| Original Article | ||
Open Vet J. 2021; 11(3): 471-482 Open Veterinary Journal, (2021), Vol. 11(3): 471–482 Original Research Dosage variability of veterinary drug products, containing furosemide, linked to tablet splittingLauretta Maggi1, Valeria Friuli1*, Paola Perugini1, Giorgio Musitelli1 and Luigi Venco21Department of Pharmaceutical Sciences, University of Pavia, Pavia, Italy 2Clinica Veterinaria Lago Maggiore, Dormelletto, Italy *Corresponding Author: Dr Valeria Friuli. Department of Pharmaceutical Sciences, University of Pavia, Pavia, Italy. Email: valeria.friuli [at] unipv.it Submitted: 28/04/2021 Accepted: 22/08/2021 Published: 09/09/2021 © 2021 Open Veterinary Journal
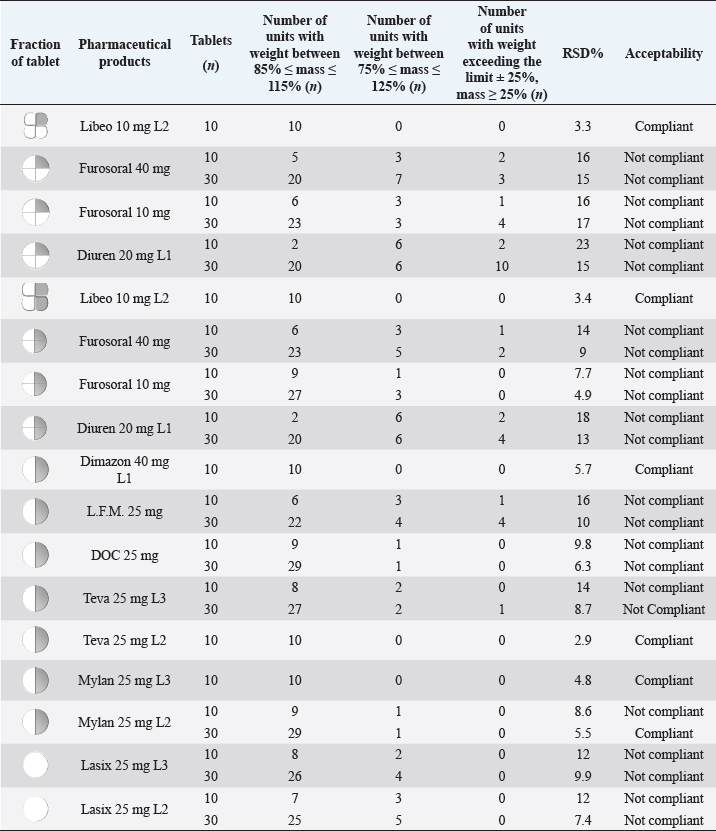
AbstractBackground: Furosemide is a potent diuretic drug widely used to treat congestive heart failure in dogs and cats, but it shows remarkable variability in bioavailability and efficacy when administered orally. In particular, a different diuretic effect can be detected after repeated administrations of the same medicinal product in the same animal. For this reason, we investigate the possible reasons for this peculiar behavior. Drug products for veterinary andhuman use are compared in terms of variability for tablet splitting, in vitro dissolution profiles (in different fluids that could simulate the gastrointestinal environment of pets), and drug distribution uniformity. Aim: To study the in vitro performances of drug products in terms of variability. Methods: Five veterinary products and five products for human use, containing different furosemide doses, are characterized. Tablets splitting uniformity, in vitro dissolution profiles in different fluids that could simulate the gastrointestinal environment of the different species, and drug content distribution, were tested. Results: The in vitro dissolution profiles of the different medicines are comparable but confirm a different dissolution rate as a function of the medium pH and volume. Many of the products considered show wide variability in the division performances of the scored tablets, and this problem could lead to the detected fluctuations in the diuretic effect. The four-leaf clover shape of a veterinary product appears to give rise to more uniform fractions. A uniform distribution of the drug in the tablets and their fractions is confirmed for all the products considered. Conclusion: The possibility of tablets splitting allows considerable dosage flexibility, but a non-uniform break of the tablets to obtain the dosage suitable for the pet’s weight, can cause dangerous over-or sub-dosing condition, especially in critical pathologies and in small breed pets. Keywords: Furosemide, Veterinary drug product, Tablet splitting, Dissolution rate, Dose variability. IntroductionThe administration of a safe and effective dose of a drug for veterinary use is a particularly critical and rather delicate aspect; but this aim is not always easy to obtain when it is necessary to divide a tablet into two or more parts for the treatment of pets, especially those with reduced weight. The division of tablets often involves problems of variability in the dosage and loss of mass (Freeman et al., 2012; Seong et al., 2019). It is, therefore, a practice generally not recommended for tablets without score marks and even prohibited in particular cases such as: coated tablets, modified release pharmaceutical forms, drugs with a low therapeutic index, with very variable bioavailability, or with a long half-life (Toutain and Koritz, 1997; Verrue et al., 2011; Pellicori and Carubelli, 2017). In chronic therapy, the effect of dose variations may not be particularly evident because, if half a tablet is underdosed, the resulting next half will be correspondingly overdosed, and could compensate for the lack, possibly without causing side effects or accumulation (provided that you divide the tablets one at a time). A completely different result could be obtained in the therapy of acute diseases in which an incomplete dose could lead to a lack of efficacy. In contrast, an excessive dose could cause dangerous side effects (Munar and Singh, 2007). This problem has been studied in human therapy, but it has never been investigated in the veterinary field. The need for dose splitting is rarer in human therapy than in veterinary treatment. Indeed, it is common practice in the veterinary field to market two or at most three divisible dosage forms to cover the wide weight range of the different breeds of dogs (from 2 to 70 kg) for which the dose is not fixed for each subject, but it is expressed in mg per kg of body weight. The division of the tablets into non-uniform parts could be due to the operator’s dexterity or formulation factors (Chou et al., 2013; Baudrit et al., 2016). In some countries, it is a common way to use by derogation of human medicines that are administered to the animal when no veterinary products are available. However, the tablets should be often fragmented to obtain the appropriate dose. This procedure could be quite dangerous because the dosages are often very different, generally much higher for humans products, and are not always whole multiples of the required dose for the pet. While breaking a tablet into two halves may seem easy, dividing it into 3, 4, or more units may even be impractical (Noviasky et al., 2006; Paparella, 2010). In addition, the oral forms for human use are registered based on pharmacokinetic and clinical efficacy studies conducted on humans, and the respective equivalent or generic medicines are validated based on bioequivalence tests in humans. These evaluations may not be suitable for ensuring the same safety, absorption, and bioavailability characteristics in different species (Toutain and Koritz,1997; Fleischer et al., 2008; Apley et al., 2017). These problems, which are related to the different physiology and the different transit time of the gastrointestinal tract (Dressman, 1986; Bourreau et al., 2004; Apley et al., 2013; Weber et al., 2017) become particularly critical for smaller animals, precisely those that need the most precise adjustment of the dose by splitting the tablets even in several pieces. The price of the pharmaceutical product is generally more linked to the production of the dosage unit rather than the content of active molecule: for many drugs, 10 tablets containing 10 mg of active have a price comparable to 10 units containing 20 mg of active (Bachynsky et al., 2002). The cost of the veterinary medicinal product is also higher because the market is significantly smaller than that of the medicinal product for human use. Therefore, the amortization of development and research costs have a greater impact on prices. The regulatory bodies have dealt with both the problem of species difference by proposing specific controls for veterinary pharmaceutical forms (Apley et al., 2013; Martinez et al., 2013) and the problem of dosage uniformity linked to the division of the tablets [Uniformity of Dosage Units <905>, USP43-NF38, 2020; Monograph 2.9.40, PhEur 9.1, 2017; Uniformity of Dosage Units 6.02, JP, 2018; (Zaid et al., 2012)]. The different Pharmacopoeias require that all solid pharmaceutical products in single doses (even fractionated tablets) pass the dosage uniformity test. However, the European Pharmacopoeia requires a specific test on the subdivision of scored tablets (Dosage forms/tablets 0478, PhEur 01, 2018). The modification of the dosage unit, which constitutes the medicinal product as it has been authorized, is always a risky practice, but can be especially problematic in emergency situations when it is necessary a particular precision in the definition and administration of the most suitable dosage to produce an effect fast and effective. In this work, we compared several commercial products in tablets, both for veterinary and human use, containing furosemide. The usual oral dose in humans is 20–120 mg in single or divided administrations per day, while in the dog is 1–4 mg/kg twice in 24 hours. This diuretic drug is widely used for its immediate efficacy in the therapy of congestive heart failure in dogs and cats (Bussadori et al., 2002) but it is also characterized by a considerable variability in the intensity of the effect due to its low solubility linked to the medium pH and to a very variable oral bioavailability when administered in the presence of food (Pellicori and Carubelli, 2017). The impact of dividing the tablets on the weight/dose variability of medicinal products for veterinary or human use was then verified. An in vitro dissolution test was also proposed, which is properly modified to better simulate the in vivo conditions found in the dog, gathering the suggestions of the Veterinary Drugs Expert Committee of the US Official Pharmacopoeia (USP) which for many years has been examining and proposing recommendations on the in vitro dissolution test and oral absorption of veterinary products (Apley et al., 2013; Martinez et al., 2013). The novelty of this work is to investigate the reasons for the wide variability in the diuretic effect of furosemide drug products when administered orally, which can be detected even in the same subject. The hypothesis is that the fractionation of the pharmaceutical form to obtain the therapeutic dose appropriate to the size of the pet could cause variability in drug administration. Moreover, we tested the possible differences in the release rate of furosemide from the drug products considered in different conditions that simulate the gastrointestinal tract of animals of different sizes and the possible problems arising from it. Materials and MethodsMaterialsFurosemide (Fabbrica Italiana Sintetici S.p.A., Vicenza, I). Drug products: Lasix® 25 mg (Sanofi-Aventis S.p.A., Milan, I) 3 different batches, coded L1, L2, L3; Furosemide Mylan 25 mg (Mylan S.p.A., Milan, I) 3 different batches, coded L1, L2, L3; Furosemide DOC 25 mg (DOC Generici S.r.l., Milan, I), Furosemide Teva 25 mg (Teva Italia S.r.l., Milan, I) 3 different batches, coded L1, L2, L3; Furosemide L.F.M. 25 mg (Laboratorio Farmacologico Milanese S.r.l., Varese, I), Diuren 20 mg (Teknofarma S.p.A., Turin, I) 2 different batches, coded L1, L2; Dimazon® 40 mg (Intervet International B.V., Boxmeer, NE) 2 different batches, coded L1, L2; Furosoral® 10 and 40 mg (Le Vet Beheer B.V., Utrecht, NE), Libeo® 10 mg (Ceva Salute Animale S.p.A., Agrate Brianza, I), 2 different batches, coded L1, L2. MethodsThe tablets were manually split into two and/or four parts following the score lines on the tablets (only Lasix 25 mg was divided into two halves, although there was no incision). The needed number of whole tablets, for each formulation, were weighed in advance using an analytical balance with 0.1 mg precision (METTLER TOLEDO AE260 DeltaRange®, Milan, I), and the average weight was determined (n=30). They were then manually divided, and the different units were weighed again and photographed (Figs. 1 and 2). The maximum and minimum percentage variation concerning the average weight was reported in the graph (Fig. 3). Uniformity of dosage unitsThe test of uniformity of dosage units was applied, following the United States Pharmacopoeia (Uniformity of Dosage Units <905>, USP43-NF38, 2020), is requested for all the solid pharmaceutical products in single doses, and therefore every split portion of the tablet are considered a single dosage unit. This test is applied in the case of uncoated tablets with an active content ≤ of 25 mg (or when the active represents less than 25% of the total mass) and provides the verification of both the variation in terms of mass and the verification of the uniformity of content in terms of the active ingredient. In this case, 10 units are tested, and if the test fails, another 20 units have been tested. For the Eu. Ph. the specific test for tablet splitting was applied: the weight of only one of the fractions obtained by dividing 30 tablets was considered. Moreover, according to the FDA guidelines (Seong et al., 2019), the percentage of weight loss after splitting was also calculated, measuring the weight difference before and after splitting. This value should not exceed 3 wt% variations concerning the average weight. For both pharmacopeias, the product is considered compliant if no more than one (fractional) unit is within the range of the expected average weight ± 15% (85% ≤ mass ≤ 115%), but, at the same time, no unit can be outside the range of the average weight ± 25% (75% ≤ mass < 125%) and, in any case, it is non-compliant if more than one unit has a weight outside the average weight ± 25% (75% ≤ mass ≤ 125%) (Uniformity of Dosage Units <905>, USP43-NF38, 2020; Dosage forms/tablets 0478, PhEur 01, 2018). The limits are the same for the two pharmacopeias, but the difference is that the USP considered the theoretical drug content instead of the weight. Moreover, USP has a further request: the relative standard deviation (RSD) should not exceed 6 wt%.
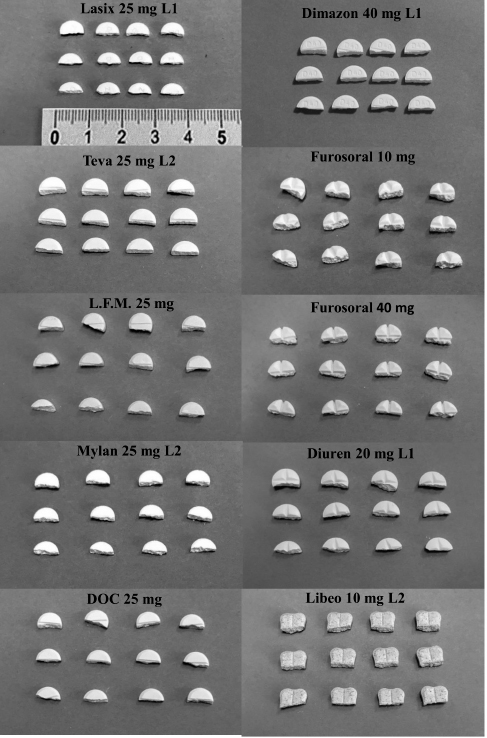
Fig. 1. Tablets split into half of the products: Lasix, Teva, L.F.M., Mylan and DOC 25 mg for human use and Dimazon 40 mg, Furosoral 10 and 40 mg, Diuren 20 mg and Libeo 10 mg for veterinary use.
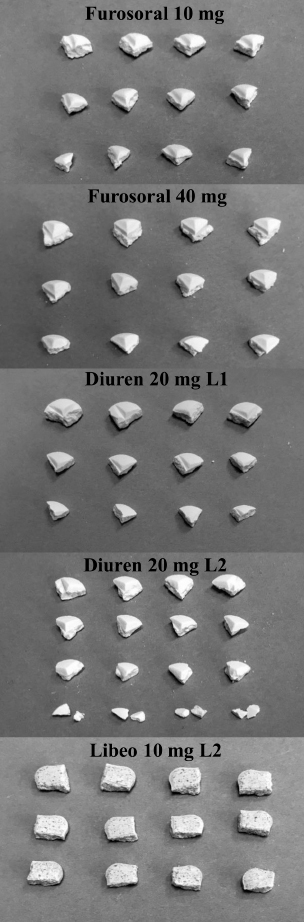
Fig. 2. Four-quarter-split tablets of veterinary products: Furosoral 10 and 40 mg, two different batches of Diuren 20 mg and Libeo 10 mg. Dissolution testThe in vitro dissolution test was performed according to the method reported in the USP furosemide tablets monography (Furosemide tablets, USP41-NF36, 2018) in 900 ml of phosphate buffer pH 5.8, at 37°C ± 0.5°C, using the dissolution apparatus 2, paddle, (Erweka DT-D6, Dusseldorf) stirring speed: 65 rpm. The active content is determined by spectrophotometry (Lambda 25; Perkin-Elmer, Milan). In this test, to compare reasonably similar doses (remembering that medicines for human and veterinary use are marketed with different doses), we simulated an administration of 20 mg of furosemide for veterinary products and 25 mg in the case of products for human use (6 replicates for each formulation). To compare the two different doses easily, the results were graphically expressed in terms of the percentage of dissolved active (to the labeled dose) as a function of time. A drug must first dissolve to be absorbed, but furosemide is a poorly soluble drug, especially at low pH values. At the same time, its solubility progressively increases with increasing the medium pH. For this reason, the dissolution test was performed in different media simulating different conditions attributable to the gastrointestinal tract: pH 5.8, as required by the US pharmacopeia, hydrochloric solution at pH 1.0 (simulating the condition of an empty stomach), deionized water pH 6.9 (neutral condition), and phosphate buffer pH 7.5 (simulating intestinal environment). Then, the dissolution tests were repeated in the same means, but using a lower volume of 500 ml instead of 900 ml, to better simulate the in vivo conditions of an animal of medium-small size.
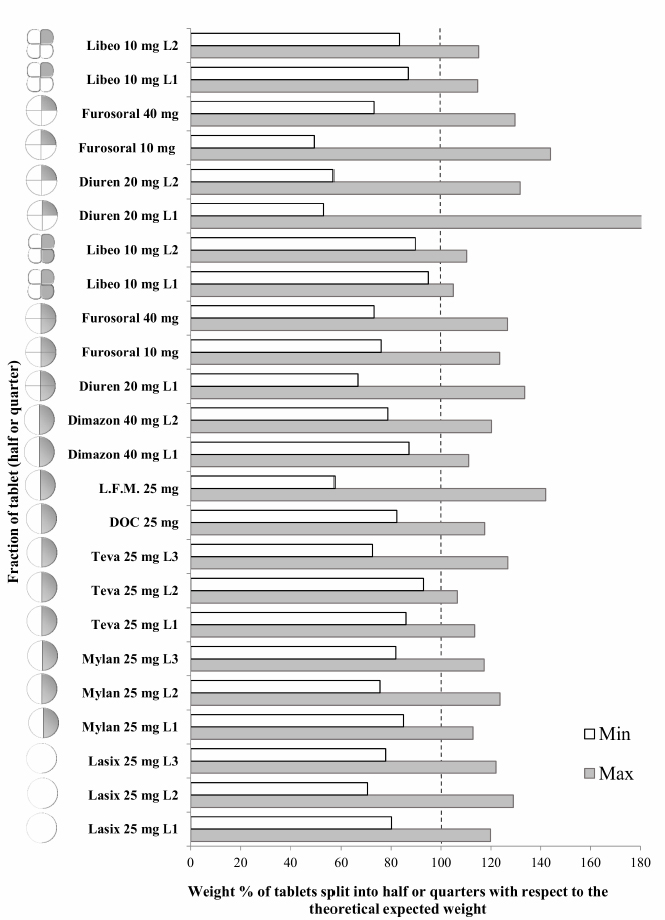
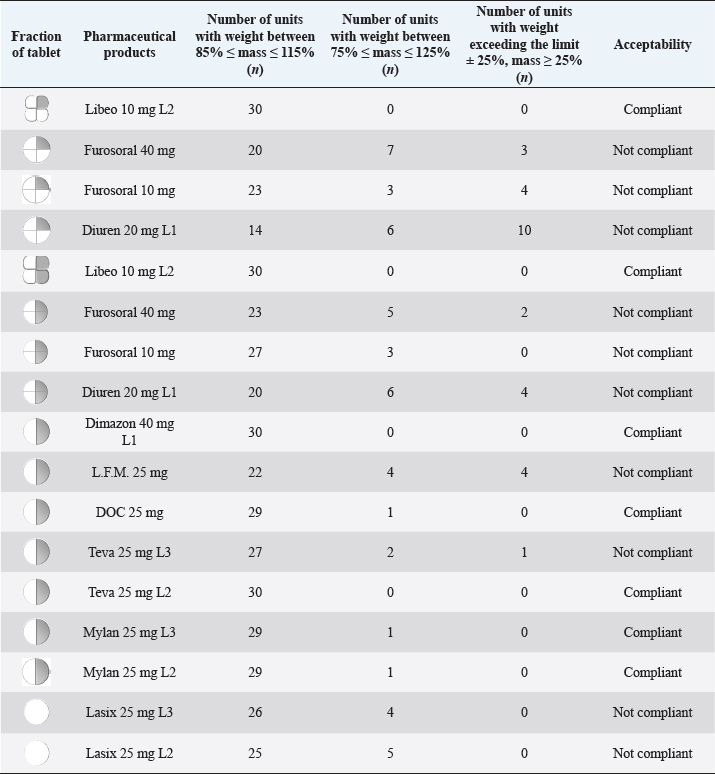
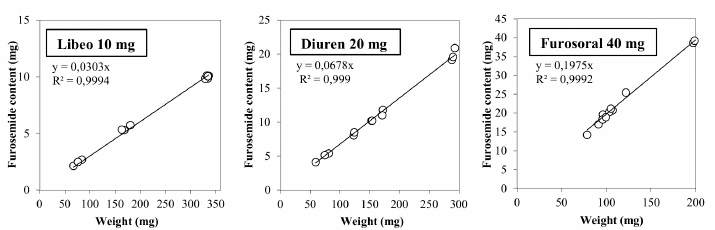
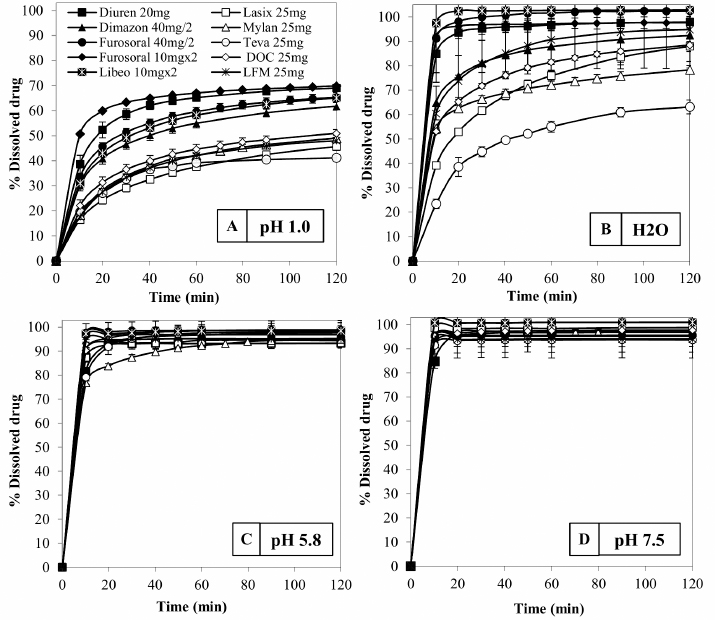
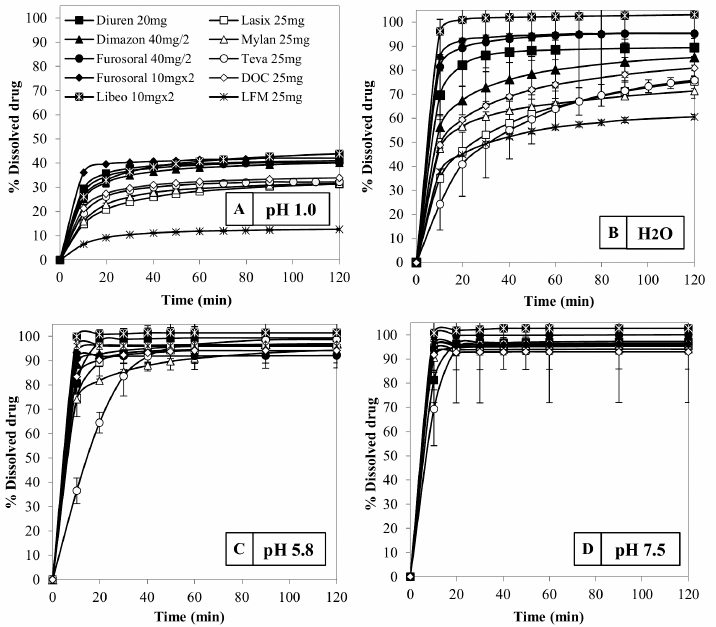
Fig. 3. Comparison of the maximum and minimum percentage change with respect to the average weight, after breaking into 2 or 4 parts of medicinal products for human and veterinary use (Lasix tablets have no incision: they have not been registered to be split). All the dissolution media were prepared following the reagents and buffer solutions section of the USP (Reagents Solutions, USP40-NF35, 2017). Uniformity of contentFinally, to evaluate the uniformity of content (Zaid et al., 2012), the furosemide concentration in the fractions of the tablets divided into two or four pieces was determined by spectrophotometry. The active content is reported in a graph as a function of the weight of the whole or split tablets, and from the linear fitting, we obtain the correlation coefficient between the two variables (n=12). If the active ingredient is uniformly dispersed in the pharmaceutical form, the correlation coefficient approaches unity. Ethical approvalNo animals were used in this study. ResultsThe images show some examples of the fractions obtained after tablet splitting (Fig. 1). It is evident that for many products, the new units after splitting were not uniform, even worse appears the result of a division into four quarters (Fig. 2). From one tablet of Dimazon 20 mg L2, we obtained many fragments instead of quarters. It should be emphasized that Lasix® 25 mg does not have a score mark for tablet division, and therefore, this product was not registered to be fractionated. It was however included in this study because it is the best-known furosemide commercial product and, in practice, it is improperly, but commonly, divided into several subunits. Figure 3 shows the extremes in weight percentages variations (with respect to the average theoretical weight), i.e., the minimum and maximum weight of the units obtained from the tablet splitting. It can be noted how different production batches can lead to completely different results: the composition being the same, this variability could probably be attributable to the applied compression force during the production process (Freeman et al., 2012; van Riet-Nales et al., 2014). The percentage of weight loss after splitting was always lower than 3% for the half tablets, while in the case of the quartering, only one batch of Diuren 20 mg showed a higher value: 7% since one tablet broke into numerous fragments (Fig. 2). According to the European Pharmacopeia, the splitting test for scored tablets provides a specific method to verify this process, Libeo 10 mg, Dimazon 40 mg, furosemide DOC, and two different lots of Furosemide Mylan 25 mg, are compliant. In contrast, at least one lot of the other products is considered not compliant (Table 1). Also, the results of the USP uniformity test of dosage units (Table 2) confirm that the fractions obtained from tablet splitting are not compliant in many cases. In most cases, the results of the two different methods are comparable, while the product DOC 25 mg passes the Eu.Ph. test, but it does not pass the USP test. In this last case, the further request in terms of RSD reduces the number of compliant products. Definitely satisfying results were obtained concerning the uniformity of content. Figure 4 shows some of the results of the furosemide content detected in the whole tablets and the fractions after splitting into two and four parts. The correlation coefficient, very close to unity, indicates an excellent uniformity in drug distribution into the pharmaceutical forms tested. The dissolution profiles in 900 ml (Fig. 5) of the four fluids considered confirm that this drug shows a remarkable variability of behavior as a function of the pH and the buffering power of the dissolution media. This variability is closely linked to the pH-dependent solubility of this molecule: very low at pH=1 (Fig. 5A), in which the drug saturates in this volume at about 45% of the 25 mg dose (products for human use) and obviously at a higher value, equal to 60% of the 20 mg dose (for veterinary products). It was impossible to compare equal doses due to the impracticability of uniformly fractionating tablets containing different multiples (i.e., in five pieces). The solubility of furosemide is much higher at pH=5.8 (Fig. 5C), fluid prescribed by the monography of the US Pharmacopoeia, and at pH=7.5 (Fig. 5D), which simulates the pH of the intestinal environment and, in these media, all the doses pass into solution in a few minutes from all the products considered. In deionized water (Fig. 5B), which has no buffering power, the dissolution curves separate considerably and allow us to observe that, while maintaining the distinct dichotomy between formulations for human and veterinary use, due to the different content of the active ingredient in the tablets, the veterinary forms show a hierarchy in the speed of dissolution, probably attributable to the different capacity of drug disaggregation/release properties linked to the composition of the different products. Furthermore, as the drug passes into the solution, it lowers the pH value of the medium, thereby depressing the further passage of more drugs into the solution. The initial water pH is 6.9 ± 0.2, while the pH is lower at the end of the dissolution process at 3.9 ± 0.3. This effect is naturally more evident for the tablets with a higher furosemide content: 25 mg (products for human use). As expected, the use of a lower dissolution volume, 500 ml (Fig. 6) that can better simulate the digestive tract of the small animals amplifies the differences mentioned above and further depresses the solubility of furosemide, especially at pH=1, that is in the conditions simulating the administration on an empty stomach (Fig. 6A). In particular, LFM formulation shows a very slow release profile in these conditions. As was already known in the literature (Beermann and Midskov, 1986), the bioavailability of furosemide shows considerable variability depending on the administration in the presence or absence of food which can significantly influence its effect. Bearing all these considerations in mind, deionized water represents a much more neutral dissolution medium than the in vivo conditions. Still, it provides extremely significant information on the behavior of the drug (Fig. 6B). A clear progressive ranking in terms of dissolution rate can be evidenced: faster Libeo 10 mg, followed by the two dosages of Furosoral, then Diuren 20 mg, Dimazon 40 mg/2, DOC 25 mg, Mylan 25 mg Lasix 25 mg Teva 25 mg, and finally LFM 25 mg. However, all the products considered show similar dissolution profiles in the other two buffered fluids used (Fig. 6C and D). Table 1. Control test of tablet splitting according to European Pharmacopoeia.
DiscussionThe drug products considered show a similar behavior in terms of in vitro dissolution profiles but confirm a difference in furosemide solubility and its solution rate as a function of the volume, pH, and buffering capacity of the medium used. This effect is particularly critical in the acid pH that simulates the administration in fasted conditions. Table 2. Evaluation of the weight uniformity of the half or quarters of tablet adopting the USP test of uniformity of dosage units.
The drug results homogeneously dispersed even in the fractionated units. However, the division of the tablets can lead to very variable dosage units in terms of weight and, therefore, of active ingredient content, even if the drug is homogeneously dispersed. Some drug products, on the other hand, show considerable variability, even inter-batch. The tests methods according to USP and EuPh regulations are similar but not always comparable.
Fig. 4. The correlation coefficient between the weight of the tablet or its fractions in 2 or 4 units and the relative content in the active principle of the three veterinary products that have a cross notch: Libeo 10 mg Diuren 20 mg and Furosoral 40 mg.
Fig. 5. Comparison of dissolution profiles of Lasix, Mylan, Teva, DOC, and LFM medicinal products containing 25 mg furosemide for human use. The veterinary medicines Diuren, Dimazon, Furosoral, and Libeo were dosed to obtain 20 mg of active: test performed in a volume of 900 ml (as prescribed by US Pharmacopoeia) in the different fluids considered.
Fig. 6. Comparison of the dissolution profiles of Lasix, Mylan, Teva, DOC, and LFM medicinal products containing 25 mg furosemide for human use. The veterinary medicines Diuren, Dimazon, Furosoral, and Libeo were dosed to obtain 20 mg of active: test performed in a reduced volume of 500 ml (to better simulate administration in pets) in the various fluids considered. One of the main reasons that human medicine is improperly administered to pets is certainly the lower cost. In addition, the purchase of medications containing higher quantities of the drug to be broken up into several units always appears very convenient to the pets owner. However, the use of products for human use, which contain a different multiple of the dose than veterinary ones, are particularly dangerous, because underestimating this aspect can lead to overdosing the drug by as much as 25% (e.g. half a tablet of Lasix 25 mg contains a quarter of a dose more than half a tablet of Diuren 20 mg). This aspect is particularly critical, especially for the animals of lower weight. Dosage variability can be dangerous in hemodynamically unstable subjects and animals of lower weight because they require the most extreme dose adjustment: dividing the tablets into several pieces. Although tablets splitting can be an advantage for dosing flexibility, it can also give rise to considerable imprecision in the actual amount of drug administered and should be discouraged when not strictly necessary. Some of the tablets considered are difficult to break, while Libeo 10 mg has a distinctive, four-leaf clover shape that facilitates the handling and breaking process. This demonstrates a sensitivity by the manufacturer to the problem of dividing tablets (Guidance for Industry, 2013) and the willingness to face it, a priori, during the formulation research and development phase (Quality by Design). However, this attention to the ease of subdivision and compliance could impact the final cost. A careful evaluation during the formulation and production phase of the dosage forms (Quality by Design) could reduce the problems related to the difficulty in handling and breaking the tablets, leading to a lack of uniformity in tablet splitting. Containment of the price of veterinary products could discourage the misuse of medicines for human use. These products generally contain high drug content and should be split, which risks causing a dangerous approximation of the desired dose. Increasing adherence to species specifications would therefore translate into an advantage both for companies and for pets. Furthermore, veterinarians should also pay particular attention to these aspects (especially in small breed dogs in which it is necessary to split the tablet) when they are forced to use drugs for human use, because there are no equivalents on the market for veterinary use, in particular for drugs with a narrow therapeutic index (anti-arrhythmic drugs, anti-epileptics). In these cases, veterinarians should consider other formulations (i.e., syrups) or different routes of administration (i.e., injectable), if available, or make aware the pet owners of the risk linked to tablet splitting and suggest discarding the non-uniform fractions. AcknowledgementsThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Conflict of interestAll authors declare that they have no competing interests. Author contributionLM and LV designed the experiment; VF supervised the execution of the experiment; LM and VF analysed the data; and LM and VF wrote the manuscript in consultation with LV, PP and GM. ReferencesApley, M., Crist, G.B., Gonzalez, M., Hunter, R.P., Martinez, M.N., Modric, S., Papich, M.G., Parr, A.F., Riviere, J.E. and Marques, M.R.C. 2013. Solubility criteria for veterinary drugs-workshop report. In Stimuli to the revision process. Rockville, MD: USP. In, pp: 1–8, vol 39(4). Apley, M., Crist, G.B., Fellner, V., Gonzalez, M., Hunter, R.P., Martinez, M., Messenheimer, J.R., Modric, S., Papich, M., Parr, A., Riviere, J. and Marques, M. 2017. Determination of thermodynamic solubility of active pharmaceutical ingredients for veterinary species: a new USP general chapter. Dissolution Technologies, pp: 36–39. Bachynsky, J., Wiens, C. and Melnychuk, K. 2002. The practice of splitting tablets: cost and therapeutic aspects. Pharmacoeconomics. 20, 339–346. Baudrit, O., Baltodano, E. and Jiménez, L. 2016. Review of the fractionation of medicines available in solid formulations (tablets). World. J. Pharm. Res. 5(8), 91–107. Beermann, B. and Midskov, C. 1986. Reduced bioavailability and effect of furosemide given with food. Eur. J. Clin. Pharmacol. 29(6), 725–727. Bussadori, C., Pradelli, D. and Domenech, O. 2002. Approccio terapeutico alle più comuni cardiopatie del cane e del gatto: ruolo cardine dei farmaci diuretici. Vet. J. 16(3), 85–93. Bourreau, J., Hernot, D., Bailhache, E., Weber, M., Ferchaud, V., Biourge, V., Martin, L., Dumon, H. and Nguyen, P. 2004. Gastric emptying rate is inversely related to body weight in dog breeds of different sizes. J. Nutr. 134(8), 2039S–2041S. Chou, C.Y., Hsu, C.C., Chiang, S.C., Ho, C.C., Chou, C.L., Wu, M.S., Chang, Y.L., Tsai, H.Y., Chen, T.J. and Chou, Y.C. 2013. Association between physician specialty and risk of prescribing inappropriate pill splitting. PLoS. One. 8(7), e70113. Dressman, J.B. 1986. Comparison of canine and human gastrointestinal physiology. Pharm. Res. 3(3), 123–131. Dosage forms/tablets 0478. 2018. European Phamacopoeia, 10th ed. Strasbourg, France: Council of Europe (01/2018:0478), pp: 937–938. Fleischer, S., Sharkey, M., Mealey, K., Ostrander, E.A. and Martinez, M. 2008. Pharmacogenetic and metabolic differences between dog breeds: their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS. J. 10(1), 110–119. Freeman, M.K., White, W. and Iranikhah, M. 2012. Tablet splitting: a review of weight and content uniformity. CPH. 27(5), 341–352. Furosemide tablets/official monographs. 2018. The United States Pharmacopeia (USP41-NF36). Rockville, MD: United States Pharmacopeial Convention, Inc, pp: 1894–1895. Guidance for Industry. 2013. Tablet scoring: nomenclature, labeling, data for evaluation. Silver Spring, MD: Food and Drug Administration, pp: 1–5. Martinez, M.N., Papich, M.G. and Riviere, J.E. 2013. Veterinary application of in vitro dissolution data and the biopharmaceutics classification system. In Stimuli to the revision process. Rockville, MD: USP. Inc, pp: 1–20, vol 30(6). Monograph 2.9.40 uniformity of dosage units. 2017. European Phamacopoeia. 9th ed. Strasbourg, France: Council of Europe (04/2017), pp: 4055–4057. Munar, M.Y. and Singh, H. 2007. Drug dosing adjustments in patients with chronic kidney disease. Am. Fam. Physician. 75, 1487–1496. Noviasky, J., Lo, V., Luft, D.D. and Saseen, J. 2006. Clinical inquiries. Which medications can be split without compromising efficacy and safety? J. Fam. Pract. 55, 707–708. Paparella, S. 2010. Identified safety risks with splitting and crushing oral medications. J. Emerg. Nurs. 36, 156–158. Pellicori, P. and Carubelli, V. 2017. Terapia diuretica nell’insufficienza cardiaca cronica: evidenze, esperienze, prospettive. G. Ital. Cardiol. 18(2), 129–138. Reagents Solutions/Buffer solutions. 2017. The United States Pharmacopeia (USP40-NF35). Rockville, MD: United States Pharmacopeial Convention, Inc, pp: 2409–2411. Seong, S.S., Shin, J.Y., Kim, D., Song, I., Sun, S., Kim, I., Park, S.K. and Ha, D. 2019. The effect of tablet splitting on the mass loss, uniformity, and stability: by hand or splitter? JAASP. 8, 7–14. Toutain, P.L. and Koritz, G.D. 1997. Veterinary drug bioequivalence determination. J. Vet. Pharmacol. Ther. 20(2), 79–90. Uniformity of Dosage Units 6.02. 2018. Japanese Pharmacopoeia. 17th ed. Tokyo, Japan: Yakuji-Nippo. Uniformity of Dosage Units <905>. 2020. The United States Pharmacopeia (USP43-NF38). Rockville, MD: United States Pharmacopeial Convention, Inc., pp: 7183. van Riet-Nales, D.A., Doeve, M.E., Nicia, A.E., Teerenstra, S., Notenboom, K., Hekster, Y.A. and van den Bemt, B.J.F. 2014. The accuracy, precision and sustainability of different techniques for tablet subdivision: breaking by hand and the use of tablet splitters or a kitchen knife. Int. J. Pharm. 466, 44–51. Verrue, C., Mehuys, E., Boussery, K., Remon, J.P. and Petrovic, M. 2011. Tablet-splitting: a common yet not so innocent practice. J. Adv. N. 67(1), 26–32. Weber, M.P., Biourge, V.C. and Nguyen, P.G. 2017. Digestive sensitivity varies according to size of dogs: a review. J. Anim. Physiol. An. N. 101(1), 1–9. Zaid, A.N., Ghoush, A.A. and Al-Ramahi, R. 2012. Evaluation of the discrepancy between the European Pharmacopoeia test and an adopted United States Pharmacopoeia test regarding the weight uniformity of scored tablet halves: is harmonization required? JPST. 66(1), 20–27. | ||
| How to Cite this Article |
| Pubmed Style Maggi L, Friuli V, Perugini P, Musitelli G, Venco L, . Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Vet J. 2021; 11(3): 471-482. doi:10.5455/OVJ.2021.v11.i3.21 Web Style Maggi L, Friuli V, Perugini P, Musitelli G, Venco L, . Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. https://www.openveterinaryjournal.com/?mno=77274 [Access: April 19, 2024]. doi:10.5455/OVJ.2021.v11.i3.21 AMA (American Medical Association) Style Maggi L, Friuli V, Perugini P, Musitelli G, Venco L, . Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Vet J. 2021; 11(3): 471-482. doi:10.5455/OVJ.2021.v11.i3.21 Vancouver/ICMJE Style Maggi L, Friuli V, Perugini P, Musitelli G, Venco L, . Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Vet J. (2021), [cited April 19, 2024]; 11(3): 471-482. doi:10.5455/OVJ.2021.v11.i3.21 Harvard Style Maggi, L., Friuli, V., Perugini, P., Musitelli, G., Venco, L. & (2021) Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Vet J, 11 (3), 471-482. doi:10.5455/OVJ.2021.v11.i3.21 Turabian Style Maggi, Lauretta, Valeria Friuli, Paola Perugini, Giorgio Musitelli, Luigi Venco, and . 2021. Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Veterinary Journal, 11 (3), 471-482. doi:10.5455/OVJ.2021.v11.i3.21 Chicago Style Maggi, Lauretta, Valeria Friuli, Paola Perugini, Giorgio Musitelli, Luigi Venco, and . "Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting." Open Veterinary Journal 11 (2021), 471-482. doi:10.5455/OVJ.2021.v11.i3.21 MLA (The Modern Language Association) Style Maggi, Lauretta, Valeria Friuli, Paola Perugini, Giorgio Musitelli, Luigi Venco, and . "Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting." Open Veterinary Journal 11.3 (2021), 471-482. Print. doi:10.5455/OVJ.2021.v11.i3.21 APA (American Psychological Association) Style Maggi, L., Friuli, V., Perugini, P., Musitelli, G., Venco, L. & (2021) Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Veterinary Journal, 11 (3), 471-482. doi:10.5455/OVJ.2021.v11.i3.21 |