churria et al. (2016) reported that all isolates of ORT were resistant to gentamicin. Most of them were resistant to enrofloxacin, erythromycin trimethoprim-sulfamethoxazole, doxycycline, and fosfomycin, while all of them were sensitive to ampicillin and florfenicol. The recent Egyptian study of Hassan et al. (2020) revealed that 100% of circulating ORT strains were non-susceptible to gentamycin, amoxycillin, and cephradine, while 100% were susceptible to colistin and doxycycline, 50% to ampicillin and streptomycin, and 16.67% to neomycin and trimethoprim. From the above-mentioned studies, it could be concluded that most ORT strains became resistant to the majority of the used antibiotics in the field (Watteyn et al., 2016; Umali et al., 2017). Therefore, vaccination may be a promising and effective strategy to counteract ornithobacteriosis. Inactivated, live, and recombinant sub-unit vaccines of ORT have been developed with variable results (Gornatti Churria et al., 2013). An early trial has been done to vaccinate day-old-broiler chickens and turkeys with autogenous inactivated oil adjuvant bacterin (Bock et al., 1997). Moreover, this type of bacterin significantly reduced ORT lesion scores after vaccination of broilers at 26-days-old (van Empel and van den Bosch, 1998). However, it has been found that vaccination of birds at 8-week-old was more effective than vaccination at 4-week-old to avoid interfering with maternal immunity (Gopala Krishna Murthy et al., 2007). Vaccination of breeders using inactivated bacterin was found to be effective and protective against the development of pathologic changes of ORT infections in the progeny (Bisshop, 2005). No cross-protection between serotypes was induced after vaccination with bacterins in oil adjuvant (Bock et al., 1995, 1997). Many types of inactivating substances and adjuvants were added to 18 ORT vaccines to choose the best one (Gopala Krishna Murthy et al., 2007). It has shown that a vaccine containing mineral oil adjuvant induced the highest immune response and the lowest respiratory lesions in vaccinated birds. Cauwerts et al. (2002) also observed decreasing mortalities and increasing production of the offspring from the vaccinated breeders. Vaccination of breeder broiler chicken flocks with inactivated ORT bacterin containing serotype A induced 39% increase in the production rate and 22.3% decrease in progeny loss (De Herdt et al., 2012). Autogenous inactivated oil adjuvant ORT bacterins showed a successful reduction of ornithobacteriosis outbreaks in Turkey (Erganis et al., 2010), Israel (Chin et al., 2013), and Iran (Ghasemipour et al., 2020). Mention if there is cross protection between different serotypes of ORT. It has been shown that inactivated or subunits vaccines of ORT mostly give low or only partial cross-protection and not always for all serotypes. However, live types vaccines can provide this cross protection (Schuijffel et al., 2005, 2006). van Empel and van den Bosch (1998) found that the vaccination of breeders against ornithobacteriosis using live vaccine provided satisfactory protection against pneumonia and airsacculitis in their progeny until 28 days of age. The protective efficacy of a live, temperature-sensitive mutant ORT vaccine against a bacterial challenge has been evaluated in day-old turkey poults (Lopes et al., 2002). The vaccine strain colonized the upper respiratory tract and recovered 13 days post vaccination with protective humoral immune response. Nevertheless, the presence of different ORT serotypes within the bacterium species represent a major challenge in vaccine production. So, the production of recombinant or sub-unit vaccines becomes urgent to induce homologous and heterologous protection along with the rapid immune response (van Empel and Hafez, 1999; Schuijffel et al., 2006). Schuijffel et al. (2005) showed that recombinant subunit vaccine containing eight encoded cross-reactive antigens induced homologous and heterologous protection against ORT challenge as well as production of protective antibodies. ConclusionDespite the continuous progress in ORT characterization in almost all countries around the world, there is a gap of knowledge and a lack of information in some aspects. Thus, more research is needed. For example, the mechanism and pathogenesis of ORT infection in the host, the development of more recent diagnostic tools, and the design of treatment and vaccination protocols are still in need. Besides, it is necessary to include some national monitoring programs for emerging respiratory affections like ornithobacteriosis to avoid the adverse economic losses caused by such infection. Conflict of interestThe author declares that there is no conflict of interest. FundingThis review article did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors. Authors’ contributionWafaa A. Abd El-Ghany collected the data, prepared and revised the manuscript, and approved the fnal version. ReferencesAbd El-Ghany, W.A. 2000. Epizootiological investigation on Ornithobacterium rhinotracheale (ORT) in chicken broilers. M.V.SC. Thesis, Cairo University, Cairo, Egypt. Alispahic, M., Christensen, H., Bisgaard, M., Hess, M. and Hess, C. 2014. MALDI-TOF mass spectrometry confirms difficulties in separating species of the Avibacterium genus. Avian Pathol. 43, 258–263. Alispahic, M., Endler, L., Hess, M. and Hess, C. 2021. Ornithobacterium rhinotracheale: MALDI-TOF MS and whole genome sequencing confirm that serotypes K, L and M deviate from well-known reference strains and numerous field isolates. Microorganisms 9, 1006. Allymehr, M. 2006. Seroprevalence of Ornithobacterium rhinotracheale infection in broiler and broiler breeder chickens in West Azerbaijan Province, Iran. J. Vet. Med. A Physiol. Pathol. Clin. Med. 53, 40–42. Amal, A.M.P. 2002. Preliminary studies on Ornithobacterium rhinotracheale infection in poultry in Upper Egypt. M.V.SC. Thesis, Assiut University, Assuit, Egypt. Amonsin, A., Wellehan, J.F.X., Li, L.L., Vandame, P., Lindeman, C., Edman, M., Robinson, R.A. and Kapur, V. 1997. Molecular epidemiology of Ornithobacterium rhinotracheale. J. Clin. Microbiol. 35, 2894–2898. Anonymous, 1995. Ornithobacterium rhinotracheale in ostrich. Khangela Quarterly Report on Animal Disease Surveillance, South Africa, January–March, pp: 11. Arns, C., Hafez, H.M., Yano, T., Monteiro, M., Alves, M., Domingues, H. and Coswig, L. 1998. Ornithobacterium rhinotracheale: DeteccË aÄo soroloÂgica em aves matrzes e Fragos de Corte. In Proceedings of the Association of Broiler Procedures, APINCO’ 98, Campinas, Brazil, pp: 56. Asadpour, Y., Banani, M. and Pourbakhsh, S.A. 2011. Isolation, identification and antibiotic sensitivity determination of Ornithobacterium rhinotracheale in slaughtering broiler chicken flocks of Guilan province. Iran. J. Vet. Res. 12, 345–349. Asadpour, Y., M.H. Bozorgmehrifard, S.A. Pourbakhsh, M. Banani and S. Charkhkar, 2008. Isolation and identification of Ornithobacterium rhinotracheale in broiler breeder flocks of Guilan province, North of Iran. Pak. J. Biol. Sci. 11, 1487–1491. Attia, S.A. 2008. Studies on Ornithobacterium rhinotracheale infection in rabbits. M.V.SC. Thesis, Kafrelshikh University, Kafrelsheikh, Egypt. Back, A., Nagaraj, K.V. and Halvorson, B.D. 1996. Preliminary studies on Ornithobacterium rhinotracheale. In Proceedings of the Turkey ORT Symposium, Minneapolis, MN, pp: 29–31. Back, A., Sprenger, S., Rajashekara, G., Halvorson, D.A. and Nagaraja, K.V. 1997. Antimicrobial sensitivity of Ornithobacterium rhinotracheale isolated from different geographic locations. In Proceedings of the North Central Avian Disease Conference, 1997 May 29–31, Des Moines, IA, vol. 48, pp: 22–24. Back, A., Rajashekara, G., Jeremiah, R., Halvorson, D. and Nagaraja, K. 1998a. Tissue distribution of Ornithobacterium rhinotracheale in experimentally infected turkeys. Vet. Rec. 143, 52–53. Back, A., Halvorson, D., Rajashekara, G. and Nagaraja, K. 1998b. Development of a serum plate agglutination test to detect antibodies to Ornithobacterium rhinotracheale. J. Vet. Diag. Invest. 10, 84–86. Banani, M., Pourbacksh, S. A. and Khaki, P. 2001. Characterization of Ornithobacterium rhinotracheale isolated from commercial chickens. Arch. Razi. Inst. 52, 27–36. Banani, M., Pourbakhsh, S.A., Erami, M., Gholamin, F. and Fatehmanesh, M. 2009. Diagnosis of Ornithobacterium rhinotracheale using polymerase chain reaction (PCR). J. Vet. Res. 64, 41–45. Barbosa, E.V., Cardoso, C.V., Silva, R.C.F., Cerqueira, A.M.F., Liberal, M.H.T. and Castro, H.C. 2019. Ornithobacterium rhinotracheale: an update review about an emerging poultry pathogen. Vet. Sci. 7(1), 3. Bisshop, S.P.R. 2005. The use of a bacterin vaccine in broiler breeders in the control of Ornithobacterium Rhinotracheale in commercial broilers. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2005, p 112. Bisgaard, M., Bojesen, A.M. and Christensen, J.P. 2008. Infections caused by species of Pasteurellaceae, Ornithobacterium and Riemerella. In Poultry diseases, 6th ed. Elsevier Science, pp: 146–148. Bock, R., Freidlin, P., Tomer, S., Manoim, M., Inbar, A., Frommer, A.,Vandamme, P. Wilding, P. and Hickson, D. 1995. Ornithobacterium rhinotracheale (ORT) associated with a new turkey respiratory tract infectious agent. In Proceedings of the 33rd Annual Convention of the Israel Branch of the World Veterinary Association, Zichron, Israel, pp: 43–45. Bock, R., Freidlin, P., Manoim, M., Inbar, A., Frommer, A., Vandamme, P. and Wilding, P. 1997 Ornithobacterium rhinotracheale (ORT) associated with a new turkey respiratory tract infectious agent in Israel. In Proceedings of the 11th International Congress of the World Veterinary Poultry Association, Budapest, Hungary, pp: 120. Bragg, R., Greyling, J. and Verschoor, J. 1997. Isolation and identification of NAD-independent bacteria from chickens with symptoms of infectious coryza. Avian Pathol. 26, 595–606. Buys, S. 1996. Ornithobacterium rhinotracheale an emerging disease in South Africa. Aerosols Newslett. World Vet. Poult. Assoc. 1996, 8–10. Chansiripornchai, N., Wanasawaeng, W. and Sasipreeyajan, J. 2007. Seroprevalence and identification of Ornithobacterium rhinotracheale from broiler and broiler breeder flocks in Thailand. Avian Dis. 51, 777–780. Canal, C.W., Leao J.A., Ferreira, D.J., Macagnan M., Pippi Salle, C.T. and Back, A. 2003. Prevalence of antibodies against Ornithobacterium rhinotracheale in broilers and breeders in Southern Brazil. Avian Dis. 47, 731–737. Canal, C.W., Leao, J.A., Rocha, S.L.S., Macagnan, M., Lima-Rosa, C.A.V., Oliveira, S.D. and Back, A. 2005. Isolation and characterization of Ornithobacterium rhinotracheale from chickens in Brazil. Res. Vet. Sci. 78, 225–230. Cauwerts, K., Herdt, P.D., Haesebrouck, F., Vervloesem, J. and Ducatelle, R. 2002. The effect of Ornithobacterium rhinotracheale vaccination of broiler breeder chickens on the performance of their progeny. Avian Pathol. 31(6), 619–624. Charlton, B.R., Channing-Santiago, S.E., Bickford, A.A., Cardona, C.J., Chin, R.P., Cooper, G.L., Droual, R., Jeffrey, J.S., Meteyer, C.U., Shivaprasad, H.L., Richard, L. and Walker, R.L. 1993. Preliminary characterization of a pleomorphic Gram-negative rod associated with avian respiratory disease. J. Vet. Diagn. Invest. 5, 47–51. Chávez, M.C., León, Y.M., Ugalde, Y.S., Cárdenas, N.B., López, A.M., Rivero, E.L. and Redondo, A.V. 2017. Evidência Sorológica, em Cuba, da Circulação de Ornithobacterium rhinotracheale em Galinhas Poedeiras com Síndrome Respiratória Crônica. XXII Congresso Latino-Americano de Avicultura. 2011. Chin, R.P. and Charlton, B.R. 2008. Ornithobacteriosis. In A laboratory manual for the isolation, identification, and characterization of avian pathogens. Eds., Dufour-Zavala, L., Swayne, D.E., Glisson, J.R., Pearson, J.E., Reed, W.M., Jackwood, M.W. and Woolcock, P.R. 5th ed. Madison, WL: The American Association of Avian Pathologists, pp: 75–76. Chin, R. and Droual, R. 1997. Ornithobacterium rhinotracheale infection. In Diseases of poultry. Ed., Calnek, B.W. 10th ed. Ames, IA: Iowa State University Press, pp: 1012–1015. Chin, R.P. and van Empel, P.C.M. 2013. Hafez, H.M. Ornithobacterium rhinotracheale infection. In Diseases of poultry. Ed., Swayne, D.E. 13th ed. Hoboken, NJ: Wiley-Blackwell, 2013, pp: 807–858. Chin, R.P., van Empel, P.C.M. and Hafez, H.M. 2008. Ornithobacterium rhinotracheale infection, 12th ed. Oxford, UK: Blackwell Publishing Ltd. Churria, C.D.G., Loukopoulos, P., Vigo, G.B., Sansalone, P., Machuca, M.A., Nievas, V. and Petruccelli, M.A. 2016. In vitro antibiotic susceptibility patterns of Ornithobacterium rhinotracheale from commercial chickens in Argentina. Int. J. Poult. Sci. 15, 293–296. Churria, C.D.G., Sansalone, P.L., Vigo G.B., Sguazza, G.H., Machuca, M.A., Origlia, J.A., Piscopo, M.V., Herrero, M. and Petruccelli, M.A. 2011. Pneumonia in broiler chicken flocks associated with β-hemolytic Ornithobacterium rhinotracheale infection. Braz. J. Vet. Pathol. 4, 243–246. Churria, C.D.G., Sansalone, P.L., Machuca, M.A., Vigo, G.B., Sguazza, G.H., Origlia, J.A., Piscopo, M.V., Herrero, M. and Petruccelli, M.A. 2012. Tracheitis in a broiler chicken flock caused by dual infection with cryptosporidium spp. (Apicomplexa: Cryptosporiidae) and non-hemolytic Ornithobacterium rhinotracheale. Braz. J. Vet. Pathol. 5, 89–93. Cobb, S.P. and Smith, H. 2015. The spread of non-OIE-listed avian diseases through international trade of chicken meat: an assessment of the risks to New Zealand. Rev. Sci. Tech. 34, 795–812. De Haro-Cruz, M.J., Ixta-Avila, L. and Guerra-Infante, F.M. 2013. Adherence of five serovars of Ornithobacterium rhinotracheale to chicken tracheal epithelial cells. Br. Poult. Sci. 54, 425–429. De Herdt, P., Broeckx, M., Vankeirsbilck, W., Van Den Abeele, G. and Van Gorp, S. 2012. Improved broiler performance associated with Ornithobacterium rhinotracheale vaccination in breeders. Avian Dis. 56, 365–368. De la Rosa-Ramos, M.A., Cruz, M.R., Villegas, E.O.L., Castro-Escarpulli, G. and Guerra-Infante, F.M. 2015. Conditions that induce biofilm production by Ornithobacterium rhinotracheale. Avian Pathol. 44, 366–369. De la Rosa-Ramos, M.A., Muñoz-Solís, K., PalmaZepeda, M., Gutierrez-Castillo, A.C., López Villegas, E.O., Guerra-Infante, F.M. and Castro-Escarpulli, G. 2018. Adherence of Ornithobacterium rhinotracheale to chicken embryo lung cells as a pathogenic mechanism. Avian Pathol. 47(2), 172–178. DeRosa, M., Droual, R., Chin, R., Shivaprasad, H. and Walker, R. 1996. Ornithobacterium rhinotracheale infection in turkey breeders. Avian Dis. 40, 865–874. DeRosa, M., Droual, R., Chin, R. and Shivaprasad, H. 1997. Interaction of Ornithobacterium rhinotracheale and Bordetella avium in turkey poults. In Proceedings of the 46th Western Poultry Disease Conference, Sacramento, CA, pp: 52–53. Devriese, L.A., De Herdt, P. and Haesebrouck, F. 2001. Antibiotic sensitivity and resistance in Ornithobacterium rhinotracheale strains from Belgian broiler chickens. Avian Pathol. 30, 197–200. Devriese, L., Hommez, J., Vandamme, P., Kersters, K. and Haesebrouck, F. 1995. In vitro antibiotic sensitivity of Ornithobacterium rhinotracheale strains from poultry and wild birds. Vet. Rec. 137, 435–436. Dudouyt, J., LeÂorat, J., van Empel, P., Gardin, Y. and CeÂline, D. 1995. Isolement d’ un nouvel pathogene chez la dinde: Ornithobacterium rhinotracheale; Conduite a tenir. In Proceedings of the JourneÂes de la Recherche Avicole, Angers, France, pp: 240–243. El-Abasy, M.A. 2008. Ornithobacterium rhinotracheale (ORT) in Muscovy and Balady ducks. In Proceedings of the 8th Scientific Conference, Egyptian Veterinary Poultry Association, pp: 133–139. El-Abasy, M.A., Youssif, A.E., Attia S.A. and El- Gohary, A.A 2016. Ornithobacterium rhinotracheale (ORT) infection in rabbits. Kafrelsheikh Vet. Med. J. 14(1), 115–133. Elbestawy, A.R. 2010. Studies on Ornithobacterium rhinotracheale (ORT) and Mycoplasma gallisepticum infections in commercial chicken flocks in El-Behera and Kafr El-Sheikh Governorates. In Proceedings of 9th Scientific Conference of the Egyptian Veterinary Poultry Association, Egypt. El-Gohary, A.A. 1998. Ornithobacterium rhinotracheale (ORT) associated with hatching problems in chicken and turkey eggs. Vet. Med. J. Giza 46, 183–191. El-Gohary, A. A. and Awaad, M.H.H. 1998. Concomitant Ornithobacterium rhinotracheale (ORT) and E. coli infection in chicken broiler. Vet. Med. J. Giza 45, 67–75. El-Gohary, A., Sultan, H. and Hafez, H.M. 1998. Isolation of Ornithobacterium rhinotracheale (ORT) and Pasteurella haemolytica from commercial larger chickens. In Proceedings of 5th Scientific Conference, Egyptian Veterinary Poultry Association, Cairo, Egypt, pp: 111–128. El-Gohary, A. and Sultan, H. 1999. Ornithobacterium rhinotracheale (ORT): infection in meat-type breeder chickens. J. Alex. Vet. Sci. 15, 253–269. Ellakany, H., Elbestawy, A., Abd-Elhamid, H., Gado, A., Nassar, A., Abdel-Latif, M., Ghanima, I.A., Abd El-Hack, M., Swelum, A. and Saadeldin, I. 2019. Effect of experimental Ornithobacterium rhinotracheale infection along with live infectious bronchitis vaccination in broiler chickens. Poult. Sci. 98(1), 105–111. Erganis, O., Hadimli, H., Kav, K., Sayin, Z. and Aras, Z. 2010. Production and development of vaccines for Ornithobacterium rhinotracheale infection in Turkeys. Eurasian J. Vet. Sci. 26(2), 101–107. Espinosa, I., Colas, M., Vichi, J., Báez, M. and Martínez, S. 2011. Isolation and identification of Ornithobacterium rhinotracheale from laying hens in farms of la Habana Province. Rev. Salud Anim. 33, 38–43. Gavrilović, P., Gavrilović, A., Vidanović, D., Parunović, J. and Jovanović, M. 2016. Comparative pathomorphological, bacteriological and serological examination of broiler breeders and pheasants experimentally infected with Ornithobacterium rhinotracheale. Avian Pathol. 45(5), 513–519. Ghanbarpour, R. and Salehi, M. 2009. Sero-prevalence and identification of Ornithobacterium rhinotracheale in broiler flocks in south-eastern Iran. Trop. Anim. Health Prod. 41, 1679. Ghasemipour, N., Goudarzi, H., Banani, M. and Asasi K. 2020. Comparison of the first Iranian native Ornithobacterium rhinotracheale vaccine with conventional vaccine: a challenge study. Vet. World 13(4), 655–660. Goovaerts, D., Vrijenhoek, M. and van Empel, P.C.M. 1998. Immuno-histochemical and bacteriological investigation of the pathogenesis of Ornithobacterium rhinotracheale infection in South Africa in chickens with osteitis and encephalitis syndrome. In Proceedings of the 16th meeting of the European Society of Veterinary Pathology, Lillehammer, Norway, pp: 81. Gopala Krishna Murthy, T.R., Dorairajan, N., Balasubramaniam, G.A., Manicavasaka Dinakaran, A. and Kalaimathi, R. 2007. The effect of vaccination of pullets against Ornithobacterium rhinotracheale infection. Avian Pathol. 36, 481–485. Gornatti Churria, C.D., Vigo, G.B., Machuca, M.A., Nievas, V.F., Nievas, W.D., Nievas, W.D., Píscopo, M.V., Herrero Loyola, M.A. and Petruccelli, M.A. 2013. Vaccines against Ornithobacterium rhinotracheale: a review. J. Vet. Sci. Med. Diagn. 2, 4. Ha, H.J., Christensen, N., Humphrey, S., Haydon, T., Bernardi, G. and Rawdon, T. 2016. The first detection of Ornithobacterium rhinotracheale in New Zealand. Avian Dis. 60, 856–859. Hafez, H. M. 1996. Current status on the role of Ornithobacterium rhinotracheale (ORT) in respiratory disease complexes in poultry. Arch. Fur Gelfugelkunde 61, 208–211. Hafez, H.M. 1997a. Serologic surveillance on Ornithobacterium rhinotracheale “ORT” in broiler breeder flocks. In Proceedings of the XIth International congress of the World Veterinary Poultry Association, Budapest, Hungary, pp: 331. Hafez, H.M. 1997b. Ornithobacterium rhinotracheale (ORT). In Putenkrankheiten. Eds., Hafez, H. M. and Jodars, S. Stuttgart, Germany: Ferdinand Enke Verlag, pp: 62–66. Hafez, H.M. 1998. Current status on the laboratory diagnosis of Ornithobacterium rhinotracheale “ORT” in poultry. Berl. Münch. Tierärztl. Wochenschr. 111, 143–145. Hafez, M.H. 2002. Diagnosis of Ornithobacterium rhinotracheale. Int. J. Poult. Sci. 1, 114–118. Hafez, H.M. and Beyer, W. 1997. Preliminary investigation on Ornithobacterium rhinotracheale (ORT) isolates using PCR- fingerprints. In Proceedings of the XIth International Congress of the World Veterinary Poultry Association, Budapest, Hungary, pp: 51. Hafez, M.H. and Schulze, D. 2003. Examinations on the efficacy of chemical disinfectants on Ornithobacterium rhinotracheale in Vitro. Arch. Fur Gelfugelkunde 67, 153–156. Hafez, H.M. and Sting, R. 1996. Serological surveillance on Ornithobacterium rhinotracheale in poultry flocks using self-made ELISA. In Proceedings of 45th Western Poultry Disease Conference, Cancun, Mexico, pp: 163–164. Hafez, H.M., Kruse, W., Emele, J. and Sting, R. 1993. Eine Atemwegsinfektion bei Mastputen durch Pasteurella-ähnliche Erreger: Klinik, Diagnostik und Therapie. In Proceedings of the International Conference on Poultry Diseases. Potsdam: German Veterinary Medical Society (DVG), pp: 105–112. Hafez, H.M. and Vandamme, P. 2011. Ornithobacterium Vandamme GXXVI. Segers, Vancanneyt, Van Hove, Mutters, Hommez, Dewhirst, Paster, Kersters, Falsen, Devriese, Bisgaard, Hinz and Mannheim 1994, 35VP. In Bergey’s manual of systematic bacteriology. Eds., Krieg, N.R., Stanley, J.T., Brown, D.R., Hedlund, B.J., Paster, N.L., et al.,.. 2nd ed. New York, NY: Springer, pp: 250–314. Hafez, H.M., Sting, R., Jodas, S. and Stadler, A. 1998. Chlamydia psittaci infections in meat turkey: investigations on the interaction with other avian infectious agents. In Proceedings of the 1st International Symposium on Turkey Diseases, Berlin, Germany, pp: 208–217. Hassan, A.K., Medhat, M., Shehata, M.A. and Bakheet AA. 2020. Phenotypic and molecular characterization of Ornithobacterium rhinotracheale isolates in broiler chickens. J. Adv. Vet. Res. 10(4), 193–199. Hassanzadeh, M., Karrimi, V., Fallah N. and Ashrafi, I. 2010. Molecular characterization of Ornithobacterium rhinotracheale isolated from broiler chicken flocks in Iran. Turk. J. Vet. Anim. Sci. 34, 373–378. Hauck, R., Chin, R.P. and Shivaprasad, H. 2015. Retrospective study on the isolation of Ornithobacterium rhinotracheale from chickens and turkeys in Central California: 294 cases (2000–12). Avian Dis. 59(1), 130–137. Heeder, C.J., Lopes, V.C., Nagaraja, K.V., Shaw, D.P. and Halvorson, D.A. 2001. Seroprevalence of Ornithobacterium rhinotracheale infection in commercial laying hens in the north central region of the United States. Avian Dis. 45, 1064–1067. Hegazy, A.M., Hassanin, O. and Ismaeil, G.I. 2015. An experimental co-infection of broilers with local isolates of Ornithobacterium rhinotracheale and Escherichia coli. Zag. Vet. J. 43, 82–94. Hinz, K.H., Blome, C. and Ryll. M. 1994. Acute exudative pneumonia and airsacculitis associated with Ornithobacterium rhinotracheale in turkeys. Vet. Rec. 135, 233–234. Hung, A.L. and Alvarado, A. 2001. Phenotypic and molecular characterization of isolates of Ornithobacterium rhinotracheale from Peru. Avian Dis. 45, 999–1005. Joubert, P., Higgins, R., Laperle, A., Mikaelian, I., Venne, D. and Silim A. 1999. Isolation of Ornithobacterium rhinotracheale from turkeys in Quebec, Canada. Avian Dis. 43, 622–626. Karimi-Dehkordi, M., Hashemi, H., Haj Salehi, M., Golami-Ahangaran, M. and Ahmadi-Dastgerdi, A. 2021. The frequency and tissue distribution of Ornithobacterium rhinotracheale in different organs of turkeys. Infect. Epidemiol. Microbiol. 7(1), 45–51. Kastelic, S., Beřcǐc, R.L., Cizelj, I., Beňcina, M., Makrai, L., Zorman-Rojs, O., Narat, M., Bisgaard, M., Christensen, H. and Beňcina, D. 2013. Ornithobacterium rhinotracheale has neuraminidade activity causing desialylation of chicken and turkey serum and tracheal mucus glycoproteins. Vet. Microbiol. 162, 707–712. Kilic, A., Timurkaan, N., Ertaş, H.B. and Yilmaz, F. 2009. Pathological examination and bacterial reisolation by culture and PCR of experimental Ornithobacterium rhinotracheale infection in broiler chickens. Revue Méd. Vét. 160(3), 140–144. Koga, Y. and Zavaleta, A.I. 2005. Intraspecies genetic variability of Ornithobacterium rhinotracheale in commercial birds in Peru. Avian Dis. 49, 108–111. Kursa, O., Tomczyk, G., Sawicka-Durkalec, A., Giza, A. and Słomiany-Szwarc, M. 2021. Bacterial communities of the upper respiratory tract of turkeys. Sci. Rep. 11, 2544. LeÂorat, J., Dudouyt, J., DoreÂ, C. and Gardin, Y. 1994. Ornithobacterium rhinotracheale: une nouvelle raison de tousser. FilieÁres Avicoles, 559, 69–70. Li, Y.Y. and Diao, Y.X. 2009. PCR method for Ornithobacterium rhinotracheale detection. Fujian J. Agric. Sci. 1, 19–23. Lopes, V.C., Back, A., Shin, H.J., Halvorson, D.A. and Nagaraja, K.V. 2002. Development, characterization, and preliminary evaluation of a temperature-sensitive mutant live vaccine in turkeys. Avian Dis. 46, 162–168. Malik, Y.S., Olsen, K., Kumar, K. and Goyal, S.M. 2003. In vitro antibiotic resistance profiles of Ornithobacterium rhinotracheale strains isolated from Minnesota turkeys during 1996–2002. Avian Dis. 47, 588–593. Marien, M., Decostere, A., Martel, A., Chiers, K., Froyman, R. and Nauwynck, H. 2005. Synergy between avian pneumovirus and Ornithobacterium rhinotracheale in turkeys. Avian Pathol. 34, 204–211. Marien, M., Decostere, A., Nauwynck, H., Froyman, R., Devriese, L. and Haesebrouck, F. 2006. In vivo selection of reduced enrofloxacin susceptibility in Ornithobacterium rhinotracheale and its resistance-related mutations in gyrA. Microb. Drug Resist. 12, 140–144. Masoud, E.A., El-Banna, H.R., El-Shafei, A.A. and Mahdy, D.M. 2015. Some studies on Ornithobacterium rhinotracheale (ORT) in broiler chickens. Egypt. J. Chem. Environ. Health 1, 653–667. Mayahi, M., Gharibi, D., Ghadimipour, R. and Talazadeh, F. 2016. Isolation, identification and antimicrobial sensitivity of Ornithobacterium rhinotracheale in broilers chicken flocks of Khuzestan, Iran. In Proceedings of Veterinary Research Forum, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran, pp: 341. Mohd-Zain, Z., Jee, T.L. and Jusoff, K. 2008. Phenotypic characteristics, antibiotic susceptibility and pathogenicity of Ornithobacterium rhinotracheale. Wseas Trans. Biol. Biomed. 7, 133–142. Montes de Oca-Jimenez, R., Veja-Sanchez, V., Morales-Erasto, V., Salgado-Miranda, C., Blackall, P.J. and Soriano-Vargas, E. 2018. Phylogenetic relationship of Ornithobacterium rhinotracheale strains. J. Vet. Med. Sci. 80, 869–873. Nagaraja, K., Back, A., Sorenger, S., Rajashekara, G. and Halvorson, D. 1998. Tissue distribution post-infection and antimicrobal sensitivity of Ornithobacterium rhinotracheale. In Proceedings of the 47th Western Poultry Disease Conference, Sacramento, CA, pp: 57–60. Numee, S., Hauck, R. and Hafez, H.M. 2012. Influence of different storage media, temperatures and time duration on susceptibility of Ornithobacterium rhinotracheale. Pak. Vet. J. 32, 438–442. Odor, E.M., Salem, M., Pop, C.R., Sample, B., Prim, M., Vance, K. and Murphy, M. 1997. Isolation and identification of Ornithobacterium rhinotracheale from commercial broiler flocks on the Delmarva Peninsula. Avian Dis. 41, 257–260. Ozbey, G., Ongor, H., Balik, D.T., Celik, V., Kilic, A. and Muz, A. 2004. Investigation on Ornithobacterium rhinotracheale in broiler flocks in Elazig province located in the east of Turkey. Vet. Med. Czech. 49, 305–311. Pan, Q., Liu, A. and He, C. 2012b. Co-infection of Ornithobacterium rhinotracheale with Streptococcus zooepidemicus. Avian Dis. 56, 680–684. Pan, Q., Liu, A., Zhang, F., Ling, Y., Ou, C., Hou, N. and He, C. 2012a. Co-infection of broilers with Ornithobacterium rhinotracheale and H9N2 avian influenza virus. BMC Vet. Res. 8, 104. Post, K.W., Murphy, S.C. Boyette, J.B. and Resseguie, P.M. 1999. Evaluation of a commercial system for the identification of Ornithobacterium rhinotracheale. J. Vet. Diagn. Invest. 11, 97–99. Rahimi, M. and Banani, M. 2007. Isolation of Ornithobacterium rhinotracheale from the chickens of a broiler farm in Kermanshah province, west of Iran. Iran. J. Vet. Res. 8, 355–359. Roger, MF. and LeÂorat, J. 1997. AÁ l’ origine de troubles respiratoires chez la dinde: Ornithobacterium rhinotracheale est mieux maõÃtriseÂ. FilieÁre Avicole Juin. 62–63. Ryll, M., Hinz, K.H., Salisch, H. and Kruse, W. 1996. Pathogenicity of Ornithobacterium rhinotracheale for turkey poults under experimental conditions. Vet. Rec. 137, 19. Ryll, M., Hinz, K-H., Neumann, U., LoÈhren, U., SuÈdbeck, M. and Steinhagen, D. 1997. Pilot study on the prevalence of the Ornithobacterium rhinotracheale infection in meat-type chickens in Northwest Germany. Berl. MuÈnch. TieraÈrztl. Wochenschr. 110, 267–271. Ryll, M., Günther, R., Hafez, M.H. and Hinz, K.H. 2002. Isolierung und Differenzierung eines Cytochromoxidase-negativen Ornithobacterium rhinotracheale-stamms aus Puten. Berl. Münch. Tierärztl. Wochenschr. 115, 274–277. Roepke, D.C., Back, A., Shaw, D.P., Nagaraja, K.V., Sprenger, S.J. and Halvorson, D.A. 1998. Isolation and identification of Ornithobacterium rhinotracheale from commercial turkey flocks in the upper Midwest. Avian Dis. 42, 219–221. Roussan, D.A., Al-Rifai, R.H., Khawldeh, G.Y., Totanji, W.S. and Shaheen, I. 2011. Ornithobacterium rhinotracheale and Mycoplasma synoviae in broiler chickens in Jordan. Rev. Sci. Tech. 30, 931–937. Salem, M., Odor, E., Sample, B., Murphy, M. and Franz, G. 1997. Ornithobacterium rhinotracheale, update and field survey in the Delmarva Peninsula. In Proceedings of the 46th Western Poultry Disease Conference, Sacramento, CA, pp: 59–60. Schuijffel, D.F., Van Empel, P.C., Pennings, A.M., Van Putten, J.P. and Nuijten, P.J. 2005. Successful selection of cross-protective vaccine candidates for Ornithobacterium rhinotracheale infection. Infect. Immun. 73(10), 6812–6821. Schuijffel, D.F., Van Empel, P.C., Segers, R.P, Van Putten, J.P. and Nuijten, P.J. 2006. Vaccine potential of recombinant Ornithobacterium rhinotracheale antigens. Vaccine 24(11), 1858–1867. Shahata, M.A., Abd El-Motelib, T.Y. and Hebat-Allah, A.M. 2006. Some studies on the incidence of Ornithobacterium rhinotracheale infection in chicken embryos and layers. Assiut Vet. Med. J. 52, 243–257 Shihata, A.B. and Ibraheem, O.B. 2004. Ornithobacterium rhinotracheale (ORT) in some birds and rabbits at Sharkia Governorate. In Proceedings of the 6th Scientific Conference, Egyptian Veterinary Poultry Association, pp: 288–298. Siddique, M., Zia, T. and Rehman, S.U. 2008. Outbreak of Ornithobacterium rhinotracheale (ORT) infection in chickens in Pakistan. Archiv für Geflügelkunde Eur. Poult. Sci. 72, 202–206. Soriano, V.E., Vera, N.A., Salado, C.R., Fernández, R.P. and Blackall, P.J. 2003. In vitro susceptibility of Ornithobacterium rhinotracheale to several antimicrobial drugs. Avian Dis. 47, 476–480. Szabó, R., Wehmann, E., Makrai, L., Nemes, C., Gyuris, É., Thuma, Á.and Maqyar, T. 2017. Characterization of Ornithobacterium rhinotracheale field isolates from Hungary. Avian Pathol. 46, 506–514. Tahseen A. 1997. Ornithobacterium rhinotracheale developing into a serious infection. World Poult. Misset 13, 47–48. Tanyi, J., Bisty, K.A., Kaszanyitzky, E., VeteÂsi, F. and Dobos-KovaÂcs, M. 1995/1996. Isolation of Ornithobacterium rhinotracheale from chickens, hens and turkeys showing respiratory symptoms. Magyar AÂllatorvosok Lapja 50, 328–330. Thieme, S., Hafez, M.H., Gutzer, S., Warkentin, N., Lüschow, D. and Mühldorfer, K. 2016. Multilocus sequence typing of Ornithobacterium rhinotracheale isolated from pigeons and birds of prey revealed new insights into its population structure. Vet. Anim. Sci. 1, 15–20. Travers, A.F. 1996. Concomitant Ornithobacterium rhinotracheale and Newcastle disease infection in broilers in South Africa. Avian Dis. 40, 488–490. Tsai, H.J. and Huang, C.W. 2006. Phenotypic and molecular characterization of isolates of Ornithobacterium rhinotracheale from chickens and pigeons in Taiwan. Avian Dis. 50, 502–507. Türkyilmaz, S. 2005. Isolation and serotyping of Ornithobacterium rhinotracheale from poultry. Turk. J. Vet. Anim. Sci. 29, 1299–1304. Umali, D., Shirota, K., Sasai, K. and Katoh, H. 2017. Characterization of Ornithobacterium rhinotracheale from commercial layer chickens in Eastern Jpn. Poult. Sci. 97(1), 24–29. van Beek, P., van Empel, P.C.M., van den Bosch, G., Storm, P., Bongers, J. and du Preez, J. 1994. Ademhalingsproblemen, groeivertraging en gewrichtsontsteking bij kalkoenen en vleeskuikens door een Pasteurella-achtige bacterie: Ornithobacterium rhinotracheal e of Taxon 28. Tijdschr. Diergeneeskd. 119, 99–101. Vandamme, P., Segers, P., Vancanneyt, M., van Hove, K., Mutters, R., Hommez, J., Dewhirst, F., Paster, B., Kersters, K., Falsen, E., Devriese, L.A., Bisgaard, M., Hinz, K.H. and Mannheim, W. 1994. Ornithobacterium rhinotracheale gen. nov., sp. nov., isolated from the avian respiratory tract. Int. J. Syst. Bacteriol. 44, 24–37. van den Bosch, H. 2001. Ornithobacterium rhinotracheale: the current status. In Proceedings of the 24th Technical Turkey Conference, pp: 1. van Empel, P.C.M. 1997. Ornithobacterium rhinotracheale: an update. In Proceedings of the 52nd meeting of the Fachgruppe ‘Geflugelkrankheiten’ der Deutsche VeterinaÈr-medizinische Gesellschaft, Hannover, Germany, pp: 20–25. van Empel, P.C.M. 1998. Ornithobacterium rhinotracheale: current status and control. In Proceedings of the 1st International Symposium on Turkey Diseases, Berlin, Germany, pp: 129–137. van Empel, P. C. and Hafez, H.M. 1999. Ornithobacterium rhinotracheale: a review. Avian Pathol. 28, 217–227. van Empel, P. and van den Bosch, H. 1998. Vaccination of chickens against Ornithobacterium rhinotracheale infection. Avian Dis. 42, 572–578. van Empel, P., van den Bosch, H., Goovaerts, D. and Storm, P. 1996. Experimental infection in turkeys and chickens with Ornithobacterium rhinotracheale. Avian Dis. 40, 858–864. van Empel, P.C.M., van den Bosch, H., Loeffen, P. and Storm, P. 1997. Identification and serotyping of Ornithobacterium rhinotracheale. J. Clin. Microbiol. 35, 418–421. van Veen, L., Hartman, E. and Fabri, T. 2001. In vitro antibiotic sensitivity of strains of Ornithobacterium rhinotracheale isolated in the Netherlands between 1996 and 1999. Vet. Rec. 149, 611–613. Veiga, I.M.B., Lüschow, D., Gutzer, S., Hafez H.M. and Mühldorfer, K. 2019. Phylogenetic relationship of Ornithobacterium rhinotracheale isolated from poultry and diverse avian hosts based on 16S rRNA and rpoB gene analyses. BMC Microbiol. 19, 31. Walters, J.N. 2014 Characterization of atypical hemolytic Ornithobacterium rhinotracheale Isolates and comparison with the normal non-hemolytic phenotype. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, 120 p. Watteyn, A., Devreese, M., Plessers, E., Wyns, H., Garmyn, A., Reddy, V.R., Pasmans, F., Martel, A., Haesebrouck, F. and De Backer, P. 2016. Efficacy of gamithromycin against Ornithobacterium rhinotracheale in turkey poults pre-infected with avian metapneumovirus. Avian Pathol. 45(5), 545–551. Welchman, D.B., Ainsworth, H.L., Jensen, T.K., Boye, M., King, S.A., Koylass, M.S., Whatmore, A.M., Manvell, R.J., Ayling, R.D. and Dalton, J.R. 2013. Demonstration of Ornithobacterium rhinotracheale in pheasants (Phasianus colchicus) with pneumonia and airsacculitis. Avian Pathol. 42, 171–178. World Organization for Animal Health, 2018. O.I.E-listed diseases, infections and infestations in force in 2018. Paris, France: OIE. Wu, H., Diao, Y., Li, Y., Li, J., Sun, J., Liu, X. and Chen, L. 2010. Isolation and identification of Ornithobacterium rhinotracheale. J. Northwest Univ. 38, 1–6. Youssef, N.M.A. and Ahmed, M.H.H. 1996. Serological studies on flocks showing depressed egg production. Vet. Med. J. Giza 44, 719–726. Zahra, M., Ferreri, M., Alkasir, R., Yin, J., Han, B. and Su, J. 2013. Characterization of small-colony variants of Ornithobacterium rhinotracheale. J. Appl. Microbiol. 51, 3228–3236. | ||
| How to Cite this Article |
| Pubmed Style Wafaa A. Abd El-Ghany. An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease. Open Vet J. 2021; 11(4): 555-568. doi:10.5455/OVJ.2021.v11.i4.5 Web Style Wafaa A. Abd El-Ghany. An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease. https://www.openveterinaryjournal.com/?mno=77590 [Access: April 19, 2024]. doi:10.5455/OVJ.2021.v11.i4.5 AMA (American Medical Association) Style Wafaa A. Abd El-Ghany. An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease. Open Vet J. 2021; 11(4): 555-568. doi:10.5455/OVJ.2021.v11.i4.5 Vancouver/ICMJE Style Wafaa A. Abd El-Ghany. An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease. Open Vet J. (2021), [cited April 19, 2024]; 11(4): 555-568. doi:10.5455/OVJ.2021.v11.i4.5 Harvard Style Wafaa A. Abd El-Ghany (2021) An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease. Open Vet J, 11 (4), 555-568. doi:10.5455/OVJ.2021.v11.i4.5 Turabian Style Wafaa A. Abd El-Ghany. 2021. An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease. Open Veterinary Journal, 11 (4), 555-568. doi:10.5455/OVJ.2021.v11.i4.5 Chicago Style Wafaa A. Abd El-Ghany. "An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease." Open Veterinary Journal 11 (2021), 555-568. doi:10.5455/OVJ.2021.v11.i4.5 MLA (The Modern Language Association) Style Wafaa A. Abd El-Ghany. "An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease." Open Veterinary Journal 11.4 (2021), 555-568. Print. doi:10.5455/OVJ.2021.v11.i4.5 APA (American Psychological Association) Style Wafaa A. Abd El-Ghany (2021) An updated comprehensive review on ornithobacteriosis: A worldwide emerging avian respiratory disease. Open Veterinary Journal, 11 (4), 555-568. doi:10.5455/OVJ.2021.v11.i4.5 |