| Original Article | ||
Open Vet J. 2022; 12(6): 830-838 Open Veterinary Journal, (2022), Vol. 12(6): 830–838 Original Research Features of the clinical picture of keratitis in horses with different forms of the course of the diseaseYuliya Lazareva1*, Maria Rayisyan2 and Ekaterina Mironova31Department of Biology and General Genetics, I. M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia 2Department of Regulatory Relations of Circulation of Medicines and Medical Devices, I. M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia 3Department of Polyclinic Therapy, I. M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia Submitted: 15/06/2022 Accepted: 20/10/2022 Published: 13/11/2022 *Corresponding Author: Yuliya Lazareva. Department of Biology and General Genetics, I. M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation. Email: yuliyalazareva455 [at] rambler.ru © 2022 Open Veterinary Journal
AbstractBackground: Keratitis is a common cause of eye diseases in horses, often resulting in complete loss of vision. Aim: The purpose of this article is to study the features of the clinical picture of primary and secondary keratitis in horses, depending on the form of the course of the disease. Methods: The study was conducted in 2019 at 22 private horse breeding farms. The study involved 80 horses with keratitis, which were divided into two equal groups depending on the diagnosis of primary or secondary keratitis. Results: The effectiveness of the therapies was compared 1, 3, and 6 months after the start of treatment. Following a 1-month therapy, only a minority of horses with primary keratitis had their eye functions fully restored, the number of horses with macula was two times higher (p ≤ 0.05), the number of horses with nebula was seven times higher (p ≤ 0.001), and leukomas were reported in most of the cases ( p ≤ 0.0001). Conclusion: The effectiveness of the therapy for keratitis is directly related to the peculiarities of treatment and adherence to the regimen. Keywords: Keratitis, Tear production, Leukoma, Macula, Nebula. IntroductionVisual-spatial perception occurs through complex interactions between the eye components. These components perform two main functions – receiving the stimulus in the form of light and sending it to the brain (Seen and Tong, 2017). Another component of the visual system is the medium that refracts the light rays. One of the main components contributing to the refractive power of the eye is the cornea. The physiological functioning of the cornea depends on its transparency and water content (Hellander-Edman et al., 2013; Ventsova and Safonov, 2021). The morphometric criteria include the shape of the cornea (sphericity and asphericity). Changes in corneal parameters can lead to visual impairment (Hellander-Edman et al., 2019). The vulnerability of the cornea is determined by the fact that this fibrous component of the eye protrudes forward relative to other eye components, and, therefore, has more opportunities for exposure to traumas and infections (Kamaev et al., 2012; Safonov, 2022a, 2022b). Corneal diseases are the most common among diseases of the anterior part (or segment) of the eye. In particular, veterinary experts claim corneal pathologies to be the most common among horses (Kim et al., 2019). These pathologies can be induced by injuries, which are associated with the invasion of bacterial infection (the primary form of corneal pathology), as well as by impaired corneal metabolism and microcirculation, which can lead to corneal necrosis (Bucolo et al., 2017; Safonov et al., 2021). Depending on the etiology, the course of the disease can vary. In the case of the primary form, the disease develops rapidly and presents with symptoms of conjunctivitis, cataract, and parenchymatous keratitis. In the case of the secondary form, a chronic course of the disease is generally observed, which is characterized by the secretion of serous exudate from the conjunctival sac and the presence of keratitis (Zernii et al., 2017). Therefore, there is a need to examine the clinical picture of vascular keratitis in horses, depending on the course of the disease (Sandmeyer et al., 2014). As long as pathological changes in the cornea are complex and diverse in their manifestations, there are multiple known forms of keratopathies. This article provides information on the relationship between the etiology of the disease (primary and secondary forms of keratitis, corneal abscess, bullous keratopathy, and marginal vascular keratitis), on the one hand, and cytomorphological parameters, on the other hand (Göncü et al., 2015). The classifications of keratopathies are based on risk factors leading to the onset and progression of corneal pathologies, as well as on the results of functional tests such as Schirmer’s and Norn’s tests and tests with dyes, which reflect the severity and dynamics of the pathological changes on the corneal surface. Considering the characteristics of keratopathy, this disease is commonly split into two categories: primary form, which is the result of a corneal trauma that promotes the growth of microorganisms, and secondary form, which is triggered by the destruction of the eye tissues (Dennis et al., 2019). Considering the foregoing, a conclusion is made that primary keratitis is characterized by an injury-induced infection, and secondary keratitis develops due to the destruction of the tissues lining the outer shell of the eyeball. Such pathologies as ulcerative keratitis, non-ulcer keratouveitis, autoimmune keratolysis, stromal abscess, and various forms of microabscesses are mostly preceded by a minor corneal injury (Dhawan et al., 2011). In horses, a minor injury to the eye can occur upon contact with cereals, seed husks, and sawdust in stables (Suter et al., 2018). Later, purulent infection is observed at the sight of injury, caused by cocci, Pseudomonas aeruginosa, Proteus, and pathogenic fungi. Another reason for the development of complications can be penetrating corneal traumas when the infected tissues are epithelized with their further localization in the corneal stroma (Tabibian et al., 2015). Finally, the long-term use of antibiotics or corticosteroids can also cause keratitis (Zernii et al., 2017). A separate factor contributing to the development of keratopathies in horses is broken anatomical barriers in the eyeball. This occurs when an infection enters the corneal tissue affected by dystrophy (Voelter-Ratson et al., 2014). The cornea is prone to damage caused by purulent and ulcerative processes. This is explained by the fact that the cornea is susceptible to infections and injuries, as well as by its anatomy, physiology, and the influence of external factors. Corneal diseases do not have apparent symptoms and progress slowly (Wada et al., 2010). The recovery processes are linked to the risk of isolation of pathogenic microorganisms in the stratified squamous epithelium and subsequent development of deep interstitial keratitis (Famose, 2015). In case of complications, corneal abscesses and penetrating lesions of the stroma are observed, leading to corneal perforation (Bucolo et al., 2017). Keratitis is a common equine disease that is known and prevalent in many countries of the world. Among the different forms of keratitis, ulcerative keratitis is one of the most widespread, which is characterized by the presence of ulcers in the cornea and keratitis localization in the deep layers of the parenchyma. It is associated with the risk of corneal abscesses, which may lead to visual impairment in case of incorrect or delayed therapy (Dhawan et al., 2011). Despite the seriousness of the disease, the literature contains very limited data about it. Generally, this is fragmentary information about the etiology of the disease, some of its symptoms, and treatment methods (Dhawan et al., 2011; Zernii et al., 2017). The pathogenesis of corneal ulcers in a horse may be based on: 1) traumatic damage to the cornea and subsequent microflora invasion and 2) pathological processes in the cornea leading to disruption of metabolic and microcirculatory processes in the cornea and further its invasion. Clinical forms of ulcerative keratitis in horses are polymorphic. It is known that pathognomonic signs affect the anterior segment of the eye and are characterized by disturbance of integrity, smoothness, sphericity, transparency, luster, and specularity of the cornea, i.e., its optical properties (Dhawan et al., 2011). Ulcerative keratitis develops as an autoimmune disease with a fairly high mortality rate (up to 40% after several years), usually due to infarcts. Ulcerative keratitis may account for up to 20% of all keratitis cases (Brukhanov, 2005). Secondary keratitis is more dangerous because it leads to complete loss of vision in one or both eyes. This is due to the formation of scars that irreversibly change the optical properties of the cornea (Brukhanov, 2005). There are no papers devoted to the peculiarities of different forms of keratitis in horses, just as there is no complete clinical picture of this disease described. This determined the relevance of this article. The authors suggest that the type of corneal tissue healing will determine the outcome of ulcerative keratitis in horses. Keratitis is a fairly common disease in horses, the incidence of which can range from 6% to 12% per year. The highest rates of keratitis are registered in Russia (up to 39% in young animals, and up to 20% in adult animals), so this region may be a convenient model area for studying this disease (Brukhanov, 2005). The purpose of the study is to perform a comparative analysis of the peculiarities of different forms of keratitis in horses. The objectives of the study include the following: a) to examine the processes of corneal healing in horses suffering from primary keratitis; b) to investigate the same issue for horses suffering from secondary keratitis; and c) to compare the effectiveness of therapies for primary and secondary keratitis given to horses. Materials and MethodsSampleThe study was conducted in 2019 in the Moscow region (Russia). The sample included 80 randomly selected horses diagnosed with ulcerative keratitis. Veterinarians retained by 22 private stables were consulted to identify the eligible horses. The sample was divided into two equal parts: 40 horses with the primary form of keratitis (Group 1) and 40 horses with the secondary form of keratitis (Group 2). All enrolled horses were kept under the same conditions, received the same care, and were fed the same diet. In neglected cases of the primary form of ulcerative keratitis, there was found a progression of the above clinical signs: corneal opacity and development of inflammatory process. The primary form of ulcerative keratitis in the majority of observed cases was characterized ophthalmoscopically by the presence of persistent organic changes on the cornea. Diagnostic clinical signs in the secondary form of ulcerative keratitis were: disturbance of smoothness, luster, specularity, and transparency of the cornea. It should be noted that to date there is no data on the predisposition of horses of a particular breed to the disease of keratitis. Perhaps the incidence of disease in horses is related to a particular region and its climatic features (high humidity, which promotes the spread of fungal and bacterial infections). Study designThe study followed the principle of random selection. If animals did not meet the criteria for the same care, diet, and housing, they were excluded from the study. In addition, the study did not include animals that were suffering from other serious chronic diseases, apart from keratitis. This study was performed in accordance with the generally accepted norms regarding attitudes toward animals. The study was approved at a meeting of the Ethics Committee at the Moscow State University named after M.V. Lomonosov, minutes No. 3340-21. Tests and vital dyes increased the effectiveness of the therapy. They made it possible to establish the severity and dynamics of the pathological changes in the morphology of corneal and conjunctival tissues after the use of the therapy. General characteristics of the scar tissue formed due to an inflammatory process were the primary efficacy endpoint, with a type of epithelialization and type of scarring taken into account. The effectiveness of the therapy was evaluated in both groups 1, 3, and 6 months after the start of therapy. Therapy methodsA set of methods was applied, including general clinical examination. The latter included collecting preliminary data about the animal (its medical history), looking at its physical state, examining hair, skin, and subcutaneous tissue layer, accessible mucous membranes, and lymph nodes, as well as recording body temperature. The area affected by the pathological process was examined separately. For this purpose, the method of slit lamp biomicroscopy was applied. The instrument used combines a slit lamp and a condensing lens. This method allows for examining different parts of the eye, including the cornea, depending on the focus of a narrow light beam. The front and back surfaces of the cornea were examined. The area affected by the pathology was localized, and the severity of the destructive processes was determined. In addition, Schirmer’s test was used to assess the total conjunctival tear production. To perform the test, a strip of filter paper was placed into each lower conjunctival fornix. The level of tear production was determined based on the length of paper moistened by tears. The normal value for horses is 15–25 mm. In addition, Norn’s test was done to evaluate the stability of the tear film. To stain the lacrimal fluid, a solution of 1% fluorescein sodium was used. Thereafter, using a slit lamp and a blue filter, the time of the first tear film breakup after the animal blinked was recorded. In addition, vital dyes were used to assess the horse’s general eye health. Denatured emulsified placenta (manufactured by Biotekhindustriya, Russia) was used as therapy. This drug was used in combination with Taufon (4% concentration). The two drugs were mixed in the amount of 0.5 ml each. The study animals were given one drop of the preparation four times a day until the opaque scar tissue appeared normal. Statistical data processing was performed using Statistica, v. 10.0 (StatSoft Inc., USA). The Student’s t-test for independent samples was applied to compare the number of animals suffering from different forms of keratitis. The level of significance of the results obtained was set at p ≤ 0.05. The number of study animals in both groups is expressed as a percentage for the purpose of unification and convenience in comparing the data obtained. Ethical approvalThis study was performed in accordance with the generally accepted norms regarding attitudes toward animals. The study was approved at a meeting of the Ethics Committee at the Moscow State University named after M.V. Lomonosov, minutes No. 3340-21. ResultsOverall restorationDepending on the type of keratitis diagnosed in horses, different therapies were administered, which were aimed at stopping the spread of infection, eliminating or minimizing microbial flora, stimulating the repair processes, and scar tissue remodeling or epithelialization. Figure 1 shows the results of the therapy by different types of scar healing in horses diagnosed with keratitis. The study found that 2 horses with primary keratitis and 26 horses with secondary keratitis had their corneal tissue healed by the formation of a layer of squamous epithelium. The healing process involved the active division of squamous epithelial cells, with the layer of them slowly covering the wound area. These results differ from those obtained for horses suffering from secondary keratitis (p ≤ 0.01 and p ≤ 0.05, respectively, for the first and second types of healing in the two groups). The second type of healing is due to the formation of a granule barrier and is characterized by the invasion of blood vessels into the corneal tissue. The type of healing was found to affect ulcer formation. In the case of superficial injuries, horses with keratitis of both types, intact stroma, and no invasion of blood vessels into the corneal tissue had their wounds healed without any scarring (Fig. 2). Absolute restoration of the morphological and physiological parameters of the cornea (shape and transparency) was observed, followed by restoration of visual functions.
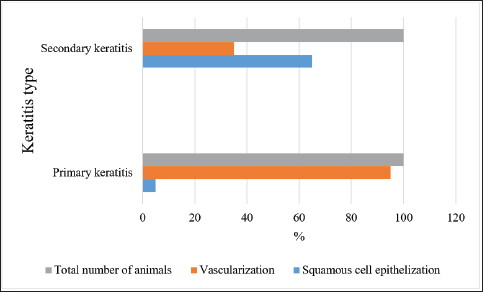
Fig. 1. Scarring characteristics for horses in both groups diagnosed with keratitis.
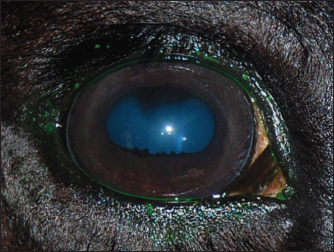
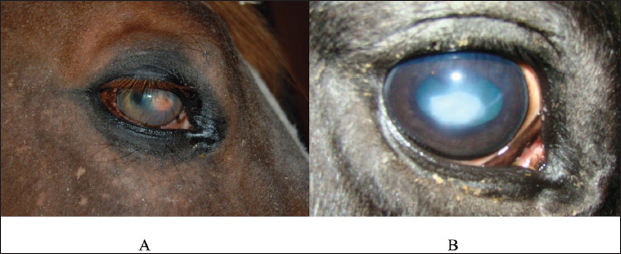
Fig. 2. A case of restoration of the optical and morphological parameters of the cornea. Morphological changesIf the healing process followed the second pattern, a scar made of connective tissue cells was formed. The scars were characterized by different densities and localization in the tissue (depth). In particular, four horses with primary keratitis and eight horses with secondary keratitis (p ≤ 0.05) had nebulas (Fig. 3). Maculae were prevalently observed in horses suffering from primary keratitis, with cases being seven times less frequent in horses suffering from secondary keratitis (p ≤ 0.01, Fig. 4). Macula is a slight opacity of the cornea. In addition to nebulae and maculae, leukoma formation was reported in both groups. Leukoma is characterized by a dense scar involving deep corneal tissues (Figs. 5 A and B). Leukomas were found in half of the horses in the first group and only in one-tenth of the horses in the second group (p ≤ 0. 001).
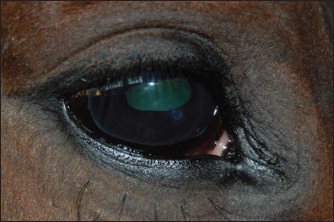
Fig. 3. Nebular corneal opacity.
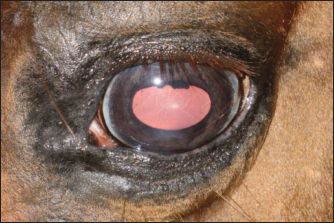
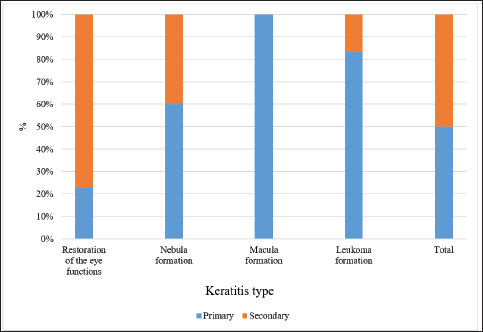
Fig. 4. Macular lesion. A short time after onset (1 month), keratitis produced different outcomes in the two groups. The data are presented in Figure 6.
Fig. 5. A. Leukoma characterized by scar formation consisting of connective tissue. B. Leukoma triggered by ulcerative keratitis.
Fig. 6. Results of the 1-month therapy in horses suffering from primary and secondary keratitis. According to the data in Figure 6, only a minority of horses with primary keratitis showed restoration of their eye functions, the number of horses with macula was two times higher (p ≤ 0.05), the number of horses with nebula was seven times higher (p ≤ 0.001), and the number of horses with leukoma was the highest (p ≤ 0.0001). The results differed for the horses with secondary keratitis, as most of them restored their visual functions, 8 times fewer horses had macula (p ≤ 0.001), and 16 times fewer horses had nebula and macula (at p ≤ 0.0001 with a favorable outcome). Tear related functionIn addition, the stability of the tear film and the rate of tear production were evaluated. In those cases when the formation of the macula was observed, the tear film breakup time was significantly lower as compared to other corneal opacities, except for leukoma (p ≤ 0.05 with full recovery, macula). In horses with leukoma, the appearance of the first dry spot in the tear film took two times less time (p ≤ 0.01, Table 1). The quantitative assessment of tear production (basic and reflex types) indicated a decrease in tear production in horses suffering from severe keratitis. These data are presented in Table 2. According to Table 2, horses with leukoma had the smallest tear volume as compared to horses having other manifestations of keratitis (p ≤ 0.01). Tear production in horses with macula was inferior to tear production in horses with nebula and restoration of the eye functions (p ≤ 0.05). Table 1. Tear film breakup time, in seconds, by different keratitis outcomes.
Table 2. Tear production in horses suffering from different forms of keratitis (based on the results of Schirmer’s test).
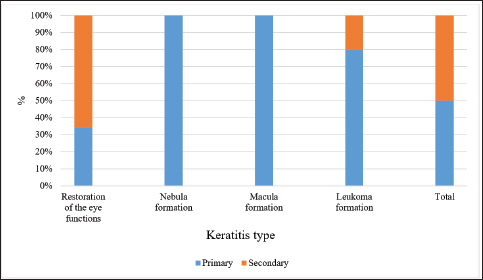
Fig. 7. Results of the 3-month therapy in horses with primary and secondary keratitis. Vision restorationAnalysis of the effectiveness of the therapy demonstrated that remission occurred in most cases. Figure 7 presents data regarding the results of the 3-month treatment. Horses with primary keratitis had their vision restored in a quarter of cases, while the number of horses with secondary keratitis that had their vision restored was 3.5 times greater (p ≤ 0.01). The incidence of nebular opacities in the study groups was similar (p ≥ 0.05). The same observations were obtained for macular opacities. The incidence of leukoma significantly differed between the groups, with leukoma being registered five times more often in the first group than in the second one (p ≤ 0.001). The long-term effects of therapyA 6-month therapy produced changes that depended on the type of keratitis and therapy given to horses (Fig. 8). Following a 6-month period, half of the horses with primary keratitis had their corneal functions restored, with this outcome registered among horses with secondary keratitis much more often (p ≤ 0.05). Notably, the incidence of nebula, macula, and leukoma formation in horses with primary keratitis was significantly higher as compared to horses with secondary keratitis (p ≤ 0.05). A comparative analysis of the results of treatment of primary and secondary keratitis showed that a correctly chosen treatment regimen (aimed to reduce inflammation) yielded complications in horses suffering from primary keratitis at a rate three times higher than in horses suffering from secondary keratitis (p ≤ 0.05). The authors attribute this to the fact that primary keratitis caused deep corneal lesions in more than 80% of cases. Due to this, the regeneration process involved the blood vessels located both in deep layers of the cornea and on its surface. A subsequently developed leukoma led to nutritional deficiency of the cornea. Additionally, the long-term results of the Norn’s test were favorable for all horses (over 10 seconds). The Schirmer’s test also showed positive changes in the long-term period (13 to 15 mm), with the only exception of leukoma (10–13 mm, p ≤ 0.05 in comparison with other categories).
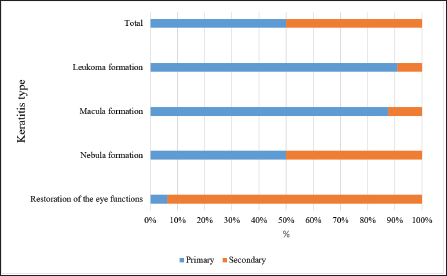
Fig. 8. Results of the 6-month therapy in horses with primary and secondary keratitis. DiscussionThis work examined primary and secondary types of keratitis, which are quite common among horses. Keratitis can be caused by injuries of varying severity. The results of the study correlated with other studies (Uchino et al., 2015; Famose, 2016) dedicated to the evaluation of the effectiveness of therapy. Nevertheless, the course of keratitis had been poorly understood at the time of taking the decision to conduct this study. A detailed analysis of the literature data regarding keratitis is presented below. The presence of blood vessels in the corneal tissues indicates the presence of chronic inflammation (Marchegiani et al., 2021). It is also known that blood vessels can be located both on the surface of the cornea and in its deep layers (Page et al., 2020). The localization of blood vessels can be a criterion for diagnosing the inflammatory process. Depending on the severity of the inflammatory processes, therapy can be either simple or complex. In case of an unremarkable inflammation, the leukocytes stop infiltrating the cornea, and the latter becomes cloudy and forms a nebula (Brooks et al., 2026). In case of complications, the infection occurs, which can lead to necrotic processes in the stroma, corneal infiltration, and purulent discharge, accompanied by scar formation (Gallhoefer et al., 2016). The diagnosis of secondary keratitis is established in horses quite often. This disease is prevalently qualified as chronic (Lombardo et al., 2015). If so, it does not induce a regenerative effect. Secondary keratitis is characterized by a sudden onset and slow progression, with periodic exacerbations of pathological symptoms. Recently, corneal ulcers, bullous keratitis, and marginal vascular keratitis have been included in the secondary keratitis category (Reed et al., 2013). In the case of corneal ulceration, the elimination of squamous stratified epithelium and upper layers of the stroma occurs. This is a consequence of microcirculatory disorders enhanced by protease activities (Sondergaard et al., 2013). A corneal ulcer is characterized by subacute or chronic inflammation in the anterior segment of the eye and a small amount of serous exudate secreted from the conjunctival sac. A corneal ulcer has an irregular shape and uneven edges that disrupt stromal stratified epithelium in the course of the lysis process in desmosomes, and the latter promotes adhesion of the above two layers (Meek and Hayes, 2013). As to bullous keratitis, the onset of degenerative processes is in the endothelium, which subsequently loses its barrier function. In connection with the above, the cornea accumulates an excessive amount of fluid and loses its optical functions. At the beginning of the disease, stromal edema develops which manifests as an increase in stromal thickness. Then edema absorbs excess fluid and starts to destruct collagen fibers. This triggers the release of collagenase, an enzyme that contributes to endothelial dysfunction by forming a layer of endothelial cells along the back of the cornea. During this process, the stroma accumulates fluid, and subsequently, the cornea becomes excessively hydrated. Stromal edema is characterized by the presence of a large number of epithelial cells in the intercellular space, the formation of cysts, and fading of membranes. At later stages, the areas soaked with fluid raise above the surface, and detachment of the squamous epithelium from the stroma occurs. Further detachment leads to bulla formation, accompanied by de-epithelialization of the cornea (Andrew et al., 1998). Finally, a distinct feature of marginal vascular keratitis is ischemia of the lateral (peripheral) sections of the stroma. That is why superficial vessels grow into the stroma and leukoma is formed (Sondergaard et al., 2013). In parallel, the infiltrate accumulates in the cells making up the cornea, which results in corneal syndrome. As this study has demonstrated, the effectiveness of keratitis therapy is directly related to the characteristics of the treatment and adherence to the regimen (Meek and Hayes, 2013). Further studies need to be devoted to keratitis in horses. Investigation of primary keratitis is of special importance since it is more dangerous in terms of consequences. For horses, as for most mammals, vision is the main sense allowing spatial orientation. In addition, vision is also important from the practical point of view (for example, for racehorses). Therefore, preventative measures should be implemented to protect horses and every effort should be made to find a more effective therapy. ConclusionThe type of healing predetermined the outcome of keratitis observed. The depth of the injury was of paramount importance. Horses with primary keratitis had a layer of squamous epithelial cells formed, ulcers healed without a trace, and corneal functions completely restored. Secondary keratitis involved vascularization, and the healing process was accompanied by the formation of scars characterized by different depths: leukomas, maculae, and nebulae. Quantitative and qualitative analysis of the effectiveness of the therapy proved the suitability of the tests for the stability of the tear film and rate of tear production. After 6 months, horses with primary keratitis had their corneal functions restored in 50% of cases, while horses with secondary keratitis had the same outcome in 85% of cases (p ≤ 0.05). Remarkably, the incidence of nebula, macula, and leukoma was significantly higher in horses with primary keratitis as compared to horses with secondary keratitis (15% and 5%, p ≤ 0.05). Almost all horses showed a trend toward full recovery 6 months after the start of the therapy, although the number of horses with a consistently severe eye condition was three times higher in the primary keratitis group than in the secondary keratitis group (p ≤ 0.05). A direct relationship was found between the size of the scar and tear film breakup time (Pearson’s correlation coefficient 0.84, p ≤ 0.05), as well as the amount of tear fluid (0.78, p ≤ 0.05). Thus, the extent and depth of the lesion determine the seriousness of the infection, and, consequently, the duration and nature of the disease. The practical value of the data obtained consists of the possibility of timely diagnosis of the type of keratitis, which will allow many horses to preserve vision in the primary type of keratitis. It follows that in horses, depending on the type of keratitis and the duration of the disease, the outcome can be predicted with high probability. The future focus should be on possible therapy for secondary keratitis, depending on the disease duration. FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Conflict of interestThe authors declare that there are no conflicts of interest related to this article. Availability of data and materialData will be available on request. Authors’ contributionsYL conceived and designed the analysis; MR collected the data; EM contributed data or analysis tools; YL performed the analysis; MR and EM wrote the paper. All authors read and approved the final manuscript. ReferencesAndrew, S.E., Brooks, D.E., Smith, P.J., Gelatt, K.N., Chmielewski, N.T. and Whittaker, C.J. 1998. Equine ulcerative keratomycosis: visual outcome and ocular survival in 39 cases (1987–1996). Equine. Vet. J. 30(2), 109–116. Brooks, D.E., Matthews, A. and Clode, A.B. 2016. Diseases of the cornea. In Equine ophthalmology. Ed., Gilger, B.C. New York, NY: John Wiley & Sons, pp: 252–368. Brukhanov, A.A. 2005. Immunocorrection and treatment of conjunctivitis in cattle. Ph.D. Thesis of the Candidate of Veterinary Sciences. Ural State Academy of Veterinary Medicine, Troitsk. Bucolo, C., Fidilio, A., Platania, C.B.M., Geraci, F., Lazzara, F. and Drago, F. 2017. Antioxidant and osmoprotecting activity of taurine in dry eye models. J. Ocul. Pharmacol. Ther. 34(1–2), 188–194. Dennis, D.A., Pinard, C.L., Kenney, D.G., Salcedo, R.J. and Trout, D.R. 2019. Normograde nasolacrimal placement of an ocular-lavage system for treatment of equine eye diseases. Can. Vet. J. 60(7), 744–748. Dhawan, S., Rao, K. and Natrajan, S. 2011. Complications of corneal collagen cross-linking. J. Ophthalmol. 2011, 869015. Famose, F. 2015. Evaluation of accelerated collagen cross-linking for the treatment of melting keratitis in ten cats. Vet. Ophthalmol. 18(2), 95–104. Famose, F. 2016. Evaluation of accelerated corneal collagen crosslinking for the treatment of bullous keratopathy in eight dogs (10 eyes). Vet. Ophthalmol. 19(3), 250–255. Gallhoefer, N.S., Spiess, B.M., Guscetti, F., Hilbe, M., Hartnack, S., Hafezi, F. and Pot, S.A. 2016. Penetration depth of corneal crosslinking with riboflavin and UV-A (CXL) in horses and rabbits. Vet. Ophthalmol. 19(4), 275–284. Göncü, T., Akal, A., Adıbelli, F.M., Çakmak, S., Sezen, H. and Yılmaz, Ö.F. 2015. Tear film and serum prolidase activity and oxidative stress in patients with keratoconus. Cornea 34(9), 1019–1023. Hellander-Edman, A., Makdoumi, K., Mortensen, J. and Ekesten, B. 2013. Corneal cross-linking in 9 horses with ulcerative keratitis. BMC. Vet. Res. 9(1), 128. Hellander-Edman, A., Strom, L. and Ekesten, B. 2019. Corneal crosslinking (CXL) – a clinical study to evaluate CXL as a treatment in comparison with medical treatment for ulcerative keratitis in horses. Vet. Ophthalmol. 22(4), 552–562. Kamaev, P., Friedman, M.D., Sherr, E. and Muller, D. 2012. Photochemical kinetics of corneal cross-linking with riboflavin. Invest. Ophthalmol. Vis. Sci. 53(4), 2360–2367. Kim, J., Ji, D.B., Takiyama, N., Bae, J. and Kim, M.S. 2019. Corneal collagen cross-linking following superficial keratectomy as treatment for corneal endothelial cell dystrophy in dogs: preliminary clinical study. Vet. Ophthalmol. 22(4), 440–447. Lombardo, M., Pucci, G., Barberi, R. and Lombardo, G. 2015. Interaction of ultraviolet light with the cornea: clinical implications for corneal crosslinking. J. Cataract. Refract. Surg. 41(2), 446–459. Marchegiani, A., Bazzano, M., Cassarani, M.P., Arcelli, R., Orzalesi, C., Lombardo, G., Spaterna, A. and Gialletti, R. 2021. Efficacy of riboflavin/UV-A corneal phototherapy as stand-alone treatment for ulcerative keratitis in horses. Vet. Med-Czech. 66(8), 321–329. Meek, K.M. and Hayes, S. 2013. Corneal cross-linking – a review. Ophthalmic. Physiol. Opt. 33(2), 78–93. Page, L., Allbaugh, R.A., Mochel, J.P., Peraza, J., Bertram, M. and Sebbag, L. 2020. Impact of diurnal variation, sex, tear collection method, and disease state on tear protein levels in dogs. Vet. Ophthalmol. 23(6), 994–1000. Reed, Z., Thomasy, S.M., Good, K.L., Maggs, D.J., Magdesian, K.G., Pusterla, N. and Hollingsworth, S.R. 2013. Equine keratomycoses in California from 1987 to 2010 (47 cases). Equine. Vet. J. 45(3), 361–366. Safonov, V. 2022a. Comparison of LPO-AOS indices and biochemical composition of animal blood in biogeochemical provinces with different levels of selenium. Biol. Trace. Element. Res. 200(5), 2055–2061. Safonov, V. 2022b. Dependence of antioxidant and biochemical status on selenium content in the blood of animals. Adv. Anim. Vet. Sci. 10(2), 263–269. Safonov, V., Ermakov, V., Danilova, V. and Yakimenko, V. 2021. Relationship between blood superoxide dismutase activity and zinc, copper, glutathione and metallothioneines concentrations in calves. Biomath 10(2), ID–2111247. Sandmeyer, L.S., Bauer, B.S. and Grahn, B.H. 2014. Diagnostic ophthalmology. Can. Vet. J. 55(1), 1263–1264. Seen, S. and Tong, L. 2017. Dry eye disease and oxidative stress. Acta. Ophthalmol. 96(4), e412–e420. Sondergaard, A.P., Ivarsen, A. and Hjortdal, J. 2013. Reduction of stromal swelling pressure after UVA-riboflavin cross-linking. Invest. Ophthalmol. Vis. Sci. 54(3), 1625–1634. Suter, A., Voelter, K., Hartnack, S., Spiess, B.M. and Pot, S.A. 2018. Septic keratitis in dogs, cats, and horses in Switzerland: associated bacteria and antibiotic susceptibility. Vet. Ophthalmol. 21(1), 66–75. Tabibian, D., Richoz, O. and Hafezi, F. 2015. PACK-CXL: corneal crosslinking for treatment of infectious keratitis. J. Ophthalmic. Vis. Res. 10(1), 77–80. Uchino, Y., Kawakita, T., Miyazawa, M., Ishii, T., Onouchi, H., Yasuda, K., Ogawa, Y., Shimmura, S., Ishii, N. and Tsubota, K. 2015. Correction oxidative stress induced inflammation initiates functional decline of tear production. PLoS One 10(5), e0127720. Ventsova, I. and Safonov, V. 2021. Biochemical screening of lipid peroxidation and antioxidant protection in imported cows during adaptation. Adv. Anim. Vet. Sci. 9(8), 1203–1210. Voelter-Ratson, K., Monod, M., Unger, L., Spiess, B.M. and Pot, S.A. 2014. Evaluation of the conjunctival fungal flora and its susceptibility to antifungal agents in healthy horses in Switzerland. Vet. Ophthalmol. 17(Suppl 1), 31–36. Wada, S., Hobo, S. and Niwa, H. 2010. Ulcerative keratitis in thoroughbred racehorses in Japan from 1997 to 2008. Vet. Ophthalmol. 13(2), 99–105. Zernii, E.Y., Baksheeva, V.E., Yani, E.V., Philippov, P.P. and Senin, I.I. 2017. Therapeutic proteins for treatment of corneal epithelial defects. Curr. Med. Chem. 26(3), 517–545. | ||
| How to Cite this Article |
| Pubmed Style Lazareva Y, Rayisyan M, Mironova E, . Features of the clinical picture of keratitis in horses with different forms of the course of the disease. Open Vet J. 2022; 12(6): 830-838. doi:10.5455/OVJ.2022.v12.i6.7 Web Style Lazareva Y, Rayisyan M, Mironova E, . Features of the clinical picture of keratitis in horses with different forms of the course of the disease. https://www.openveterinaryjournal.com/?mno=58721 [Access: April 25, 2024]. doi:10.5455/OVJ.2022.v12.i6.7 AMA (American Medical Association) Style Lazareva Y, Rayisyan M, Mironova E, . Features of the clinical picture of keratitis in horses with different forms of the course of the disease. Open Vet J. 2022; 12(6): 830-838. doi:10.5455/OVJ.2022.v12.i6.7 Vancouver/ICMJE Style Lazareva Y, Rayisyan M, Mironova E, . Features of the clinical picture of keratitis in horses with different forms of the course of the disease. Open Vet J. (2022), [cited April 25, 2024]; 12(6): 830-838. doi:10.5455/OVJ.2022.v12.i6.7 Harvard Style Lazareva, Y., Rayisyan, M., Mironova, E. & (2022) Features of the clinical picture of keratitis in horses with different forms of the course of the disease. Open Vet J, 12 (6), 830-838. doi:10.5455/OVJ.2022.v12.i6.7 Turabian Style Lazareva, Yuliya, Maria Rayisyan, Ekaterina Mironova, and . 2022. Features of the clinical picture of keratitis in horses with different forms of the course of the disease. Open Veterinary Journal, 12 (6), 830-838. doi:10.5455/OVJ.2022.v12.i6.7 Chicago Style Lazareva, Yuliya, Maria Rayisyan, Ekaterina Mironova, and . "Features of the clinical picture of keratitis in horses with different forms of the course of the disease." Open Veterinary Journal 12 (2022), 830-838. doi:10.5455/OVJ.2022.v12.i6.7 MLA (The Modern Language Association) Style Lazareva, Yuliya, Maria Rayisyan, Ekaterina Mironova, and . "Features of the clinical picture of keratitis in horses with different forms of the course of the disease." Open Veterinary Journal 12.6 (2022), 830-838. Print. doi:10.5455/OVJ.2022.v12.i6.7 APA (American Psychological Association) Style Lazareva, Y., Rayisyan, M., Mironova, E. & (2022) Features of the clinical picture of keratitis in horses with different forms of the course of the disease. Open Veterinary Journal, 12 (6), 830-838. doi:10.5455/OVJ.2022.v12.i6.7 |