| Case Report | ||
Open Vet J. 2021; 11(2): 289-294 Open Veterinary Journal, (2021), Vol. 11(2): 289–294 Case Report Autologous blood patch pleurodesis treatment for persistent pneumothorax: A case series of five dogs (2016–2020)Marie-Laure Théron*, Tomas Lahuerta-Smith, Sébastien Sarrau, Bruno Ben-Moura and Antoine HidalgoClinique vétérinaire de référé, Vetivia, Biarritz, France Submitted: 10/03/2021 Accepted: 25/05/2021 Published: 14/06/2021 *Corresponding Author: Marie-Laure Théron. Clinique vétérinaire de référé, Vetivia, Biarritz, France. Email: ml.theron.vet [at] gmail.com © 2021 Open Veterinary Journal
AbstractBackground: Autologous blood patch pleurodesis (ABP) has been described as a treatment for persistent pneumothorax in the dogs and among humans. Although the treatment of persistent or recurring spontaneous pneumothorax is classically surgical, it cannot always be performed due to medical or financial constraints. This case series describes the clinical course, etiology, and outcome of five dogs with persistent pneumothorax treated with ABP. Case Description: Five client-owned dogs with persistent pneumothorax are presented. Two dogs had pneumothorax due to congenital pulmonary bullae, one due to thoracic trauma, another due to lungworm infection, and a fifth with unknown cause in the context of a relapsing subcutaneous haemangiosarcoma. Around 5 ml/kg of non-coagulated blood was aseptically collected from the jugular vein and injected via a thoracotomy tube immediately into the pleural cavity of dogs with persistent pneumothorax. The procedure was successful in four out of five dogs after one procedure, therefore a success rate of 80%. A repeat of the pleurodesis was attempted in the fifth dog, 12 hours after the first injection due to the recollection of the pneumothorax. Still, the dog died during anesthesia in preparation for the procedure. No complications that could be directly linked to ABP occurred. Conclusion: ABP is a simple, rapid, inexpensive, effective, and safe procedure that can be useful for treating persistent pneumothorax that does not respond to conservative treatment and where surgical exploration cannot be carried out. Pneumothorax secondary due to trauma and congenital pulmonary bullae seem to respond well to ABP. Keywords: autologous blood patch pleurodesis, dog, pleurodesis, pneumothorax. IntroductionPneumothorax is a potentially life-threatening condition if left untreated, in which air accumulates within the pleural space and interferes with pulmonary function. Pneumothorax may occur as a primary or secondary disease. Primary spontaneous pneumothorax due to pulmonary bullae has been well described in dogs (Lipscomb et al., 2003). Secondary pneumothorax may occur following trauma, iatrogenic intervention, or secondary to underlying lung pathology such as neoplasia, pneumonia, or parasitic disease (Puerto et al., 2002; Sobel and Williams, 2009). Pneumothorax can be managed conservatively by thoracocentesis (percutaneous or via thoracostomy tube) or surgically by thoracotomy or thoracoscopy (Puerto et al., 2002). Even though surgical therapy via bullectomy or partial or complete pulmonary lobectomy is the treatment of choice in many cases, this is not always possible. This is usually the case in animals with diffuse pulmonary pathology, dogs with poor general status, or due to the owner’s financial constraints (Puerto et al., 2002; Oppenheimer et al., 2014). Pleurodesis is a technique that has been used in human medicine for the last 30 years in the context of persistent pneumothorax (Dugan et al., 2017; Hallifax et al., 2017). The principle of this procedure is to inject within the pleural space a substance capable of inducing inflammation and sclerosis, leading to adhesions that will seal the lung from leaking air. Many agents such as talc, silver nitrate, tetracycline, bleomycin, and interferon have been used for this. These agents require opposition between the visceral and parietal pleura to induce the formation of adhesions. The inflammatory response can result in side effects, including fever, dyspnea, pain, and acute respiratory distress syndrome. When comparing these substances, autologous blood represents a quick and safe way to treat pneumothorax with fewer complications (Andreetti et al., 2007; Aihara et al., 2011; Manley et al., 2012; Evman et al., 2016). The most common complication following autologous blood patch pleurodesis (ABP) in people is empyema and occurs approximately in 10% of cases (Manley et al., 2012). In most patients, antimicrobial therapy resolves the infection. ABP has been used successfully as a treatment for pneumothorax following surgery and in cases of primary and secondary pneumothorax in humans and dogs. One study carried out on 31 humans with secondary spontaneous pneumothorax treated with ABP had a success rate of 93.5% (29/31) (Evman et al., 2016). Andreetti et al. (2007) performed ABP procedures on 25 people with air leaks on the sixth postoperative day after lobectomy. All patients had a resolution of pneumothorax 72 hours later. There is little information regarding ABP in veterinary medicine. ABP pleurodesis has been described in nine dogs with persistent pneumothorax due to various causes, which was successful in eight out of nine dogs (Merbl et al., 2010; Oppenheimer et al., 2014). Pneumothorax resolved immediately after one administration in six dogs. Infections following ABPs were encountered in two out of the nine dogs and resolved in one dog. The objective of this case series was to provide additional information to increase the knowledge of this technique, which is effective, easy to perform, and cheap for dogs that do not respond well to conservative therapy and where surgery is not a viable option. Cases DetailsThe medical records of five client-owned dogs treated with ABP from 2016 to 2020 were reviewed. Persistent pneumothorax was defined as persistent air leakage into the pleural cavity that did not respond to conservative management after 3 or more days. This included rest and repeated percutaneous thoracocentesis. The ABP procedure was standardized for all dogs. Animals were premedicated with 0.1 to 0.3 mg/kg of butorphanol. Anesthesia was induced with propofol titrated to effect and then maintained with isoflurane after endotracheal intubation. One or two chest tubes were placed aseptically, depending on the localization of the pneumothorax. If this was reduced to one hemithorax, the chest tube was placed ipsilaterally. In cases of diffused lung pathology, one tube was placed in each hemithorax. All dogs had the air removed from the chest before the procedure via the chest tube. Around 5 ml/kg of whole blood was collected from the jugular vein via an 18- or 20-gauge needle (depending on the dog’s size) attached to an extension tube and a three-way stopcock into 50 or 20 ml syringes (depending on the volume) that contained no additives. Once the syringe was full, the blood was immediately injected into the thoracic cavity via the thoracostomy tube. This procedure was repeated until the total volume was retrieved and injected. At the end of the procedure, the thoracostomy tube was flushed with 10 ml of sterile saline solution to avoid clotting inside the chest tube and ensure all the blood was kept inside the thoracic cavity. The dog was changed of position twice immediately after infusion (right and left lateral recumbency) to obtain a homogenous distribution of blood within the pleural cavity. Half of the total volume of blood was injected into each hemithorax in dogs with bilateral thoracostomy tubes. The chest tubes were not used for 4 hours following the injection. Subsequently, all dogs were monitored for changes in respiratory rate, and the chest tube was emptied every 8 hours to assess for resolution or recollection of the pneumothorax. Survey thoracic X-rays were obtained 24 hours and 1 week after each procedure. Antibiotic prophylaxis was not deemed necessary. Case details for each of the five dogs included in the study are listed in Table 1. The etiology of the pneumothorax were congenital pulmonary bullae in two dogs, traumatic in one (road traffic accident), lungworm infection in one, and unknown in the remaining dog. Dog number 1 presented a large pulmonary bulla in the right cranial pulmonary lobe (Fig. 1). Dog number 2 presented four bullae: two in the medial aspect of the left cranial lung lobe (17 mm each), one in the caudal aspect of the left caudal lung lobe (26 mm), and another in the caudal border of the left caudal lung lobe (36 mm) (Fig. 2). Dog number 3 presented a large bulla measuring 33 mm in the medial aspect of the right middle lung lobe and around 10 blebs measuring from 3 to 6 mm in the ventral border of both right and left cranial lung lobes. Dog number 4 developed multiple small bullae and a larger one measuring over 10 cm, 24 hours after treatment of lungworm with spot on Imidacloprid and Moxidectin, which were responsible for a moderate unilateral pneumothorax (Fig. 3). Despite the severity of the pneumothorax, dog number 5 did not show any lesions on the computed tomography (CT) scan that could clearly explain its origin. Only one chest tube was placed in dogs 1, 2, 3, and 4. Two were placed in dog 5. Table 1. Summary of patient data from the five dogs that underwent autologous blood patch pleurodesis.
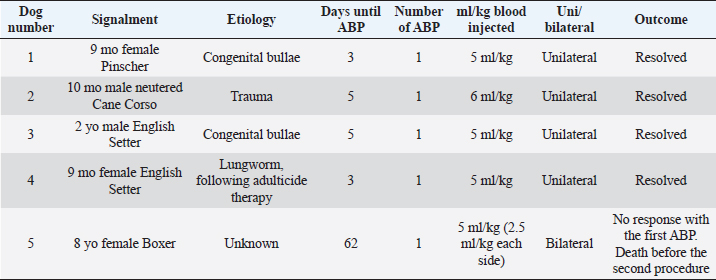
Fig. 1. CT transverse image of the thorax of the dog number 1 with pneumothorax showing a large pulmonary bulla in the right cranial lung lobe (arrow).
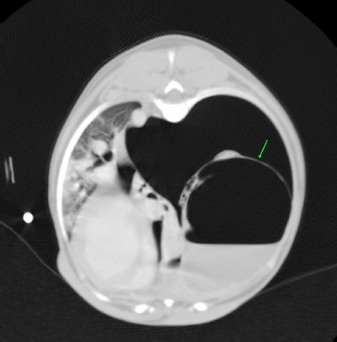
Fig. 2. CT transverse image of the thorax of the dog number 2 with pneumothorax secondary to multiple bullae after a road traffic accident. A bulla in the right hemithorax and another in the left are indicated in the image (arrows).
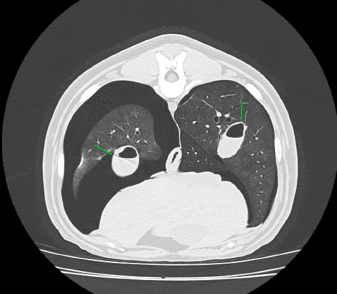
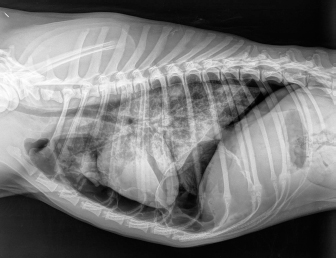
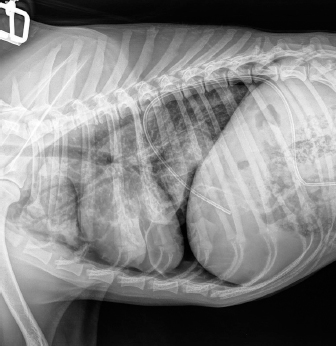
Fig. 3. Right-lateral thoracic radiograph of a 9-month-old female dog (dog number 4) suffering from a pneumothorax secondary to lungworm infection. The duration of pneumothorax until the ABP was performed ranged between 3 and 62 days. Pneumothorax resolved after one procedure in four out of five dogs. A second procedure was attempted in the remaining animal, but the dog did not survive induction of anesthesia. Dog number 5, which did not respond to the ABP procedure, was an 8-year-old Boxer with a 62-day-long history of pneumothorax and previous resection of two subcutaneous hemangiosarcomas (HSA). A first subcutaneous HSA was excised 8 months prior from the cervical region. Local recurrence of the tumor 6 months later prompted further investigation via CT scan of the thorax and abdominal ultrasound. These did not show the presence of metastases, but did however reveal a moderate pneumothorax with no visible cause within the thoracic cavity and no clinical repercussions on the dog. The mass was then re-excised surgically, and metronomic chemotherapy with cyclophosphamide (10 mg/m2/day) was started after the surgery. Seven weeks after the second surgery, the dog was presented for dyspnea and thoracic survey X-rays showed worsening air collection. Therefore, a bilateral ABP was carried out to resolve the pneumothorax. Twenty-four hours later, there was no improvement of the condition, and a second procedure was proposed. However, the dog sadly did not survive the induction of anesthesia for venepuncture and died during the induction of anesthesia. In all other cases, CT in three dogs and thoracic radiography in one (dog 4) revealed solitary or multiple bullae in several lung lobes. Owners were reluctant to perform an exploratory thoracotomy due to the need to perform multiple lung lobectomies or partial lobectomies in young dogs and the financial aspects of these procedures. Moreover, in dogs number 2, 3, and 4, due to the presence of multiple bullae, ABP was deemed to be the treatment of choice over surgical thoracotomy. No complication due to ABP was observed. Control thoracic X-rays were obtained 24 hours and 1 week after the procedures, which did not show any signs of relapse in dogs 1 through to 4 (Fig. 4). DiscussionABP pleurodesis seems to be an effective, safe, and easily performed procedure for treating persistent pneumothorax in dogs where conservative management has failed and where surgery cannot be performed due to financial or medical considerations. We observed a success rate of 80% after a single administration; however, these results must be interpreted cautiously due to the small number of dogs included in the study. These values are similar to those described previously in the literature, such as a success rate of 89% in the case series published by Oppenheimer et al. (2014) in eight dogs. It is also noteworthy that published success rates for surgical treatment of persistent pneumothorax via thoracotomy and lung lobectomy is 88% in veterinary medicine (Puerto et al., 2002). While thorax exploration by thoracotomy or thoracoscopy to find air leakage is still the first choice for treatment of persistent pneumothorax, ABP offers a solution in dogs who are poor surgical candidates or when owners decline surgery due to financial constraints. Even though surgical treatment was refused in dogs 1–4 due to financial reasons, dogs 2–4 were not considered to be ideal candidates for surgery due to the presence of multiple bullae distributed in various lung lobes. Median sternotomy with multiple partial lobectomies would have been necessary, which seemed to be excessively invasive, particularly in dogs 2 and 4, which were younger than 10 months old.
Fig. 4. Right-lateral thoracic radiographic of the same dog in Figure 3, 24 hours after autologous blood patch pleurodesis. Dog number 5 was the only candidate that failed to respond to ABP. Sudden death during induction of anesthesia for the second ABP procedure could be explained by the development of re-expansion pulmonary edema after the removal of air from the chest, considering that pneumothorax was present for 60 days. The exact cause of the pneumothorax was not clear in this dog. In the absence of an identifiable cause on thoracic CT scan and the context of subcutaneous HSA, many hypotheses could be formulated to explain the origin of the pneumothorax, such as a diffuse pneumopathy due to HSA metastases, pulmonary thromboembolism, or lungworm infection as the animal lived in an endemic area. In a study of 12 dogs with pneumothorax secondary to bullae and blebs, none of the dogs responded to conservative treatment with a thoracocentesis or thoracostomy tube drainage (Lipscomb et al., 2003). Thoracotomy with partial or complete lung lobectomy of one or many lung lobes was needed to resolve the pneumothorax. Although the outcome following surgery was excellent, this is an invasive procedure that needs an experienced surgeon. All the dogs in our study (three dogs) and those in the study published by Oppenheimer et al. (2014) (two dogs), cases in which the pneumothorax was secondary to congenital bullae or trauma, had a good response to ABP with definitive resolution after only one administration. The underlying cause of the pneumothorax might determine the response to ABP, and congenital bullae and trauma seem to be a good indication for ABP. ABP procedure should be proposed before a surgical exploration in these cases of persistent pneumothorax. However, if there is no response to ABP, surgery must be performed. ABP could also be a therapeutic option for spontaneous pneumothorax secondary to lungworm infection. We defined persistent pneumothorax as a pneumothorax that did not respond to conservative treatment after at least 3 days. A delay of up to 10 days can be considered before an ABP procedure is performed (Rivas de Andrés et al., 2000). However, the latest British Thoracic Society guidelines recommend surgical intervention as a treatment option in patients with secondary spontaneous pneumothorax when air leak persistently for more than 48 hours (MacDuff et al., 2010). In the only case series published in the veterinary literature, persistent pneumothorax was defined as a leakage of air that did not respond to 2 days or more of conservative management (Oppenheimer et al., 2014). Furthermore, financial aspects in veterinary medicine force us to make waiting periods shorter than in human medicine: 10 days of hospitalization with the care needed to attend an animal with chest tubes and regular thoracic radiographs are much more expensive than an ABP procedure. Evman et al. (2016) also considered the financial impact of performing ABP in patients with pneumothorax that did not respond to conservative treatment after 48 hours to limit the costs of hospitalization. Finally, Aghajanzadeh et al. (2010) have reported a lower success rate of blood pleurodesis in patients in whom the pneumothorax lasted longer than 10 days. All of this data indicates that in dogs preparing to undergo ABP, a maximum delay of 3 days should be considered. We cannot exclude that some of the dogs included in this case series may have improved with a longer period of conservative treatment alone. The precise mechanism via which ABP works is still uncertain. Sealing off the air leak by ABP is believed to work as the coagulated blood adheres to the affected site and provides an immediate mechanical blood patch effect (Manley et al., 2012; Evman et al., 2016). This mechanism explains the rapid resolution of pneumothorax after the procedure. Our choice of using 5 ml/kg is based on the volume injected described in the case series of Oppenheimer et al. (2014), which showed efficacy in treating persistent pneumothorax. There is no consensus in human medicine regarding the volume of blood needed to be injected during ABP to be efficacious. An experimental study in rats showed that the instillation of 3 ml/kg of blood leads to more effective pleurodesis after 1 month than when a volume of 2 ml/kg is used. When 1 ml/kg of blood was instilled, there was no evidence of macroscopic adhesion 1 month later (Ozpolat et al., 2010). Therefore, a volume of at least 3 ml/kg of blood seems necessary for the pleurodesis to be effective. Even though empyema (Manley et al., 2012; Oppenheimer et al., 2014) has been reported as a common complication of ABP, we did not administer prophylactic antibiotics as the instillation of blood does not favor infection if aseptic techniques are strictly applied. Although the cause of death behind dog number 5 is still not certain, the second ABP may have played a role. No complications or adverse effects were noted in the other three dogs. We are aware of the difficulty of drawing strong conclusions regarding indications and success rates of ABP with such a small sample size. However, with the broader experience in human medicine, the results from our study, and those from Oppenheimer et al. (2014), it seems ABP is a simple, painless, inexpensive, and effective treatment for dogs with persistent pneumothorax and should be considered for treatment of this condition. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionAll authors contributed to the study. All authors read and approved the final manuscript. ReferencesAghajanzadeh, M., Hemati, H., Moghaddamnia, M.R. and Aghajanzadeh, G. 2010. Autologous blood pleurodesis for treatment of prolonged air leak in secondary spontaneous pneumothorax. Indian J. Thorac. Cardiovasc. Surg. 25, 188–191. Aihara, K., Handa, T., Nagai, S., Tanizawa, K., Watanabe, K., Harada, Y., Chihara, Y., Hitomi, T., Oga, T., Tsuboi, T., Chin, K. and Mishima, M. 2011. Efficacy of blood-patch pleurodesis for secondary spontaneous pneumothorax in interstitial lung disease. Intern. Med. 50, 1157–1162. Andreetti, C., Venuta, F., Anile, M., De Giacomo, T., Diso, D., Di Stasio, M., Rendina, E.A. and Furio Coloni, G. 2007. Pleurodesis with an autologous blood patch to prevent persistent air leaks after lobectomy. J. Thorac. Cardiovasc. Surg. 133, 759–762. Dugan, K.C., Laxmanan, B., Murgu, S. and Hogarth, D.K. 2017. Management of persistent Air Leaks. Chest. 152, 417–423. Evman, S., Alpay, L., Metin, S., Kıral, K., Demir, M., Yalçinsoy, M., Baysungur, V. and Yalçinkaya, I. 2016. The efficacy and economical benefits of blood patch pleurodesis in secondary spontaneous pneumothorax patients. Kardiochir. Torakochirurgia. Pol. 13, 21–25. Hallifax, R.J., Yousuf, A., Jones, H.E., Corcoran, J.P., Psallidas, I. and Rahman, N.M. 2017. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: a systematic review. Thorax. 72, 1121–1131. Lipscomb, V.J., Hardie, R.J. and Dubielzig, R.R. 2003. Spontaneous pneumothorax caused by pulmonary blebs and bullae in 12 dogs. J. Am. Anim. Hosp. Assoc. 39, 435–445. MacDuff, A., Arnold, A. and Harvey, J. 2010. Management of spontaneous pneumothorax: british thoracic society pleural disease guideline 2010. BTS Pleural Disease Guideline Group. Thorax. 65(Suppl. 2), ii18–31. Manley, K., Coonar, A., Wells, F. and Scarci, M. 2012. Blood patch for persistent air leak: a review of the current literature. Curr. Opin. Pulm. Med. 18, 333–338. Merbl, Y., Kelmer, E., Shipov, A., Golani, Y., Segev, G., Yudelevitch, S. and Klainbart, S. 2010. Resolution of persistent pneumothorax by use of blood pleurodesis in a dog after surgical correction of a diaphragmatic hernia. J. Am. Vet. Med. Assoc. 237, 299–303. Oppenheimer, N., Klainbart, S., Merbl, Y., Bruchim, Y., Milgram, J. and Kelmer, E. 2014. Retrospective evaluation of the use of autologous bloodpatch treatment for persistent pneumothorax in 8 dogs (2009–2012). J. Vet. Emerg. Crit. Care 24, 215–220. Ozpolat, B., Gazyagci, S., Gozubuyuk, A., Ayva, S. and Atinkaya, C. 2010. Autologous blood pleurodesis in rats to elucidate the amounts of blood required for reliable and reproducible results. J. Surg. Res. 161, 228–232. Puerto, D.A., Brockman, D.J., Lindquist, C. and Drobatz, K. 2002. Surgical and nonsurgical management of and selected risk factors for spontaneous pneumothorax in dogs: 64 cases (1986–1999). J. Am. Vet. Med. Assoc. 220, 1670–1674. Rivas de Andrés, J.J., Blanco, S. and De la Torre, M. 2000. Postsurgical pleurodesis with autologous blood in patients with persistent air leak. Ann. Thorac. Surg. 70, 270–272. Sobel, K.E. and Williams, J.E. 2009. Pneumothorax secondary to pulmonary thromboembolism in a dog. J. Vet. Emerg. Crit. Care. 19, 120–126. | ||
| How to Cite this Article |
| Pubmed Style Théron M, Lahuerta-Smith T, Sarrau S, Ben-Moura B, Hidalgo A, . Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020). Open Vet J. 2021; 11(2): 289-294. doi:10.5455/OVJ.2021.v11.i2.13 Web Style Théron M, Lahuerta-Smith T, Sarrau S, Ben-Moura B, Hidalgo A, . Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020). https://www.openveterinaryjournal.com/?mno=62888 [Access: April 23, 2024]. doi:10.5455/OVJ.2021.v11.i2.13 AMA (American Medical Association) Style Théron M, Lahuerta-Smith T, Sarrau S, Ben-Moura B, Hidalgo A, . Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020). Open Vet J. 2021; 11(2): 289-294. doi:10.5455/OVJ.2021.v11.i2.13 Vancouver/ICMJE Style Théron M, Lahuerta-Smith T, Sarrau S, Ben-Moura B, Hidalgo A, . Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020). Open Vet J. (2021), [cited April 23, 2024]; 11(2): 289-294. doi:10.5455/OVJ.2021.v11.i2.13 Harvard Style Théron, M., Lahuerta-Smith, T., Sarrau, S., Ben-Moura, B., Hidalgo, A. & (2021) Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020). Open Vet J, 11 (2), 289-294. doi:10.5455/OVJ.2021.v11.i2.13 Turabian Style Théron, Marie-Laure, Tomas Lahuerta-Smith, Sébastien Sarrau, Bruno Ben-Moura, Antoine Hidalgo, and . 2021. Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020). Open Veterinary Journal, 11 (2), 289-294. doi:10.5455/OVJ.2021.v11.i2.13 Chicago Style Théron, Marie-Laure, Tomas Lahuerta-Smith, Sébastien Sarrau, Bruno Ben-Moura, Antoine Hidalgo, and . "Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020)." Open Veterinary Journal 11 (2021), 289-294. doi:10.5455/OVJ.2021.v11.i2.13 MLA (The Modern Language Association) Style Théron, Marie-Laure, Tomas Lahuerta-Smith, Sébastien Sarrau, Bruno Ben-Moura, Antoine Hidalgo, and . "Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020)." Open Veterinary Journal 11.2 (2021), 289-294. Print. doi:10.5455/OVJ.2021.v11.i2.13 APA (American Psychological Association) Style Théron, M., Lahuerta-Smith, T., Sarrau, S., Ben-Moura, B., Hidalgo, A. & (2021) Autologous blood-patch pleurodesis treatment for persistent pneumothorax: A case series of 5 dogs (2016 -2020). Open Veterinary Journal, 11 (2), 289-294. doi:10.5455/OVJ.2021.v11.i2.13 |