| Original Article | ||
Open Vet J. 2023; 13(4): 473-480 Open Veterinary Journal, (2023), Vol. 13(4): 473–480 Original Research Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depressionDimple Shet1, Nayanatara Arun Kumar1, Megha Gokul1, Rekha Durgadas Kini1, Aradhana Marathe2, Sowndarya Kollampare2 and Vandana Blossom31Department of Physiology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India 2Department of Biochemistry, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India 3Department of Anatomy, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India *Corresponding Author: Nayanatara Arun Kumar. Department of Physiology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India. Email: nayanatara.arun [at] manipal.edu Submitted: 29/06/2022 Accepted: 24/03/2023 Published: © 2023 Open Veterinary Journal
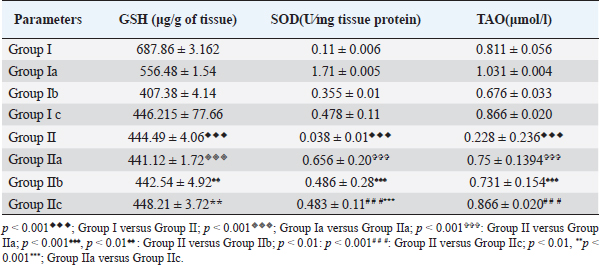
AbstractBackground: Proper nutrition and balanced diet have a profound influence on mental well-being. Nutritional psychiatry plays an important role in influencing a healthy mind and body. The animal model of chronic unpredictable stress has been considered the effective model to explore research on anxiety and depression. Aim: The present study aimed to explore the protective role of cod liver oil on various biochemical and neuronal analyses in the hippocampus tissue of the Wistar rat model of comorbid depression. Methods: Healthy adult albino rats of Wistar strain weighing (120–160 g) were divided into control groups and experimental groups. These groups were further categorized into various subgroups based on stress exposure, cod liver oil, and antidepressant treatment. Six animals were taken in each group. The duration of stress exposure was for 15 days. After the experimentation procedure, the animals were anesthetized and hippocampus was dissected for the estimations of various biochemical and neurological parameters. Results: The combination of cod liver oil with the antidepressant significantly (p < 0.001) decreased the lipid peroxidation level. Total antioxidant (TAO) and superoxide dismutase (SOD) levels significantly increased (p < 0.001) in the hippocampus. Treatment of cod liver oil during the stress exposure increased (p < 0.001) the neuronal count. Conclusion: Cod liver oil proved to be an effective antidepressant agent by increasing the antioxidants and promoting neurogenesis in the hippocampus. Keywords: Depression, Cod liver oil, Antidepressant, Hippocampus, Antioxidants. IntroductionPsychiatric diseases seem to be a burden both at national and global levels, causing an impact on the quality of life (Whiteford et al., 2015). Long-term stressful situations have been linked with depression and anxiety (Yang, 2015). The duration and the incidence of stress periods are essential for the understanding of the neurobiological phenomenon. Food has a significant role in the maintenance of physical and mental health. Recent trends rely on the protective role of the essential constituents of natural compounds present in the diet against disease development. Till date, no effective therapeutic strategy has been found in the management of stress without side effects. Animal models of stress have been used as experimental tools in understanding the human psychopathology. The chronic unpredictable stress model (CUS) is expansively used to study various psychological disorders (Nayanatara et al., 2005, 2014; Gokul et al., 2019). The biochemical and neurological alterations are important biomarkers, which could help to understand and manage stress-induced health issues. The central nervous system modulates responses related to stress by activating various brain regions. Forebrain stress systems have come under considerable scrutiny in recent years (Wilson, 2017). Several forebrain structures, such as hippocampus, prefrontal cortex, and amygdala, are known to influence stress responses (Wilson, 2017). The functional changes in the hippocampus have been directly linked with the stress response (Wilson, 2017). Till date, no individual therapeutic agent has been found to treat stress-induced disturbances targeting distinct brain tissue. Therefore, there is a need for an agent, which is safe and effective in the treatment of adverse stress effects as a crucial priority. Dietary antioxidants have been shown to protect neurons against various neurodegenerative disorders (Wilton, 1995). Food rich in antioxidants and vitamins plays an important role having influencing neurological outcomes. Cod liver oil contains omega-3 fatty acids, eicosapentaenoic acid, docosahexaenoic acid vitamins A and D (Wilton, 1997). Various health benefits have been associated with the cod liver oil intake (Khare et al., 2008; Bazazzadegan et al., 2017). Literature is lacking on the role of cod liver oil on CUS with its impact on hippocampus. The beneficial role of cod liver supplements in various diseased conditions provoked us to focus on the antidepressant role of targeting on brain antioxidant levels and neuronal count in the Wistar rat model of comorbid depression. Materials and MethodsAnimalsHealthy adult albino rats of Wistar strain weighing (120–160 g) were selected for the study. The animals were procured from our institutional animal house, All the animals were housed in polypropylene cages with husk as bedding material and a standard pallet diet and water ad libitum. All the animals were maintained at 25°C ± 2°C temperature with 12 hours of light and dark cycles. Further, rats were provided with ad libitum access to laboratory water and food (commercial rat pellets from VRK Nutritional Solutions, India) CUS procedureCUS procedure included the exposure of animals to varieties of unpredictable stressors at different durations to minimize predictability and habituation (Nayanatara et al., 2005; Nayanatara et al., 2014; Gokul et al., 2019). All the experimental stress procedures were conducted in a separate room next to the room where the rats were housed. After each stressor, animals were given a rest for 1 hour and shifted to their original cages. During restraint stress, the rats were placed in the plexiglass restrainer for 1 hour. In the rotation stress, the rats were placed on the rotating spinner (50 rpm) for 1 hour. During cage wetting stress, the rats were kept in the cage with a wet husk (5 cm high) for 4 hours. In warm and cold swimming stress, the rats were allowed to swim in a cylindrical tank (60 cm height × 30 cm diameter) filled with water at a 30 cm depth at 45°C and 8°C. Footshock (1.5 mA) stress was given for 10 minutes. The alteration in the dark and light cycle was done by placing the rats in dark in the daytime and light at night time. In tail pinch stress, the clothes pin was placed at 2 cm from the base of the tail for 20 minutes. Isolation stress was given by placing single rats in a different home cage for 4 hours. Overcrowding stress was done by placing six to seven rats in a single small cage for 3–4 hours. Cage tilting involved the placement of rats in a cage and tilting at an inclination of 45°. In the heat stress, the rats were exposed to a hot air stream. In cold stress, the rats were exposed to the cold chamber at 8°C for 1 hour. Overnight food and water deprivation were also involved in this stress procedure. Animal groupingAnimals were divided into two major groups, the control groups and experimental groups (n=6; n is the number of animals in each individual group). Control groupThe control groups of animals were not exposed to any type of stress. The control group was subdivided into various subgroups. Normal control group (Group I; n=6)—This group of rats was not exposed to any experimental stress exposure. Cod-liver oil control group (Group Ia; n=6)—These rats were treated with cod liver oil via oral gavage (5 ml/kg body weight) but were not exposed to any stress procedures. Antidepressant control group (Group Ib; n=6)—This group of rats was treated with the antidepressant imipramine orally (5 mg/kg/body weight) but were not exposed to any experimental stress procedures (Bolz, 2015). Cod-liver oil + Antidepressant control group (Group Ic; n=6)—This group of control rats was treated with cod liver oil (5 mg/kg/body weight) and antidepressant (5 mg/kg/body weight). Experimental groupThese rats are exposed to stressors, and based on the treatment, they were subdivided into various subgroups. CUS- (Group II; n=6)—These rats were subjected to 15 days CUS. CUS + Cod liver oil (Group IIa; n=6)—Cod liver oil treatment was given to these rats during CUS exposure. CUS + Antidepressant (Group IIb; n=6)—These rats were treated with imipramine during the exposure to CUS. CUS + Cod liver oil + Antidepressant (Group II c; n=6)—These rats were treated with cod liver oil and imipramine during the exposure to CUS. Dissection of brain tissuesAt the end of the 15 days, the animals were anesthetized (ketamine and xylazine; 50 mg/ml of ketamine and 20 mg/ml of xylazine). Brain dissection was performed as per the method of Glowinski and Iverson (1966). Hippocampus was separated for histological and biochemical analysis. Determination of oxidative stress in the hippocampusLipid peroxidation assayHomogenate of the hippocampus (1 ml) was mixed with 2.5 ml of ice-cold trichloroacetic acid and centrifuged for 10 minutes. 2 ml of the supernatant of the homogenate was mixed with 0.67% of thiobarbituric acid. Serum malondialdehyde (MDA) concentrations were measured (Rao et al., 2000). Reduced glutathione1 ml of the hippocampus homogenate was mixed with 1 ml of metaphosphoric acid and kept for incubation for 5 minutes at room temperature (Koracevic et al., 2001). The sample was centrifuged at 5,000 rpm for 15 minutes at 4°C. Further, 2.7 ml of phosphate buffer and 0.2 ml of 5,5’dithio-bis-nitrobenzoic acid were added to the homogenate. The yellowish color obtained was noted at 412 nm. The values were expressed as μg/g of tissue. Estimation of superoxide dismutase (SOD) activityMarklund and Marklund (1974) method was followed. For the test sample, 0.1 ml of homogenate of hippocampus tissue was treated with 2.5 ml of Tris–HCl buffer of pH 8.2. A kinetic endpoint method was used where the change in the autoxidation of pyrogallol was observed for 3 minutes for each sample at 420 nm. A control sample was run in the same way, without taking tissue homogenate. The total protein concentration for tissue was calculated by Lowry’s method (using the Folin-Ciocalteu reagent). Absorbance was at 420 nm, and the values were expressed as units/minute/mg of tissue protein. Estimation of total antioxidants (TAO)Each sample had its own control in which Fe-EDTA mixture, hydrogen peroxide, and sodium benzoate were added after 20% acetic acid. Negative control was prepared for each series except the sample homogenate was replaced with 0.1 M sodium phosphate buffer, pH 7.4. Uric acid (1 Mm/l) was used as the standard (Martí et al., 1994). The reaction mixture was kept for incubation at 37°C for 60 minutes, then 20% acetic acid and 0.8% thiobarbituric acid (TBA) were added and again kept for incubation for 10 minutes at 100°C and cooled using an ice bath. The absorbance was measured at 532 nm. The TAOs level is expressed as μmol/l. Neuronal assay of the hippocampusThe brain tissue was stored in formalin (10%) and the paraffin blocks were made. Coronal sections of the dentate gyrus (DG), frontal cortex, and hippocampus were prepared and the staining procedure was done with crystal violet stain. The quantitative analysis was performed under light microscopy (Madhyastha et al., 2002). Quantification of neuronsThe quantifiable investigation of the neurons was performed under light microscopy. In the frontal cortex section, the neurons were counted in 300 × 300 µ areas. In the DG, a 150 × 150 µ area was selected for counting. The various regions of Hippocampus were selected for quantifications. Neurons were quantified using imaging software NIS Elements (Blossom et al., 2020). Statistical analysisAll the data were expressed as mean ± standard deviation Student t-test was used to do the comparison between the two groups. p < 0.05 was considered statistically significant. Ethical approvalThe present experimental procedures were reviewed and approved by the Institutional Animal Ethical (IAEC) at Kasturba Medical College, Mangalore (KMC/MNG/IAEC/16-2019). The guidelines proposed by the committee for control and supervision of experimentation on animals, the Government of India, were strictly followed accordingly. ResultsExposure to CUS significantly increased (p < 0.001) the lipid-peroxidation level (Fig. 1). Combination of cod liver oil with the antidepressant significantly decreased (p < 0.001) the lipid peroxidation level when compared to the rats treated with cod liver oil. Further, reduced glutathione, SOD, and the TAO level significantly declined (p < 0.001) in the animals exposed to CUS (Table 1). However, in the treated rats, antioxidant levels and SOD significantly increased (p < 0.001). Neuronal count significantly declined (p < 0.001) in the stressed rats. A significant rise (p < 0.001) in the neuronal count was observed in the treated groups (Figs. 2–6 and Table 2). DiscussionLong-term stress is the prime factor for major and minor health issues. The activation of the stress system leads to disturbances in the normal physiological mechanisms reverting homeostasis. The physiological, biochemical, and neurological alterations have been considered the important biomarkers of stress, helping to understand and manage stress-induced health issues (Stratakis and Chrousos, 1995; Schneiderman et al., 2005; Lucassen et al., 2014). Chronic stress targets brain areas leading to various psychological disorders (Stratakis and Chrousos, 1995; Nayanatara et al., 2011; Nayanatara et al., 2012). Various antidepressants are clinically used for therapeutic use. However, they induce various side effects (Cryan et al., 2002; Bondi et al., 2008). The antidepressant role of imipramine is well documented in the literature and it is also known to be clinically effective. (Katz et al., 1982; Willner et al., 2014). Imipramine inhibits the reuptake of noradrenaline and serotonin, increasing their content in the synapse, and augmenting adrenergic and serotonergic transmission (Katz et al., 1982; Willner et al., 2014). To explore the potential antidepressant role of cod liver oil in this study, we treated a separate group of rats with the antidepressant imipramine.
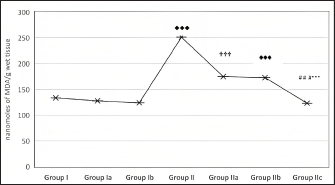
Fig. 1. Effect of CUS on MDA level of the hippocampus in control groups and experimental groups. p < 0.001♦♦♦; Group I versus Group II; p < 0.001✞✞✞: Group II versus Group IIa; p < 0.001♠♠♠: Group II versus Group IIb; p < 0.001# # #: Group II versus Group IIc; p < 0.001***; Group IIa versus Group IIc. Table 1. Effect of CUS on biochemical parameters of the hippocampus in control groups and experimental group.
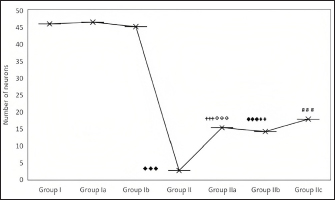
Fig. 2. Effect of CUS on the neuronal count in the DG in control groups and experimental groups. p < 0.001♦♦♦; Group I versus Group II; ◈◈◈; Group Ia versus Group IIa; p < 0.001✞✞✞: Group II versus Group IIa; p < 0.001♠♠♠: Group II versus Group IIb; p < 0.001# # #: Group II versus Group IIc; p < 0.001***; Group IIa versus Group IIc; † † † Group Ic versus Group IIc. In the present study, stress exposure significantly increased lipid-peroxidation, decreased GSH, and decreased SOD and TAO levels in the hippocampus. Stress-mediated hippocampal injury has been linked to various pathological variations (Wilson, 2017). The decline in the antioxidant enzyme activities might be explained using up of antioxidant enzymes developed because of the rise in MDA level following stress exposure. CUS produces functional stimulation of the hypothalamic-pituitary-adrenal (HPA) axis elevating glucocorticoids. Hippocampus has much more glucocorticoid receptors and thus it is more prone to excessive stress (Wilson, 2017). Hippocampal dysfunction has also been directly linked with the etiology of depression (Wilson, 2017). Cod liver oil and imipramine increased the SOD, GSH, and TAO levels in the hippocampus. Further, it also declined the increased lipid peroxidation. The antidepressant role of cod liver oil might be attributed to the antioxidant properties of unsaturated fatty acids in cod liver oil exerting its protective role in decreasing lipid peroxidation and increasing antioxidant activities. The influential role of omega-3 fatty acids on various functions of the brain has been well documented in the literature (Gertsik et al., 2012). Omega-3 fatty acids have been shown beneficial in reducing symptoms of mood disorders (Smith et al., 2011). Cod liver oil is rich in vitamin A and vitamin D, besides the essential omega-3 fatty acids (Trofimiuk et al., 2011). Vitamin A has a potent role in the maintenance of many essential biological processes (Livrea et al., 1995). The antioxidant role of vitamin A could be mainly because of the hydrophobic chain of polyene units. Previous research studies have also examined the antioxidant activity of vitamin A in exerting protective effects against neurodegenerative and cardiovascular diseases (Lee et al., 2009; Huang et al., 2011). Vitamins present in the cod liver oil might have exerted their protective role in decreasing the stress-mediated inflammatory response, decreasing free radical production. The present study is consistent with the previous study reports augmenting antioxidant activity in various oxidative stress animal models like streptozotocin-induced diabetes in rats (Hunkar et al., 2002; Ohtake et al., 2002). The potent antioxidant activity of omega-3 fatty acids has also been well-documented (Diggle, 2002; Mesa et al., 2004; El-Mesery et al., 2009; Al-Gayyar et al., 2012). Essential protective components in the cod liver oil might restore the redox balance in the stress-induced oxidative damage to the hippocampus.
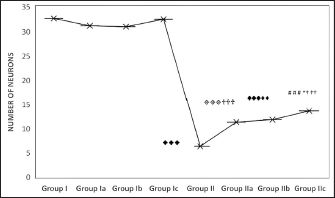
Fig. 3. Effect of CUS on the neuronal count in the frontal cortex in the control group and experimental groups. p < 0.001♦♦♦; Group I versus Group II; ◈◈◈; Group Ia versus Group IIa; p < 0.001✞✞✞: Group II versus Group IIa; p < 0.001♠♠♠: Group II versus Group IIb; p < 0.001# # #: Group II versus Group IIc; p < 0.001***; Group IIa versus Group IIc; † † † Group Ic versus Group IIc.
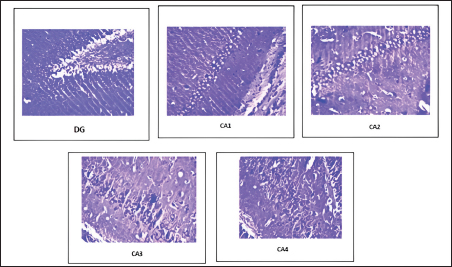
Fig. 4. Distribution of neuronal count in different regions of the hippocampus in the CUS group (Group II).
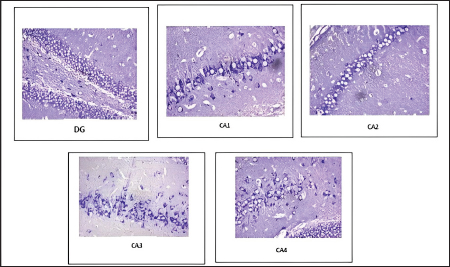
Fig. 5. Distribution of neuronal count in different regions of the hippocampus in CUS-induced cod liver oil treated group (Group IIa). In the present study, CUS decreased hippocampal neurogenesis. Chronic stress exposure might have caused the generation of free radicals affecting hippocampal neurogenesis. Previous study reports also documented increased inflammatory responses in brain tissue (Kim et al., 2015; Blossom et al., 2020). Cod liver oil treatment increased neuronal count in the dental gyrus, frontal cortex, and different regions of the hippocampus. The observed neurogenesis was similar to the action of the antidepressant imipramine. The neurogenesis could be attributed to the essential components present in the cod liver oil (Wilton, 1997), augmenting the production of neurons in stressed rats. Based on the study results, cod liver oil could be used in the management of anxiety and depression. The antidepressant action of the cod liver might be mainly because of the unsaturated fatty acids, eicosapentaenoic acid, docosahexaenoic acid, and antioxidant vitamins. It exerted a role similar to the antidepressant imipramine. Thus proving its potential role. The anxiolytic and antidepressant-like effects of cod liver oil might be mediated through multiple molecular and cellular pathways. However, detailed studies are needed to know the mechanism involved in various stress-induced neurodegenerative disease models. This might help in exploring the new potential therapeutic targets in the management of stress-induced depression and anxiety involving a dietary approach.
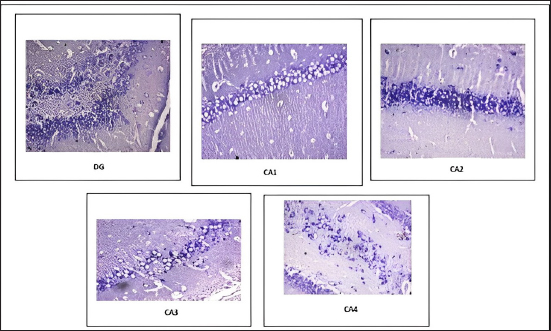
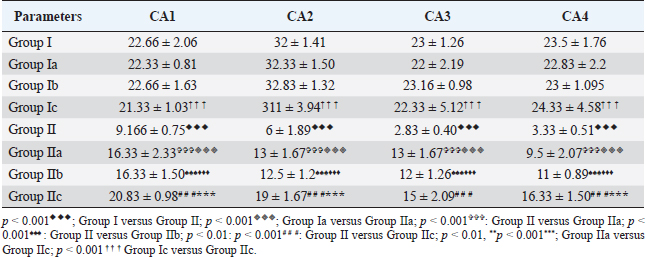
Fig. 6. Distribution of neuronal count in different regions of the hippocampus in CUS-induced cod liver oil treated group (Group IIb). Table 2. Effect of CUS on neuronal count on various regions of hippocampus in control groups and experimental groups.
Conflict of interestAll authors declare that there is no conflict of interest. AcknowledgmentsWe are very thankful to sincere gratitude to the Manipal Academy of Higher Education for providing us with all the facilities for the smooth conduction of the research. Author’s contributionsDr. Nayanatara Arun Kumar designed the experiments and preparation of the manuscript. Miss. Dimple Shet performed the experimental procedures. Mrs. Aradhana Marathe, Mrs. Sowndarya K, and Mrs. Vandana Blossom helped with biochemical and histological analysis, and Dr. Rekha D Kini and Mrs. Megha Gokul helped in the analysis of the results. All authors read and approved the final version of the manuscript. ReferencesAl-Gayyar, M.M., Shams, M.E. and Barakat, E.A. 2012. Fish oil improves lipid metabolism and ameliorates inflammation in patients with metabolic syndrome: impact of nonalcoholic fatty liver disease. Pharm. Biol. 50, 297–303. Bazazzadegan, N., Dehghan Shasaltaneh, M., Saliminejad, K., Kamali, K., Banan, M. and Khorram Khorshid, H.R. 2000. Effects of herbal C12 compound (IMOD) on behavior and expression of Alzheimer’s disease related genes in streptozotocin-rat model of sporadic Alzheimer’s disease. Adv. Pharm. Bull. 7(3), 491–494. Blossom, V., Gokul, M., Kumar, N.A., Kini, R.D., Nayak, S. and Bhagyalakshmi, K. 2020. Chronic unpredictable stress-induced inflammation and quantitative analysis of neurons of distinct brain regions in Wistar rat model of comorbid depression. Vet. World. 13(9), 1870–1874. Bolz, L., Heigele, S. and Bischofberger, J. 2015. running improves pattern separation during novel object recognition. Brain Plast. 1(1), 129–141. Bondi, C.O., Rodriguez, G., Gould, G.G., Frazer, A. and Morilak, D.A. 2008. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33(2), 320–331. Cryan, J.F., Markou, A. and Lucki, I. 2002. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 23(5), 238–245. Diggle, C.P. 2002. In vitro studies on the relationship between polyunsaturated fatty acids and cancer: tumour or tissue specific effects? Prog. Lipid Res. 41, 240–253. El-Mesery M, Al-Gayyar M, Salem H, Darweish M and El-Mowafy A. 2009. Chemopreventive and renal protective effects for docosahexaenoic acid (DHA): implications of CRP and lipid peroxides. Cell Div. 4, 6. Gertsik, L., Poland, R.E., Bresee, C. and Rapaport, M.H. 2012. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 32, 61–64. Glowinski, J. and Iversen, L.L. 1966. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J. Neurochem. 13(8), 655–669. Gokul, M., Arun Kumar, N., Durgadas Kini, R., Blossom, V., Kodavanji, B., Noojibail, A., Murali, N. and Vishwanath Rai, S.P. 2019. Evaluation of biomarkers of stress in chronic stress-exposed comorbid depression model Wistar rats. J. Basic Clin. Physiol. Pharmacol. 30(5); doi: 10.1515/jbcpp-2018-0215. Huang, W.B., Fan, Q. and Zhang, X.L. 2011. Cod liver oil: a potential protective supplement for human glaucoma. Int. J. Ophthalmol. 4, 648–651. Hünkar, T., Aktan, F., Ceylan, A., Karasu, C. and Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. 2002. Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotocin-diabetic rats. Cell Biochem. Funct. 20(4), 297–302. Khare, S., Asad, M., Dhamanigi, S.S. and Prasad, V.S. 2008. Antiulcer activity of cod liver oil in rats. Indian J. Pharmacol. 40(5), 209–214. Kim, E.J., Pellman, B. and Kim, J.J. 2015. Stress effects on the hippocampus: a critical review. Learn Mem. 22(9), 411–416. Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S. and Cosic, V. 2001. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 54(5), 356–361. Livrea, M.A., Tesoriere, L., Bongiorno, A., Pintaudi, A.M., Ciaccio, M. and Riccio, A.1995. Contribution of vitamin A to the oxidation resistance of human low density lipoproteins. Free Rad. Biol. Med. 18, 401–409. Lucassen, P.J., Pruessner, J., Sousa, N., Almeida, O.F., Van Dam, A.M., Rajkowska, G., Swaab, D.F. and Czéh, B. 2014. Neuropathology of stress. Acta Neuropathologica 127(1), 109–135. Madhyastha, S., Somayaji, S.N., Rao, M.S., Nalini, K. and Bairy, K.L. 2002. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can. J. Physiol. Pharmacol. 80(11), 1076–1084. Marklund, S. and Marklund, G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47(3), 469–474. Martí, O., Martí, J. and Armario, A. 1994. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol. Behav. 55(4), 747–753. Mesa, M.D., Buckley, R., Minihane, A.M. and Yaqoob, P. 2004. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on the oxidizability and thrombogenicity of low-density lipoprotein. Atherosclerosis 175, 333–343. Nayanatara, A.K., Nagaraja, H.S. and Anupama, B.K. 2005. The effect of repeated swimming stress on organ weights and lipid peroxidation in rats. Thai J. Physiol. Sci. 18(1), 3–9. Nayanatara, A.K., Tripathi, Y., Nagaraja, H.S., Jegananthan, P.S., Ramaswamy, C., Ganaraja, B. and Asha, K. 2011. The Involvement of amygdala nucleus in chronic stress induced changes on ingestive behavior of wistar rats. Int. J. Appl. Biol. Pharmac. Technol. 2(3), 339–346. Nayanatara, A.K., Tripathi, Y., Nagaraja, H.S., Jeganathan, P.S., Ramaswamy, C., Ganaraja, B., Sheila, R.P. and Asha, K. 2012. Effect of chronic immobilization stress on some selected physiological and lipid parameters in Wistar Albino Rats. Res. J. Pharmac. Biol. Chem. Sci. 3(1), 34–42. Nayanatara, A.K., Gokul, M., Kini, R.D., Bhagyalakshmi, K., Anupama, N., Shetty, S.B., Vinodini, N.A., Bhat, R., Pai, S.R. and Kaupu, R. 2014. Effect of chronic unpredictable stressors on reproductive parameters in male wistar rats: role of ascorbic acid. Int. J. Biol. Med. Res. 5(4), 4644–4647. Ohtake, T., Kimura, M., Takemura, H. and Hishida, A. 2002. Effects of dietary lipids on daunomycin-induced nephropathy in mice: comparison between cod liver oil and soybean oil. Lipids 37(4), 359–366. Willner, P., Belzung, C. and Scheel-Kruger, J. 2014. Resistance to antidepressant drugs: the case for a more predisposition-based and less hippocampocentric research paradigm. Behav. Pharmacol. 25, 352–371. Katz, R. and Baldrighi, G. 1982. A further parametric study of imipramine in an animal model of depression. Pharmacol. Biochem. Behav. 16, 969–972. Rao, G.M., Rao, A.V., Raja, A., Rao, S. and Rao, A. 2000. Lipid peroxidation in brain tumours. Clin. Chem. Acta. 302(1–2), 205–211. Schneiderman, N., Ironson, G. and Siegel, S.D. 2005. Stress and health: psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 1, 607–628. Sherif, I.O. and Al-Gayyar, M.M. 2015. Cod liver oil in sodium nitrite induced hepatic injury: does it have a potential protective effect? Redox Rep. 20(1), 11–16. Stratakis, C.A. and Chrousos, G.P. 1995. Neuroendocrinology and pathophysiology of the stress system. Ann. N. Y. Acad. Sci. 771, 1–18. Trofimiuk, E. and Braszko, J.J. 2011. Long-term administration of cod liver oil ameliorates cognitive impairment induced by chronic stress in rats. Lipids 46, 417–423. Whiteford, H.A., Ferrari, A.J., Degenhardt, L., Feigin, V. and Vos, T. 2015. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One 10(2), e0116820. Wilson, D.W., Nash, P., Buttar, H.S., Griffiths, K., Singh, R., De Meester, F., Horiuchi, R. and Takahashi, T. 2017. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: an overview. Antioxidants 6(4), 81. Wilton, P. 1995. Cod-liver oil, vitamin D and the fight against rickets. CMAJ. 152(9), 1516–1517. Yang, L., Zhao, Y., Wang, Y., Liu, L., Zhang, X., Li, B. and Cui, R. 2015. The effects of psychological stress on depression. Curr. Neuropharmacol. 13(4), 494–504. | ||
| How to Cite this Article |
| Pubmed Style Shet D, Kumar NA, Gokul M, Kini RD, Marathe A, Kollampare S, Blossom V. Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression. Open Vet J. 2023; 13(4): 473-480. doi:10.5455/OVJ.2023.v13.i4.9 Web Style Shet D, Kumar NA, Gokul M, Kini RD, Marathe A, Kollampare S, Blossom V. Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression. https://www.openveterinaryjournal.com/?mno=70724 [Access: April 25, 2024]. doi:10.5455/OVJ.2023.v13.i4.9 AMA (American Medical Association) Style Shet D, Kumar NA, Gokul M, Kini RD, Marathe A, Kollampare S, Blossom V. Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression. Open Vet J. 2023; 13(4): 473-480. doi:10.5455/OVJ.2023.v13.i4.9 Vancouver/ICMJE Style Shet D, Kumar NA, Gokul M, Kini RD, Marathe A, Kollampare S, Blossom V. Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression. Open Vet J. (2023), [cited April 25, 2024]; 13(4): 473-480. doi:10.5455/OVJ.2023.v13.i4.9 Harvard Style Shet, D., Kumar, . N. A., Gokul, . M., Kini, . R. D., Marathe, . A., Kollampare, . S. & Blossom, . V. (2023) Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression. Open Vet J, 13 (4), 473-480. doi:10.5455/OVJ.2023.v13.i4.9 Turabian Style Shet, Dimple, Nayanatara Arun Kumar, Megha Gokul, Rekha Durgadas Kini, Aradhana Marathe, Sowndarya Kollampare, and Vandana Blossom. 2023. Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression. Open Veterinary Journal, 13 (4), 473-480. doi:10.5455/OVJ.2023.v13.i4.9 Chicago Style Shet, Dimple, Nayanatara Arun Kumar, Megha Gokul, Rekha Durgadas Kini, Aradhana Marathe, Sowndarya Kollampare, and Vandana Blossom. "Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression." Open Veterinary Journal 13 (2023), 473-480. doi:10.5455/OVJ.2023.v13.i4.9 MLA (The Modern Language Association) Style Shet, Dimple, Nayanatara Arun Kumar, Megha Gokul, Rekha Durgadas Kini, Aradhana Marathe, Sowndarya Kollampare, and Vandana Blossom. "Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression." Open Veterinary Journal 13.4 (2023), 473-480. Print. doi:10.5455/OVJ.2023.v13.i4.9 APA (American Psychological Association) Style Shet, D., Kumar, . N. A., Gokul, . M., Kini, . R. D., Marathe, . A., Kollampare, . S. & Blossom, . V. (2023) Protective role of cod liver oil on hippocampal oxidative damage and neuronal count in Wistar rat model of comorbid depression. Open Veterinary Journal, 13 (4), 473-480. doi:10.5455/OVJ.2023.v13.i4.9 |