| Case Report | ||
Open Vet J. 2022; 12(5): 762-767 Open Veterinary Journal, (2022), Vol. 12(5): 762–767 Case Report Effective treatment with mitotane for a canine case of presumed ectopic Cushing’s syndrome-related pheochromocytomaKonatsu Miura1*, Hiroshi Sunahara2, Yusuke Sakai3, Saneyuki Isshiki3, Moe Hasegawa3 and Mayumi Oda11Oda Animal Hospital, Hiroshima, Japan 2Laboratory of Surgery, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan 3Laboratory of Veterinary Pathology, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan *Corresponding Author: Konatsu Miura. Oda Animal Hospital, Hiroshima, Japan. Email: konatsum0425 [at] gmail.com Submitted: 08/06/2022 Accepted: 12/09/2022 Published: 10/10/2022 © 2022 Open Veterinary Journal
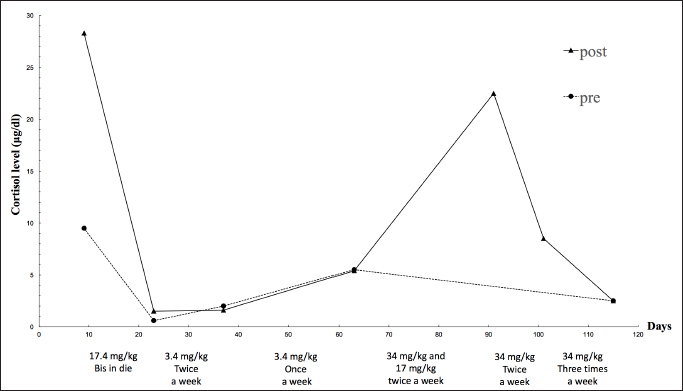
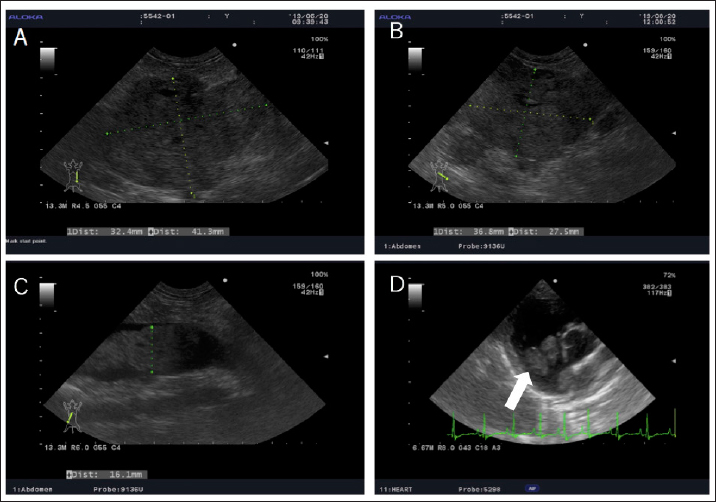
AbstractBackground: In humans, ectopic Cushing’s syndrome (ECS) is characterized by hypercortisolemia, which is caused by small lung carcinoma, bronchial carcinoids, and pheochromocytoma. In dogs, only a few cases of ECS associated with pheochromocytoma have been reported to date. Case Description: Herein, we describe a canine case of malignant pheochromocytoma that is presumed to be the cause of ECS. An 11-year-old, castrated, male Toy Poodle with hypercortisolemia was diagnosed with an adrenal tumor (AT) and treated with mitotane. Although repeated adrenocorticotropic hormone stimulation tests revealed improvement in the dog’s condition by mitotane treatment, its condition started declining 197 days post-diagnosis, and he died on day 280. The necropsy revealed the AT was a pheochromocytoma, not an adrenocortical tumor. However, because of no pathological change in the pituitary gland and the other adrenal gland, pheochromocytoma was presumed to be the cause of ECS. Conclusion: This is the first report that describes the effectiveness of mitotane against presumed ECS-related pheochromocytoma. Keywords: Dog, Ectopic Cushing’s syndrome, Hypercortisolemia, Pheochromocytoma. IntroductionHyperadrenocorticism (Cushing’s syndrome) is a symptom caused by the excess secretion of glucocorticoids (Iwasaki et al., 2011; Nelson and Couto, 2011; Tsujimoto et al., 2015). The symptoms are usually attributed to the hypersecretion of adrenocorticotropic hormone (ACTH) by pituitary tumors [pituitary-dependent hyperadrenocorticism (PDH)] and adrenal tumors (ATs). In dogs with hyperadrenocorticism, PDH accounts for 90%, while ATs and abnormal corticotropin-releasing hormone secretion in the hypothalamus are rarely observed (Iwasaki et al., 2011; Nelson and Couto, 2011). In the cases of ATs, cortisol is secreted autonomously without any regulation and its levels become excessive (Nelson and Couto, 2011). Cases of ectopic Cushing’s syndrome (ECS) caused by extrapituitary ACTH-producing tumors have been reported in humans. In these cases, ACTH is secreted by small-cell lung cancer, bronchial carcinoids, pancreatic islet cell tumors, and pheochromocytoma (White et al., 2000; Shindou et al., 2012; Folkestad et al., 2014; Gabi et al., 2018). In dogs, only a few cases of ECS due to pheochromocytoma has been reported (Galac et al., 2005; Lee et al., 2020). However, there has been no report that described a canine or a human case of ECS-related pheochromocytoma with anti-tumor medication. We experienced a canine case of ECS-related pheochromocytoma with long-term mitotane treatment and showed improved cortisol levels and survived 280 days. We describe detailed long-term follow-up and pathological examination at autopsy in detail. Case DetailsAn 11-year-old, castrated male Toy Poodle, weighing 7.4 kg, that had a right inguinal hernia and a skin lesion received a medical examination at Oda Animal Hospital. He had no major medical history. A physical examination revealed abdominal distension, right inguinal hernia, and epidermal collarette on the entire dorsal skin. The dog also showed Cushing body shape, polydipsia, and polyuria. Thus, we suspected Cushing’s syndrome and the serum cortisol levels are summarized in Figure 1. In addition, a Grade II/VI systolic murmur was heard from the left ventricle. We speculated that the inguinal hernia was caused by muscle weakness due to Cushing's syndrome and that the heart murmur was derived from heart disease such as valve disease. The skin was preliminarily diagnosed as simple pyoderma. We proposed an imaging test, a urine test, and a blood test, but due to the owner's financial constraints, we decided to perform only the abdominal echography and epidermal curettage test on that day. Abdominal echography showed a mixed echogenic intraperitoneal tumor continuous to the left adrenal gland, occupying more than 50% of the abdominal cavity, and the right adrenal gland was not visible. In addition, findings suggestive of tumor invasion into the caudal vena cava were obtained, but the details could not be confirmed because the tumor was too large (Fig. 2A). Therefore, we suspected a huge functional primary AT. The owner was unsure whether to give aggressive treatment or not, so I decided to ask her to come home and think about it. By epidermal curettage test on the dorsal epidermal collarette, we found bacteria thought to be Staphylococci. At the owner’s request, we prescribed cephalexin (Relexepet, 20 mg/kg, PO, BID, Virbac, Kanagawa, Japan) for the dorsal epidermal collarette. On day 9, the dog developed polyuria, but the exact amount of urine passed was not examined. We explained that by knowing the cortisol levels in the body, it might be possible to reduce the volume of the tumor from a medical point of view, and consent was obtained. As the ACTH stimulation test revealed high cortisol levels (pre-stimulation level, 9.5 μg/dl and post-stimulation level, 28.3 μg/dl), we suspected that the intraperitoneal tumor was adrenocortical adenocarcinoma and started mitotane (LYSODREN tablets, Bristol Myers Squibb, New York, NY) administration at 17.4 mg/kg BID according to references (Nelson and Couto, 2011). The changes in cortisol level and mitotane dose are summarized in Figure 1. On day 23, the polydipsia improved and the ACTH stimulation test revealed that the cortisol level decreased (pre-stimulation level, 0.6 μg/dl and post-stimulation level, 1.5 μg/dl). Thus, we thought that the effect of mitotane reduced the AT and reduced cortisol levels. Therefore, the mitotane dose was changed to 34 mg/kg and administered twice a week according to references (Nelson and Couto, 2011). On day 37, as the cortisol levels were found to remain low (pre-stimulation level, 2.0 μg/dl and post-stimulation level, 1.6 μg/dl), mitotane administration was reduced to once a week. On day 63, the dog started showing other symptoms such as a decrease in appetite and a whitish nasal discharge. Direct physical examination revealed a stridor. Although the dog’s general condition did not deteriorate, the cortisol level was higher than last time (pre-stimulation level, 5.5 μg/dl and post-stimulation level, 5.4 μg/dl) and mitotane was administered twice a week at alternate doses of 34 and 17 mg/kg. Palpation also revealed the disappearance of abdominal distension. Abdominal ultrasonography showed that the tumor was entirely isolated around the left kidney, and it had clearly reduced in size compared to that at the initial diagnosis. Nevertheless, tumor formation in the posterior vena cava was clear. A tumor thrombus with a maximum minor axis of 16.1 mm occupying more than 90% of the posterior vena cava was observed. The longest diameter of the tumor thrombus could not be measured because the tumor thrombus was quite long and could not be traced by echo examination (Fig. 2B and C). On day 91, the dog’s appetite had markedly reduced and polydipsia did not recur. However, the post-stimulation cortisol level returned to a high level (22.5 μg/dl). Thus, the mitotane dose was increased to 34 mg/kg and administered twice a week. In addition, as symptomatic treatment, metoclopramide (0.2 mg/kg, PO, BID, Nichi-Iko Pharmaceutical Co. Ltd., Tokyo, Japan), ranitidine (Takepron, 2.1 mg/kg, PO, BID, Glaxo SmithKline plc, London, Greek), and cyproheptadine (Periactin, 0.5 mg/kg, PO, BID, Nichi-Iko Pharmaceutical Co. Ltd., Tokyo, Japan) were prescribed to improve the decreased appetite. On day 101, as the appetite had not recovered and the cortisol level had reduced to 8.5 μg/dl, the mitotane administration was increased to three times a week at 34 mg/kg. On day 115, the general condition remained unchanged, and an ACTH stimulation test was performed. Since the cortisol level was below 2.5 μg/dl both pre and post, the dose of mitotane was reduced again to twice a week. On day 143, decreased appetite was also observed only in the morning, and the cortisol level was 1.5 μg/dl, so the dose of mitotane was continued as before.
Fig. 1. Changes in mitotane dose and the serum cortisol levels examined by ACTH stimulating tests. The broken line and the solid line show the cortisol levels before and after ACTH stimulation respectively. The texts under the graph indicate the mitotane dose in each period. However, on day 197, the dog visited because of a severe decline both in appetite and activity, and the left eyeball protrusion. A physical examination revealed external strabismus and protrusion of the left eye and deformity of the fourth premolar to the jawbone. A Grade II/VI systolic murmurs were heard from the right chest wall and left basal portion of the heart. Chest echography revealed wart-like structures in the right atrium (Fig. 2D). Blood tests showed elevated white blood cell count (21,800/μl), elevated C-reactive protein (CRP) (3.7 mg/dl), and mildly elevated GPT (100 U/l). Based on blood tests and echography, the possibility that warts in the right atrium were complicated by bacterial endocarditis could not be ruled out, and there was a high possibility that the nasal discharge was likely due to a bacterial infection, so, enrofloxacin (Baytril, 5 mg/kg, PO, SID, Bayer Yakuhin, Ltd., Osaka, Japan) was prescribed. However, the nasal discharge remained severe and the symptoms of polydipsia recurred. No further treatment was desired by the owner. Finally, the dog died on day 280.
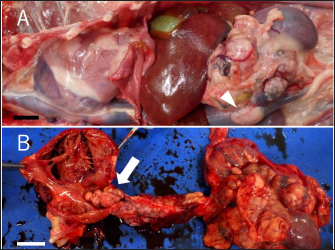
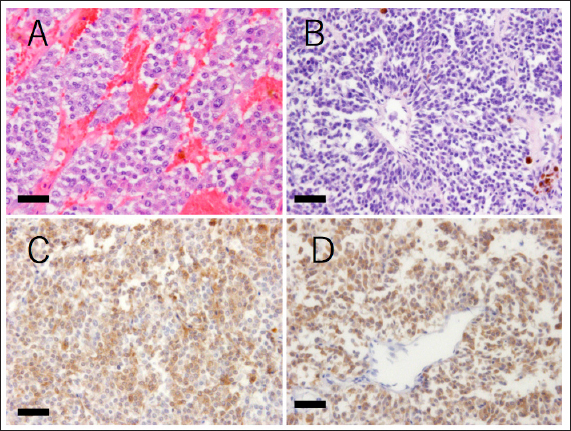
Fig. 2. Echography images of the abdominal and cardiac regions. (A) An intra-abdominal tumor with continuously mixed echogenicity is found in the left adrenal gland, occupying most of the abdominal cavity on day 1. (B and C) At day 63, the size of the tumor mass, particularly, the size of the tumor plug in the posterior vena cava, is clearly reduced. (D) Wart-like structures (shown by an arrow) are also found in the right atrium on day 203. A necropsy was performed at the Laboratory of Veterinary Pathology, Yamaguchi University. A gross examination of the abdominal cavity revealed that a peritoneal tumor originated in proximity to the left kidney and was considered an adrenal origin. And the tumor had invaded the dorsal-ventral wall of the left lumbar region. It adheres to the subcutaneous muscle layer, but does not invade the muscle layer (Fig. 3A). The tumor had proliferated in the vascular lumen between the renal vein and the posterior vena cava, and extended to the right atrium (Fig. 3B). The wart-like structures in the right atrium observed by echography were considered as tumor tissues macroscopically. In addition, the dog’s face was deformed because of the presence of a tumor occupying the nasal cavities and extending from the oral cavity. The tumor dominated the space from the hard palate to the nasal cavities and infiltrated the nasal cavities, pharyngeal cavities, and retrobulbar space. The pituitary gland and right adrenal gland were normal macroscopically. However, in the cut surface of the right adrenal gland, there was one white nodule with a diameter of about 5 mm, which was considered to be a cortical hyperplasia lesion. Histological examination revealed the abdominal mass comprised polyhedral to spindle cells forming a nest and pseudorosette (Fig. 4A and B). Although immunohistochemical staining was performed using rabbit anti-human chromogranin A (poly, ready to use; Nichirei Bioscience, Tokyo, Japan), mouse anti-human melan A (clone A103, 1:200; Dako, Carpinteria, CA), and rabbit anti-ACTH (ready to use; Zymed Laboratories, San Francisco, CA) only chromogranin A returned a positive result (Fig. 4C and D). Therefore, the intraperitoneal tumor was diagnosed as a pheochromocytoma. In addition to the vena cava and abdominal wall, micrometastatic foci were found in the liver. No histological abnormality was found in the brain and pituitary gland. The right adrenal gland, in the macroscopically observed white nodule, chromogranin-A-positive medulla-derived tumor cells, similar to those in the left adrenal gland, proliferate while compressing the cortex. The nasal tumor was diagnosed as chondrosarcoma, and negative for chromogranin A. The tricuspid valve becomes moderately thickened with mucoid generation, but the myocardium was unchanged.
Fig. 3. Gross finding of the abdominal region. (A) A large tumor mass (arrowhead) locates in the cranial region of the left kidney. The mass attached to and invaded the left renal vein deeply and invades the lumen of vena cava. (B) The tumor tissue invades also the vena cava and grew upstream to the right ventricle. The whole surface and the cut section of the mass are white or dark red. Bars indicate 2 cm. DiscussionTo date, only a few cases of ECS-related pheochromocytoma have been reported in dogs (Galac et al., 2005; Lee et al., 2020; Arias et al., 2021). In one case, the left AT was surgically removed and the cortisol level returned to the normal range without any medication (Lee et al., 2020). In the present case, the ACTH stimulation tests revealed that the cortisol levels increased in an ACTH-dependent manner. This is a characteristic feature of Cushing’s syndrome. However, the abdominal mass was pheochromocytoma, not an adrenocortical tumor, and immunostaining was negative for the ACTH marker. In humans, it has been reported that there are cases of ectopic ACTH-producing tumors that are negative for anti-ACTH antibodies by immunostaining (Folkestad et al., 2014). Immunostaining with anti-CRH antibodies is used for CRH-producing tumors in humans, but it is difficult to distinguish between them in dogs because it is currently difficult to use. Thus, we considered that pheochromocytoma was the cause of ECS in this case. In addition, transient tumor regression was observed by treatment with mitotane, a drug effective for ACTH-producing tumors (Waszut et al., 2017). Thus, we suspected that the pheochromocytoma was ACTH-producing and caused ECS. By treatment with mitotane, the cortisol level decreased significantly and the size of the left adrenal mass was reduced. This suggests that mitotane exerts some effects on ectopic ACTH-producing tumor cells. The dog survived for 280 days after the tumor diagnosis. This survival period was significantly longer than those reported in other cases of typical pheochromocytoma with the invasion of the caudal vena cava (Barthez et al., 1997; Barrera et al., 2013; Musser et al., 2018). We consider this prolonged survival period was also attributed to mitotane treatment. To our knowledge, this is the first case report which describes the use and effectivity of mitotane in ECS-related pheochromocytoma. The findings of this case suggest that mitotane treatment enables long-term survival in dogs with ECS-related pheochromocytomas, which are quite hard to treat surgically. However, there is a case that a combination of cortical and medullary ATs may be simultaneously present (Appelgrein et al., 2020). We consider a possibility that adrenocortical tumor and pheochromocytoma coexisted, and only the cortical tumor reduced in size due to mitotane, leaving only the pheochromocytoma.
Fig. 4. Histopathological findings of the tumor tissue. (A and B) Hematoxylin and eosin stain. The mass comprises variously shaped tumor cells. The cells are polyhedral with large eosinophilic cytoplasm or are spindle-shaped with scant cytoplasm. The polyhedral cells are arranged in nests or chords and are separated by fine septa or capillary collagen. The spindle cells are scattered and form pseudorosettes in some regions. (C and D) Anti-chromogranin A immunohistochemistry. Both types of tumor cells are positive for chromogranin A, a marker of neuroendocrine cells (detected in brown). Bars indicate 50 μm. In addition, based on the references and the owner's intentions, the patient was treated with mitotane, but we should think about whether there were differences in tumor response with the dose or with another medicine, such as trilostane. More information is needed to confirm the effect of mitotane against ECS-related tumors including pheochromocytoma. Conflict of interestThe authors declare that there is no conflict of interest. AcknowledgmentsWe express our sincere thanks to the owner of the dog for donating her canine patient’s remains for our pathological examination. ReferencesAppelgrein, C., Hosgood, G., Drynan, E. and Nesbitt, A. 2020. Short-term outcome of adrenalectomy in dogs with adrenal glamd tumours that did not receive pre-operative medical management. Aust. Vet. J. 98(9), 449–454. Barrera, J.S., Bernard, F., Ehrhart, E.J., Withrow, S.J. and Monet, E. 2013. Evaluation of risk factors for outcome associated with adrenal gland tumors with or without invasion of caudal vena cava and treated via adrenalectomy in dogs:86 cases (1993-2009). J. Am. Vet. Med. Assoc. 15, 1715–1721. Barthez, P.Y., Marks, S.L., Woo, J., Feldman, E.C. and Matteucci, M. 1997. Pheochromocytoma in dogs: 61 cases (1984-1995). J. Vet. Intern. Med. 11, 272–278. Arias, E.A.S., Castillo, V.A., Louiset, E. and Lefebvre, H. 2021. Cushing's syndrome caused by intra-adrenocortical adrenocorticotropic hormone in a dog. J. Vet. Intern. Med. 36, 264-271. Folkestad, L., Andersen, M.S., Nielsen, A.L. and Glintborg, D. 2014. A rare cause of Cushing’s syndrome: an ACTH-secreting phaeochromocytoma. BMJ Case Rep. 2014, bcr2014205487. Gabi, J.N., Milhem, M.M., Tover, Y.E., Karem, E.S., Gabi, A.Y. and Khthir, R.A. 2018. Severe Cushing syndrome due to an ACTH-producing pheochromocytoma: a case presentation and review of the literature. J. Endocr. Soc. 2, 621–630. Galac, S., Kooistra, H.S., Voorhout, G., Van, Den, Ingh, T.S., Mol, J.A., Van, den, Berg, G. and Meji, B.P. 2005. Hyperadrenocorticism in a dog due to ectopic secretion of adrenocoticotropic hormone. Domest. Anim. Endocrinol. 28, 338–348. Iwasaki, T., Tsujimoto, H. and Hasegawa, A. 2011. Textbook of veterinary internal medicine. Tokyo, Japan: Buneidou. Lee, S., Lee, A., Chai, S.H., Lee, S., Kweon, O.K. and Kim, W.H. 2020. Ectopic Cushing’s syndrome associated with a pheochromocytoma in a dog: a case report. BMC Vet. Res. 16, 35. Musser, M.L., Taikowski, K.L., Johannes, C.M. and Bergman, P.J. 2018. Retrospective evaluation of toceranib phosphate (Palladia®) use in the treatment of inoperable, metastatic, or recurrent canine pheochromocytomas: 5 dogs (2014–2017). BMC Vet. Res. 14, 272. Nelson, R.W. and Couto, C.G. 2011. Small animal internal medicine, 4th ed. Tokyo, Japan: Interzoo Publishing Co., Ltd. Shindou, T., Masumori, N., Tanaka, T., Kitamura, H., Takahashi, S., Sunaga, D., Tsuchihashi, K. and Tsukamoto, Y. 2012. A case of ACTH-producing pheochromocytoma treated by laparoscopic adrenalectomy. Jpn. J. Endourol. 25, 337–340. Tsujimoto, H., Koyama, S., Okusa, K. and Kaneshima, T. 2015. A guidebook of medical treatments for sick cats and dogs 2015: my methods. Tokyo, Japan: Interzoo Publishing Co., Ltd. Waszut, U., Szyszka, P. and Dworakowska, D. 2017. Understanding mitotane mode of action. J. Physiol. Pharmacol. 68, 13–26. White, A., Ray, D.W., Talbot, A., Abraham, P., Thody, A.J. and Bevan, J.S. 2000. Cushing’s syndrome due to phaeochromocytoma secreting the precursors of adrenocorticotropin. J. Clin. Endocrinol. Metab. 85, 4771–4775. | ||
| How to Cite this Article |
| Pubmed Style Miura K, HS, Sakai Y, . Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.. Open Vet J. 2022; 12(5): 762-767. doi:10.5455/OVJ.2022.v12.i5.22 Web Style Miura K, HS, Sakai Y, . Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.. https://www.openveterinaryjournal.com/?mno=54130 [Access: April 19, 2024]. doi:10.5455/OVJ.2022.v12.i5.22 AMA (American Medical Association) Style Miura K, HS, Sakai Y, . Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.. Open Vet J. 2022; 12(5): 762-767. doi:10.5455/OVJ.2022.v12.i5.22 Vancouver/ICMJE Style Miura K, HS, Sakai Y, . Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.. Open Vet J. (2022), [cited April 19, 2024]; 12(5): 762-767. doi:10.5455/OVJ.2022.v12.i5.22 Harvard Style Miura, K., , H. S., Sakai, Y. & (2022) Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.. Open Vet J, 12 (5), 762-767. doi:10.5455/OVJ.2022.v12.i5.22 Turabian Style Miura, Konatsu, Hiroshi Sunahara, Yusuke Sakai, and . 2022. Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.. Open Veterinary Journal, 12 (5), 762-767. doi:10.5455/OVJ.2022.v12.i5.22 Chicago Style Miura, Konatsu, Hiroshi Sunahara, Yusuke Sakai, and . "Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.." Open Veterinary Journal 12 (2022), 762-767. doi:10.5455/OVJ.2022.v12.i5.22 MLA (The Modern Language Association) Style Miura, Konatsu, Hiroshi Sunahara, Yusuke Sakai, and . "Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.." Open Veterinary Journal 12.5 (2022), 762-767. Print. doi:10.5455/OVJ.2022.v12.i5.22 APA (American Psychological Association) Style Miura, K., , H. S., Sakai, Y. & (2022) Effective treatment with mitotane for a canine case of Ectopic Cushing's syndrome-related pheochromocytoma.. Open Veterinary Journal, 12 (5), 762-767. doi:10.5455/OVJ.2022.v12.i5.22 |