| Case Report | ||
Open Vet J. 2022; 12(5): 618-621 Open Veterinary Journal, (2022), Vol. 12(5): 618–621 Case Report Evaluation of the efficacy of transdermal administration of maropitant in managing vomiting in catsYoucef Boukaache*, Marie-Laurence Ferret, Valérie Delteil-Prévotat Karim Khoukh, Andra Enache, Dorothée Iwaszkiw, Sébastien Bertin and Fabien BrunoDelpech Pharmacy, Paris, France *Corresponding Author: Youcef Boukaache. Delpech Pharmacy, Paris, France. Email: veterinaire [at] delpechparis.com Submitted: 24/01/2022 Accepted: 08/08/2022 Published: 06/09/2022 © 2022 Open Veterinary Journal
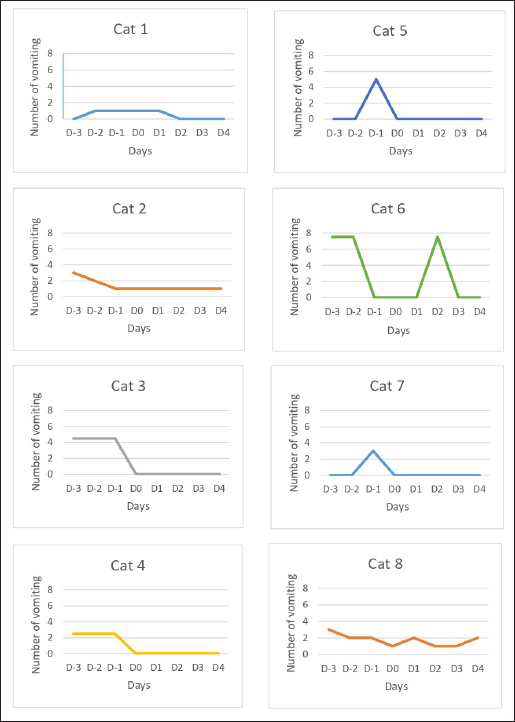
AbstractBackground: Antiemetic maropitant is a widely used medication for treating acute and chronic vomiting in cats. It is available as tablets or injectable solution (Cerenia®). With the oral and injectable routes being especially difficult to pursue in cats experiencing vomiting and nausea, the transdermal administration might be an efficient alternative. The aim of this study was to demonstrate the antiemetic effect of maropitant administered via the transdermal route in cats. Case Description: There were 8 cats enrolled in this study, weighing between 2 and 7 kg, more than 6 months old, and experiencing at least 2 episodes of vomiting in the last 72 hours. Compounded transdermal maropitant was prepared using finely ground Cerenia® tablets, dissolved in propylene glycol and incorporated in the commercial liposomal base Pentravan® (Fagron®, Thiais, France). The uniformity of content was determined using the high performance liquid chromatography (HPLC) method. The product was administered at a dosage of 4 mg/cat once a day (QD), applied on the inner pinna of the ear for five consecutive days. Monitoring and evaluation of vomiting frequency and nausea were performed. A significant decrease in vomiting frequency was observed in 6 of the 8 enrolled cats. A reduction in nausea, associated with an improvement of appetite, was observed in some cases. Conclusion: Transdermal application of maropitant to cats experiencing vomiting seems to be efficient and a good alternative to existing oral medication, taking into account the difficulty of oral administration in these cases. This work provides preliminary clinical results of the efficacy of transdermal maropitant in cats. Further studies are necessary to determine dosing and pharmacokinetics. Keywords: Cat, Compounding, Maropitant, Transdermal, Vomiting. IntroductionVomiting in cats has been associated with a vast range of conditions, categorized into alimentary tract conditions (food sensitivity, gastritis, inflammatory bowel disease, infections, etc.), nonalimentary tract abdominal diseases (hepatobiliary, pancreatic, renal disease, etc.), and systemic diseases (cardiorespiratory, neurologic, metabolic disorders, etc.) (Batchelor et al., 2013). The management of vomiting in cats includes medication and dietary measures. The available antiemetic drugs for cats are phenothiazines (chlorpromazine, prochlorperazine), metoclopramide, selective 5HT3 antagonists (ondansetron, granisetron, and dolasetron), and maropitant (Batchelor et al., 2013). Maropitant is a selective neurokinin 1 receptor antagonist that works by inhibiting the binding of substance P, one of the main neurotransmitters involved in vomiting. It is effective against the nervous and humoral causes of vomiting (Quimby et al., 2015). In cats, maropitant is indicated in the treatment and prevention of vomiting and nausea. It is available as tablets and as an injectable solution, making compliance to the therapy difficult for both the owner and the animal. The transdermal pen is a device that delivers a transdermal base containing an active ingredient of interest, as well as a penetration enhancer, allowing absorption into the blood flow, at precise volume and dose. There are already several molecules which were studied and used in compounded preparations for transdermal administration in cats: mirtazapine (Benson et al., 2016), methimazole (Hoffmann et al., 2003), buspirone (Chávez et al., 2016), amlodipine (Helms 2007), atenolol (Mohamed et al., 2020), fluoxetine (Ciribassi et al., 2003), phenobarbital (Krull et al., 2019), and gabapentin (Slovak and Costa, 2021). The compliance rate for oral forms in chronic diseases is only 76%, which means that 24% of pets are not treated correctly. 60% of pet owners would pay more for drugs with good palatability or easy to administer (American Animal Hospital Association, 2009). The transdermal form meets this demand because of its ease of administration by the owner and its noninvasive character for the animal. Case DetailsThis study was carried out on eight cats. The inclusion criteria were the age of more than 6 months and 2 episodes of vomiting in the 72 hours prior to the transdermal treatment. The selected candidates were cats weighing between 2 and 7 kg, with the mean age of 11.45 years, with a range from 7 months to 19 years. There were five male cats and three female cats. The animals selected for the study were cats received at the veterinary clinics for vomiting related to different pathologies (most often pancreatitis). The compounded transdermal maropitant was made using tablets of Cerenia® finely ground into powder, which was dissolved in propylene glycol and then incorporated into the commercial liposomal base Pentravan® (Fagron®, Thiais, France). The formulation was made by the same compounding pharmacy, under the same conditions of manufacture. The uniformity of the content of the compounded formula was determined using an in-house high performance liquid chromatography (HPLC) method, validated according to the ICH guidelines Q2(R1) for linearity, specificity, accuracy, precision, robustness, and limit of detection / limit of quantification (LOD/LOQ). The transdermal pen (MD® PEN, Medisca®) is a device that comprises a cartridge filled with the cream, which is inserted in a tube that represents the body of the pen. The base of the device must be pressed to deliver the cream through a silicone tip. The device contains 6.5 ml of cream and delivers precise and repeatable doses of 0.1 ml for every pressing of the base. The starting dose used for the transdermal application has been chosen based on the usual oral dose of 1 mg/kg (Hickman et al., 2008). We calculated a median dose of 4 mg/cat to standardize the manufacturing process and the stability conditions of the preparation. The owner or the veterinarian had to administer on the inner pinna of the ear a dose of transdermal cream at 4 mg/cat (concentration of 4 mg/0.1 ml) daily for five consecutive days. The number of vomiting was then quantified by the owner or the veterinarian from day 1 to day 5. Any side effects or additional treatments or tests were also notified. The results show a decrease of 63% in the number of vomiting during the administration of the maropitant transdermal preparation for five consecutive days, as well as a noticeable improvement in appetite. The evolution of vomiting from day -3 to day 5 for the eight cats is detailed in Figure 1. The first cat (Cat 1; Fig. 1) treated with transdermal maropitant experienced two vomiting 72 hours prior to the treatment, continued with one vomiting per day the first 2 days and then the vomiting stopped. Four of the eight cats (Cat 3, Cat 4, Cat 5, and Cat 7; Fig. 1) passed from 13, 7, 5 and 3, respectively, to zero vomiting from day 1 of the maropitant transdermal treatment; another one of the cats (Cat 2; Fig. 1) passed from 6 vomiting in the 72 hours prior to the initiation of maropitant to 1 vomiting per day for the 5 days of the trial. One of the cats (Cat 6; Fig. 1) enrolled passed from 15 episodes of vomiting in the 72 hours prior to the administration of maropitant to 0 vomiting per day in the first 2 days of the treatment; after experiencing 7 vomiting on the third day, the veterinarian decided to increase the dose to 2 applications (one application every 12 hours) and the vomiting episodes decreased to zero per day for the next 2 days. The administration of transdermal maropitant proved no efficacy for one 19-year-old cat (Cat 8; Fig. 1) that experienced 7 vomiting prior to the administration of maropitant and continued with 2 vomiting per day on days 1 and 4 and 1 vomiting per day on days 2 and 3 of the treatment. The cat was reportedly suffering from chronic kidney failure. DiscussionChronic vomiting in cats is one of the most common health concerns experienced by owners nowadays (Sorrell, 2021). In this cases, the oral or injectable routes can prove to be difficult to accomplish, therefore, the transdermal administration appears to be a more easy and suitable solution (Davidson, 2003). This is the first case report studying the efficacy of the transdermal administration of maropitant against vomiting in cats. The transdermal application of maropitant at a dose comparable to the oral dose (4 mg/cat) for five consecutive days resulted in a reduction of vomiting in six of the eight cats included in this report. There were no adverse effects reported for the transdermal administration, which is similar to the studies for the oral route for maropitant (Quimby et al., 2015). The influence of the appetite was subjectively evaluated by the owner and was not objectively quantified; therefore, there are no exact results to show in this study. However, this point could be developed in a future study similarly to the case of mirtazapine (Poole et al., 2019), where the participants could be weighted after the treatment. The amount of the ingested food could be also quantified. In our study, half of the owners found that the appetite increased back to normal after the treatment. This study did not attempt to assess the influence of the adjacent pathologies or physiological state of the animal on the efficacy of transdermal maropitant. Further diagnosis may be needed in cases of chronic vomiting, even though the symptoms can be intermittent. In such cases, the underlying cause must be determined. The therapeutic approach for a cat with chronic vomiting should then be developed based on identified etiology (Hauck et al., 2016). Being a quick-acting, noninvasive, easy to use device and a more convenient alternative to the oral or the injectable medication, the transdermal pen of maropitant may improve the management of both acute and chronic vomiting in cats. However, it is necessary that additional studies determine blood concentrations, bioavailability (with this base), and the duration of action in older cats and in cats with liver and renal deficiencies.
Fig. 1. Evolution of the number of vomiting before and after the administration of transdermal maropitant. Authors’ contributionsYoucef Boukaache: conceptualization, investigation, methodology, software, supervision, writing-original draft, writing-review, and editing. Marie-Laurence Ferret: conceptualization, investigation, methodology, supervision, writing-review, and editing. Valérie Delteil-Prévotat: conceptualization, investigation, methodology, and supervision. Karim Khoukh: conceptualization, investigation, methodology, supervision, writing-review, and editing. Andra Enache: conceptualization, data curation, investigation, methodology, supervision, writing-review, and editing. Dorothée Iwaszkiw: investigation and resources. Sébastien Bertin: formal analysis, methodology, project administration, writing-review, and editing. Fabien Bruno: resources and validation. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe study was financially supported by Pharmacie Delpech. ReferencesAmerican Animal Hospital Association. 2009. Compliance: taking quality care to the next level: a report of the 2009 AAHA compliance follow-up study. Lakewood, CO: American Animal Hospital Association, pp: 8–17. Batchelor, D.J., Devauchelle, P., Elliott, J., Elwood, C.M., Freiche, V., Gualtieri M., Hall, E.J., Den Hertog, E., Neiger, R., Peeters, D., Roura, X., Savary-Bataille, K. and German, A.J. 2013. Mechanisms, causes, investigation and management of vomiting disorders in cats: a literature review. J. Feline Med. Surg. 15(4), 237–265. Benson, K.K., Zajic, L.B., Morgan, P.K., Brown, S.R., Hansen, R.J., Lunghofer, P.J., Wittenburg, L.A., Gustafson, D.L. and Quimby, J.M. 2017. Drug exposure and clinical effect of transdermal mirtazapine in healthy young cats: a pilot study. J. Feline Med. Surg. 19(10), 998–1006. Chávez, G., Pardo, P., Ubilla M.J. and Marín M.P. 2016. Effects on behavioural variables of oral versus transdermal buspirone administration in cats displaying urine marking. J. Appl. Anim. Res. 44(1), 454–457. Ciribassi, J., Luescher, A., Pasloske, K.S., Robertson-Plouch, C., Zimmerman, A. and Kaloostian-Whittymore, L. 2003. Comparative bioavailability of fluoxetine after transdermal and oral administration to healthy cats. Am. J. Vet. Res. 64(8), 994–998. Davidson, G. 2003. Veterinary transdermal medications: A to Z. Int. J. Pharm. Compd. 7(2), 106. Hauck, S.R., Gisselman, K., Cordner, A. and Nicholson, A.G. 2016. Chronic vomiting in cats: etiology and diagnostic testing. J. Am. Anim. Hosp. Assoc. 52(5), 269–276. Helms, S.R. 2007. Treatment of feline hypertension with transdermal amlodipine: a pilot study. J. Am. Anim. Hosp. Assoc. 43(3), 149–156. Hickman, M.A., Cox, S.R., Mahabir, S., Miskell, C., Lin, J., Bunger, A. and McCall, R.B. 2008. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (CereniaTM) for the prevention of emesis and motion sickness in cats. J. Vet. Pharmacol. Ther. 31(3), 220–229. Hoffmann, G., Marks, S.L., Taboada, J., Hosgood, G.L. and Wolfsheimer, K.J. 2003. Transdermal methimazole treatment in cats with hyperthyroidism. J. Feline Med. Surg. 5(2), 77–82. Krull, D.P., Stephanie, A.T., Annie, V.C., Katrina, L.M. and Mark, G.P. 2019. Evaluation of Transdermal Administration of Phenobarbital in Healthy Cats. J. Am. Anim. Hosp. Assoc. 55(1), 1–7. Mohamed, S.M., Christensen, J.M. and LeBlanc, N. 2020. Transdermal formulation development and topical administration of atenolol to cats. Pharmacol. Pharm. 11, 39–53. Poole, M., Quimby, J.M., Hu, T., Labelle, D. and Buhles, W. 2019. A double- blind, placebo- controlled, randomized study to evaluate the weight gain drug, mirtazapine transdermal ointment, in cats with unintended weight loss. J. Vet. Pharmacol. Therap. 42, 179–188. Quimby, J.M., Brock, W.T., Moses, K., Bolotin, D. and Patricelli, K. 2015. Chronic use of maropitant for the management of vomiting and inappetence in cats with chronic kidney disease: a blinded, placebo-controlled clinical trial. J. Feline Med. Surg. 17(8), 692–697. Sorrell, S. 2021. Diagnosing and treating chronic vomiting in cats. In Prac. 43(7), 348–361. Slovak, J.E. and Costa, A.P. 2021. A pilot study of transdermal gabapentin in cats. J Vet Intern Med. 35(4), 1981–1987. | ||
| How to Cite this Article |
| Pubmed Style Boukaache Y, Ferret M, Delteil-Prevotat V, Khoukh K, Enache A, Iwaszkiw D, Bertin S, Bruno F, . Evaluation of the Efficacy of Transdermal Administration of Maropitant in Managing Vomiting in Cats. Open Vet J. 2022; 12(5): 618-621. doi:10.5455/OVJ.2022.v12.i5.4 Web Style Boukaache Y, Ferret M, Delteil-Prevotat V, Khoukh K, Enache A, Iwaszkiw D, Bertin S, Bruno F, . Evaluation of the Efficacy of Transdermal Administration of Maropitant in Managing Vomiting in Cats. https://www.openveterinaryjournal.com/?mno=77125 [Access: April 19, 2024]. doi:10.5455/OVJ.2022.v12.i5.4 AMA (American Medical Association) Style Boukaache Y, Ferret M, Delteil-Prevotat V, Khoukh K, Enache A, Iwaszkiw D, Bertin S, Bruno F, . Evaluation of the Efficacy of Transdermal Administration of
Maropitant in Managing Vomiting in Cats. Open Vet J. 2022; 12(5): 618-621. doi:10.5455/OVJ.2022.v12.i5.4 Vancouver/ICMJE Style Boukaache Y, Ferret M, Delteil-Prevotat V, Khoukh K, Enache A, Iwaszkiw D, Bertin S, Bruno F, . Evaluation of the Efficacy of Transdermal Administration of
Maropitant in Managing Vomiting in Cats. Open Vet J. (2022), [cited April 19, 2024]; 12(5): 618-621. doi:10.5455/OVJ.2022.v12.i5.4 Harvard Style Boukaache, Y., Ferret, M., Delteil-Prevotat, V., Khoukh, K., Enache, A., Iwaszkiw, D., Bertin, S., Bruno, F. & (2022) Evaluation of the Efficacy of Transdermal Administration of
Maropitant in Managing Vomiting in Cats. Open Vet J, 12 (5), 618-621. doi:10.5455/OVJ.2022.v12.i5.4 Turabian Style Boukaache, Youcef, Marie-Laurence Ferret, Valerie Delteil-Prevotat, Karim Khoukh, Andra Enache, Dorothee Iwaszkiw, Sebastien Bertin, Fabien Bruno, and . 2022. Evaluation of the Efficacy of Transdermal Administration of
Maropitant in Managing Vomiting in Cats. Open Veterinary Journal, 12 (5), 618-621. doi:10.5455/OVJ.2022.v12.i5.4 Chicago Style Boukaache, Youcef, Marie-Laurence Ferret, Valerie Delteil-Prevotat, Karim Khoukh, Andra Enache, Dorothee Iwaszkiw, Sebastien Bertin, Fabien Bruno, and . "Evaluation of the Efficacy of Transdermal Administration of
Maropitant in Managing Vomiting in Cats." Open Veterinary Journal 12 (2022), 618-621. doi:10.5455/OVJ.2022.v12.i5.4 MLA (The Modern Language Association) Style Boukaache, Youcef, Marie-Laurence Ferret, Valerie Delteil-Prevotat, Karim Khoukh, Andra Enache, Dorothee Iwaszkiw, Sebastien Bertin, Fabien Bruno, and . "Evaluation of the Efficacy of Transdermal Administration of
Maropitant in Managing Vomiting in Cats." Open Veterinary Journal 12.5 (2022), 618-621. Print. doi:10.5455/OVJ.2022.v12.i5.4 APA (American Psychological Association) Style Boukaache, Y., Ferret, M., Delteil-Prevotat, V., Khoukh, K., Enache, A., Iwaszkiw, D., Bertin, S., Bruno, F. & (2022) Evaluation of the Efficacy of Transdermal Administration of
Maropitant in Managing Vomiting in Cats. Open Veterinary Journal, 12 (5), 618-621. doi:10.5455/OVJ.2022.v12.i5.4 |