| Case Report | ||
Open Vet J. 2022; 12(4): 502-507 Open Veterinary Journal, (2022), Vol. 12(4): 502–507 Case Report Secretory carcinoma of the canine mammary gland with nodal and bone metastases: Case reportHalana do Carmo Silva1, Marina Possa dos Reys1 Geovanni Dantas Cassali2, Fernanda Rezende Souza2, Rodrigo dos Santos Horta3, Bruna Voltolin de Sena1, Adriano Lima Stelzer Bindaco1, Ana Carolina de Jesus Pinto1, Tayse Domingues de Souza1 and Mayra Cunha Flecher1*1Department of Veterinary Medicine, Universidade Vila Velha, Vila Velha, Brazil 2Department of General Pathology, Laboratory of Comparative Pathology, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil 3Department of Veterinary Medicine and Surgery, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil *Corresponding Author: Mayra C. Flecher. Department of Veterinary Medicine, Universidade Vila Velha, Vila Velha, Brazil. Email: mayra.flecher [at] uvv.br Submitted: 12/12/2021 Accepted: 08/07/2022 Published: 03/08/2022 © 2022 Open Veterinary Journal
AbstractBackground: Secretory carcinoma is a rare histological type of breast neoplasm in humans and dogs that is characterized by the presence of intracellular and extracellular eosinophilic secretions. Case Description: In this case report, we describe the cytological, histological, and immunohistochemical characteristics of secretory mammary carcinoma in a 10-year-old mixed-breed female dog with nodal and bone metastases. The bitch had a history of claudication and a mass in the left humeral scapular region, which revealed osteolysis of the proximal humerus on radiography. Fine-needle aspiration cytology revealed numerous neoplastic cells arranged mostly in cohesive groups but sometimes isolated, that contained cytoplasmic vacuoles and had a moderate-to-high nucleus: cytoplasm ratio with frequent karyomegaly and evident nucleoli. Histologically, the neoplasm was organized in solid, tubular structures with luminal spaces filled with eosinophilic secretions and was composed of cells with clear cytoplasm and prominent vacuoles that pushed the nuclei to the periphery, resembling signet ring cells. The extracellular and intracytoplasmic material of the epithelial cells was positive for periodic acid-Schiff staining and immunoreactive for alpha-lactalbumin. Two chemotherapy sessions were performed, but 1 month after surgery, the clinical condition worsened, and euthanasia was elected, accounting for 133 days of survival after surgical removal of the tumor. Conclusion: The bitch presented with secretory mammary carcinoma with nodal and bone metastases, and histological and immunohistochemical characteristics were important for diagnosis. The morphological and immunohistochemical characteristics of this carcinoma were similar to those observed in humans. Mammary gland secretory carcinoma with bone metastasis must be included as a differential diagnosis among canine mammary gland carcinomas showing cellular morphological characteristics of intracytoplasmic vacuolization and eosinophilic secretion. Keywords: Alpha-lactalbumin, Dog, Mammary gland carcinoma. IntroductionMammary gland tumors are very common in non-spayed female dogs, representing 50%–70% of neoplasms in this species (Nunes et al., 2018; Silva et al., 2019). Tumor occurrence is directly related to patient age and hormone exposure (Sorenmo et al., 2013). The frequency of malignant mammary neoplasms is higher than that of benign neoplasms (Nunes et al., 2018; Silva et al., 2019). The most frequent histological types of malignant neoplasms of the mammary gland in bitches are mixed tumor carcinomas, carcinomas in situ, papillary, tubular, and solid types. Special types, such as anaplastic, mucinous, basal cell, and lipid-rich carcinomas, are uncommon (Sorenmo et al., 2013; Cassali et al., 2014; Silva et al., 2019). Secretory carcinoma is a rare histological type of breast cancer in humans (Suzuki et al., 1999; Banerjee et al., 2021), initially called juvenile carcinoma because of its frequent occurrence in children (McDivitt and Stewart, 1966). The pattern of histological organization of cells may vary, but the deposition of extracellular and intracellular secretory eosinophilic material is an important feature (Li et al., 2012; Din et al., 2013), as well as the presence of cytoplasmic vacuoles. In veterinary medicine, only one case has been reported in a 3-year-old female dog, whose diagnosis was made through cytological, histopathological, and immunohistochemical staining (Cassali et al., 2020). Other mammary gland carcinomas may have cells with vacuolated cytoplasm that also produce intracellular and extracellular content such as lipid-rich, glycogen-rich, and mucinous material (Cassali et al., 2014). The differentiation between these carcinomas is made by histochemical staining with Oil Red O for lipids, periodic acid-Schiff (PAS) for structures containing carbohydrates, PAS associated with the enzyme diastase to remove all the glycogen evidenced in the staining, and immunohistochemistry (IHC) to identify the intracytoplasmic secretion product (Sorenmo et al., 2013; Cassali et al., 2014). This study aimed to report the clinical, cytological, histopathological, and immunophenotypic findings in a female dog diagnosed with mammary gland secretory carcinoma with metastases to regional lymph nodes and bone in the proximal region of the humerus. Case DetailsA 10-year-old female mixed-breed dog presented with claudication of the left thoracic limb with the manifestation of local pain, fever, apathy, prostration, hyporexia, and progressive cachexia. The animal still had a regular estrous cycle, with estruses every 6 months, and there was no history of pregnancy, pseudocyesis, or use of contraceptives. Physical examination revealed a mass of approximately 8 cm by 4 cm in the right abdominal caudal (M4) and inguinal (M5) mammary glands, a slight increase in the volume of the right inguinal lymph node, and a non-ulcerated mass adhered to the left humeral scapular region, with a 7-month evolution. Complementary tests were performed, including chest and left thoracic limb radiography and fine-needle aspiration biopsy. Radiography of the left thoracic limb revealed areas of bone lysis in the proximal portion of the humerus. There was no evidence of metastases in the abdomen or thorax on abdominal ultrasound or thoracic radiographs. The aspiration biopsy of the mammary mass revealed a high cell density, multidimensional cohesive groups of cells, cytoplasm with indistinct borders, bluish color with some pink material, a large number of vacuoles, a moderate to high nucleus: cytoplasm ratio, round to oval nuclei, predominantly single, lacy chromatin, multiple and prominent nucleoli, marked anisocariosis with frequent karyomegaly, moderate cells, and nuclear pleomorphism (Fig. 1A). The samples from the inguinal lymph node and the mass of the proximal region of the humerus consisted predominantly of the same groups of cells as the breast mass. It was concluded to be mammary carcinoma with metastasis to the inguinal lymph node and proximal region of the humerus. After aspiration biopsy, a right unilateral partial mastectomy (M3 to M5) was performed with high amputation of the left thoracic limb and lymphadenectomy of the respective regional lymph nodes (right inguinal and axillary, and left superficial cervical), and the material was removed for histopathological examination. The fragments were processed according to routine techniques by embedding in paraffin, staining with hematoxylin and eosin , and analyzing using an Eclipse E-200 light microscope (Nikon, FN20).
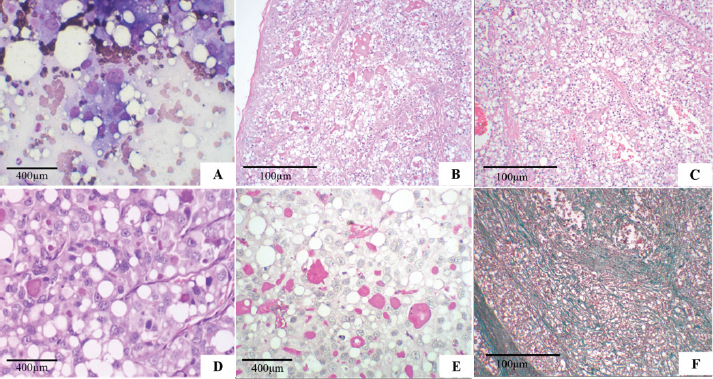
Fig. 1. Secretory carcinoma of the canine mammary gland. (A): Fine-needle aspiration cytology. There is high cellularity consisting of multidimensional groups of cells, sometimes isolated, with vacuolar cytoplasm, possibly containing amorphous pink material; large, round to ovoid nuclei, with marked anisocariosis, frequent karyomegaly (Diff-Quik, 100×). (B): Histopathology. Cells in solid arrangement with eosinophilic secretion (HE, 100×). (C): Histopathology of mammary gland carcinoma showing well-vascularized and fibrocollagenous stroma (HE, 100x). (D): Histopathology of mammary gland carcinoma showing cells with vacuolar cytoplasm, round nucleus displaced to the periphery (signet ring appearance), and prominent nucleoli (HE, 400×). (E): Histopathology. Evidence of abundant cytoplasmic vacuoles and containing homogeneous secretion material, positive with PAS (PAS, 400×). (F): Histopathology. Green stained fibrocollagenous stroma (Gomori trichrome, 100×). Macroscopically, the mammary tumor measured 8.1 × 3.9 × 4.9 cm. When cut, the mammary tumor had a solid, multinodular surface, and was whitish with multifocal to reddish coalescent areas. The mass in the proximal region of the humerus, measuring 6.6 × 5.3 × 5.7 cm, had characteristics similar to the breast mass. When cut, the mass exhibited a multinodular aspect with creaky areas and bone infiltration. The right inguinal, axillary, and left superficial cervical lymph nodes presented extensive white and firm nodular areas when cut. The histopathological examination of the four fragments showed that the mammary tumor consisted of a multilobular neoformation, was poorly delimited and infiltrative with variable and well-vascularized fibrocollagenous stroma, and was organized in solid, tubular areas with luminal spaces filled with eosinophilic secretion (Fig. 1B and C). The mammary tumor was constituted by cells with moderate and vacuolated cytoplasm, variable nucleus: cytoplasm ratio, round to oval nucleus that was central, but often peripheral (signet-ring appearance) (Fig. 1D) with loose chromatin, single to multiple, and prominent nucleoli. Marked anisocytosis and anisocariosis, marked cell and nuclear pleomorphism; seven mitotic figures in 2.37 mm² (Meuten et al., 2017). We frequently observed intracytoplasmic and extracellular homogeneous eosinophilic material positive for PAS (Fig. 1E), in addition to areas of hemorrhage and intratumoral coagulative necrosis. In the gomori trichrome, collagen was stained in green (Fig. 1F). Extensive areas of neoplastic infiltration were observed in the axillary and inguinal lymph nodes, in the subcapsular and medullary sinuses, consisting of the same cells observed in the mammary tumor, characterized as metastases of carcinoma. The mass in the proximal region of the humerus was characterized by multilobular neoplastic cell proliferation, which infiltrated the musculature and adjacent bone tissue with characteristics similar to those observed in the breast mass. Such morphological characteristics are consistent with the diagnosis of mammary gland secretory carcinoma, grade II, according to the histological grading system of Elston and Ellis (Elston and Ellis, 1991), with metastases in the analyzed lymph nodes and bone. For the IHC reaction of mammary cancer, cytokeratin AE1/AE3 1:500 (Dako®), oestrogen receptor (ER–Clone EP1) ready-to-use (Dako®), progesterone receptor (PR–Clone hRP a2) 1:50 (Invitrogen®), Ki-67 (MIB-1) 1:50 (Dako®), and alpha-lactalbumin (polyclonal) 1:500 (Invitrogen®) were used. Blocking of endogenous peroxidation and non-specific proteins was performed with a commercial solution of the Novolink® system (Leica Biosystems Newcastle Ltd., UK) according to the manufacturer’s recommendations, and the recommendations proposed by the consensus on the diagnosis, prognosis, and treatment of canine and feline mammary tumors (Cassali et al., 2019). For ER and PR, >10% nuclear labeling was considered positive, Ki-67 was evaluated in at least 1,000 neoplastic cells in high-power (400X) fields, and nuclear labeling>20% was considered positive, and alpha-lactalbumin with cytoplasmic and secretory labeling. The obtained positive membrane and cytoplasmic immunostaining for cytokeratin (Fig. 2A), Ki-67 in 40% of cells (Fig. 2B), alpha-lactalbumin positively labeled the cytoplasm and secretion (Fig. 2C), ER was negative (Fig. 2D), PR was positive in >75% (++++) (Fig. 2E). After surgery and histopathological diagnosis, two chemotherapy sessions were performed with a combination of carboplatin (10–10 mg/kg) and gemcitabine (200–200 mg/m²), with an interval of 1 week between sessions. 1 month after the surgery, the clinical condition worsened, with refractory pain in the region of the amputation scar, and euthanasia was elected, accounting for 133 days of survival after surgical removal of the tumor. The animal was autopsied after euthanasia, and samples were collected from the lungs, liver, kidneys, adrenals, spleen, pancreas, heart, intestine, and brain; however, there were no findings of metastasis or recurrence of the breast mass. Ethical approvalThe data used in this manuscript were obtained from Prof. Ricardo Alexandre Hippler of the Veterinary Hospital at Universidade Vila Velha (ES, Brazil). This case was part of routine clinical practice. The necropsy and study protocols were approved by the owner and hospital, and all procedures were performed by institutional guidelines (Universidade Vila Velha). DiscussionSecretory carcinoma is a special type of mammary gland tumor (Gamba et al., 2017) with an overall incidence of less than 0.15% in humans (Banerjee et al., 2021). In dogs, (Gamba et al., 2017), only one case has been reported to date (Cassali et al., 1999), in addition to that described in this study. In women, breast secretory carcinoma has a good prognosis with a low incidence of lymph node metastasis of only 30%, and distant metastases are also infrequent (Vasudev and Onuma, 2011; Horowitz et al., 2012; Li et al., 2012; Banerjee et al., 2021). In female dogs, metastases were observed in the lungs, kidneys, adrenals, pancreas, heart, intestine, diaphragm, and peritoneum in a 3-year-old German Shepherd dog (Cassali et al., 1999), and in the humerus and lymph nodes in a 10-year-old mixed-breed dog reported in the present study. Bone metastases are uncommon in dogs with mammary tumors, but this result may be underestimated (Craig et al., 2016). Bone metastases of carcinomas in the mammary gland and other tissues in dogs usually occur in the axial skeleton and the proximal region of long bones (Craig et al., 2016), and they preferentially occur via the hematogenous route (Theriault and Theriault, 2012; Tahara et al., 2019). It has been reported that bone metastases must be preceded by pulmonary metastases (Cooley and Waters, 1998), but as in the present report, bone metastases may occur without previous or concurrent pulmonary metastases (Trost et al., 2014).
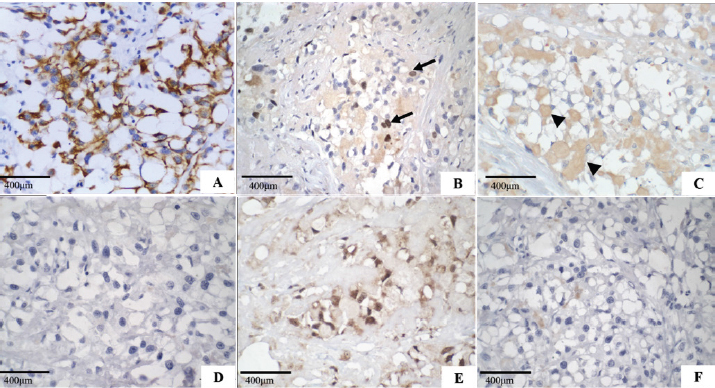
Fig. 2. Secretory carcinoma of the canine mammary gland, Immunohistochemistry. (A): Positive membrane labeling for pancytokeratin (AE1/AE3), which confirms the epithelial component of the neoplasm (IHC, 200×). (B): Nuclear labeling for Ki-67 (MIB-1) in 40% of neoplastic cells (arrow) (IHC, 400×). (C): Cytoplasmic labeling for alpha-lactalbumin, of intracytoplasmic and extracytoplasmic secretion (arrowhead) (IHC, 400x). (D): Negative oestrogen. (E): Nuclear labeling for progesterone. (F): Negative control for hormone receptors (400×). The morphological characteristics of the neoplasm described in this work are similar to those of mammary gland secretory carcinoma described in a female dog (Cassali et al., 1999), and in women (Suzuki et al., 1999; Vasudev and Onuma, 2011). It presents an infiltrative characteristic with solid and/or tubular proliferation and contains spaces full of eosinophilic secretion. The neoplasm presented here is also composed of cells with granular cytoplasm, clear eosinophilic, and prominent vacuoles that displace the nucleus to the periphery, resembling signet-ring cells. In women, microcystic, cystic, solid, and papillary patterns are observed histologically (Li et al., 2012; Din et al., 2013). However, in veterinary medicine, both the 1999 case (Cassali et al., 1999) and the one reported here, showed a solid organization. The intracytoplasmic content of secretory carcinoma cells is PAS-positive (Cassali et al., 1999; Suzuki et al., 1999; Din et al., 2013; Sharma et al., 2015), which was observed in this study, whereas in cells involved in lipid- and glycogen-rich carcinomas, PAS and diastase were negative (Cassali et al., 1999; Cassali et al., 2014). In contrast, mucinous carcinoma secretions are positive for PAS, diastase, and Alcian Blue in at least 40% of its growth, and mucin accumulation is predominantly located in the intraductal component (Cassali et al., 2014), which was not observed in this case. Alpha-lactalbumin is a diastase-resistant protein that is different from what is observed for glycogen, and intracytoplasmic evidence allows the diagnosis of secretory carcinoma (Cassali et al., 1999; Vasudev and Onuma, 2011; Cassali et al., 2014). The IHQ characterization in the present case is similar to that previously described in dogs and women (Cassali et al., 1999; Suzuki et al., 1999; Vasudev and Onuma, 2011; Din et al., 2013; Sharma et al., 2015) with positive immunolabeling for cytokeratin and alpha-lactalbumin and negative ER. However, PR differs from some cases reported in humans (Li et al., 2012; Sharma et al., 2015; Banerjee et al., 2021) and is correlated with tumor-favorable biological behavior. Ki-67 was expressed in 40% of neoplastic cells, which was not seen on the HE, because most of the neoplastic cells were in interphase; this case indicates a high rate of cell proliferation, which implies a more aggressive biological behavior and, consequently, poor prognosis. The prognosis in humans is generally favorable (Horowitz et al., 2012; Banerjee et al., 2021), with indolent clinical behavior (Li et al., 2012), and a low chance of metastasis when the tumor size is <2 cm (Li et al., 2012; Trost et al., 2014). However, it may behave more aggressively in adults than in children (Suzuki et al., 1999; Li et al., 2012). In contrast to a previous report in veterinary medicine (Cassali et al., 1999), the dog in the present study was 10 years old, which, using an age conversion table (Lebeau, 1953), corresponding to a 56-year-old woman. The tumor also had a long axis of 8 cm and high Ki-67 labeling, which may be associated with worse outcomes. The survival of the bitch in the present study (133 days post-surgery) was 83 days longer than that observed in the first report of such neoplasia in dogs (Cassali et al., 1999), but significantly lower than those found in reports of mammary gland-lipid-rich carcinoma (Espinosa De Los Monteros et al., 2003; Perossi et al., 2019), which may indicate an even more aggressive biological behavior in dogs (Tei et al., 2012; Perossi et al., 2019). Up until now, this is the second report of canine mammary gland secretory carcinoma and the first report of bone metastasis, which suggests the aggressive behavior of this neoplastic type in such species. Furthermore, secretory carcinoma must be included in the differential diagnosis of canine mammary gland carcinomas, showing cellular morphological characteristics of intracytoplasmic vacuolization and eosinophilic secretion. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsHalana do Carmo Silva, Mayra Cunha Flecher, Geovani Dantas Cassali, Tayse Domingues de Souza, Adriano Lima Stelzer Bindaco and Ana Carolina de Jesus Pinto performed the cytological, necropsy and histopathological description. Geovani Dantas Cassali and Fernanda Rezende de Souza performed immunohistochemistry. Bruna Voltolin de Sena and Rodrigo dos Santos Horta were oncologists. Halana do Carmo Silva, Mayra Cunha Flecher, Marina Possa dos Reys, Tayse Domingues de Souza and Rodrigo dos Santos Horta wrote the manuscript. All authors reviewed the manuscript and made important contributions to the manuscript. ReferencesAktepe, F., Sarsenov, D. and Özmen, V. 2016. Secretory carcinoma of the breast. J. Breast. Health. 12, 174–176. Banerjee, N., Banerjee, D. and Choudhary, N. 2021. Secretory carcinoma of the breast commonly exhibits the features of low grade, triple negative breast carcinoma- a case report with updated review of literature. Autops. Case. Rep. 11, 202–227. Cassali, G.D., Gobbi, H., Gartner, F. and Schmitt, F.C. 1999. Secretory carcinoma of the canine mammary gland. Vet. Pathol. 36, 601–603. Cassali, G.D., Jark, P.C., Gamba, C., Damasceno, K.A., Estrela-Lima, A., De Nardi, A.B., Ferreira, E., Horta, R.S., Firmo, B.F., Sueiro, F.A.R., Rodrigues, L.C.S. and Nakagaki, K.Y.R. 2020. Consensus regarding the diagnosis, prognosis and treatment of canine and feline mammary tumors - 2019. Braz. J. Vet. Pathol. 13, 555–574. Cassali, G.D., Lavalle, G.E., Ferreira, E., Estrela-Lima, A., De Nardi, A.B., Ghever, C., Sobral, R.A., Amorim, R.L., Oliveira, L.O., Sueiro, F.A.R., Beserra, H.E.O., Bertagnolli, A.C., Gamba, C.O., Damasceno, K.A., Campos, C.B., Araujo, M.R., Campos, L.C., Monteiro, L.N., Nunes, F.C., Horta, R.S., Reis, D.C., Luvizotto, M.C.R., Magalhães, G.M., Raposo, J.B., Ferreira, A.M.R., Tanaka, N.M., Grandi, F., Ubukata, R., Batschinski, K., Terra, E.M., Salvador, R.C.L., Jark, P.C., Delecrodi, J.E R., Nascimento, N.A., Silva, D.N., Silva, L.P., Ferreira, K.C.R.S., Frehse, M.S., di Santis, G.W., Silva, E.O., Guim, T.N., Kerr, B., Cintra, P.P., Silva, F.B.F., Leite, J.S., Mello, M.F.V., Ferreira, M.L.G., Fukumasu, H., Salgado, B.S. and Torres, R. 2014. Consensus for the diagnosis, prognosis and treatment of canine mammary tumours - 2013. Braz. J. Vet. Pathol. 7, 38–69. Cooley, D.M. and Waters, D.J. 1998. Skeletal metastasis as the initial clinical manifestation of metastatic carcinoma in 19 dogs. J. Vet. Intern. Med. 12, 288–293. Craig, L.E., Dittmer, K.E. and Thompson, K.G. 2016. Bones and joints. In Jubb, kennedy and palmer’s pathology of domestic animals, 5th ed. Ed., Maxie, M.G. St Louis, MO: Elsevier, pp: 1–184. Din, N.U., Idrees, R., Fatima, S. and Kayani, N. 2013. Secretory carcinoma of breast: clinicopathologic study of 8 cases. Ann. Diagn. Pathol. 17, 54–57. Elston, C.W. and Ellis, I.O. 1991. Pathological prognostic factors in breast cancer, I: the value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathol 19, 403–410. Espinosa De Los Monteros, A.E., Hellmén, E., Ramírez, G.A., Herráez, P., Rodríguez, F., Ordás, J., Millán, Y., Lara, A. and Mulas, J.M. 2003. Lipid-rich carcinomas of the mammary gland in seven dogs: clinicopathologic and immunohistochemical features. Vet. Pathol. 40, 718–723. Gamba, C.R., Ferreira, E., Salgado, B.S., Damasceno, K.Y., Bertagnolli, A.C., Nakagaki, K.Y.R. and Cassali, G.D. 2017. Neoplasias malignas. In Patologia mamária canina: do diagnóstico ao tratamento, 1st ed. Ed., Cassali, G.D. Brasil, pp: 91–114. Horowitz, D.P., Sharma, C.S., Connolly, E., Gidea-Addeo, D. and Deutsch, I. 2012. Secretory carcinoma of the breast: results from the survival, epidemiology and end results database. Breast 21, 350–353. Lebeau, A. 1953. La´geduchien et celui de l’homme. assai de statistique sur la mortalite´ canine. Bull. Acad. Vét. Fr. 26, 229–232. Li, D., Xiao, X., Yang, W., Shui, R., Tu, X., Lu, H. and Shi, D. 2012. Secretory breast carcinoma: a clinicopathological and immunophenotypic study of 15 cases with a review of the literature. Mod. Pathol. 25, 567–75. McDivitt, R.W. and Stewart, F.W. 1966. Breast carcinoma in children. J. Am. Med. Assoc. 195, 388–390. Meuten, D.J., Moore, F.M. and George, J.W. 2017. Appendix: mitotic count. In Tumors in domestic animals, 5th ed. Ed., Meuten, D.J. Ames, IA: John Wiley & Sons Inc., pp: 944–945. Nunes, F.C., Campos, C.B., Teixeira, S.V., Bertagnolli, A.C., Lavalle, G.E. and Cassali, G.D. 2018. Epidemiological, clinical and pathological evaluation of overall survival in canines with mammary neoplasms. Arq. Bras. Med. Vet. Zoo. 70, 1714–1722. Perossi, I.F.S., Martinelli, P.E.B., Bonato, L., Lima, G.P., Bertolo, P.H.L., Costa, R.R.M.E., Gómez, J.L.A., De Nardi, A.B. and Vasconcelos, R.O. 2019. Lipid rich carcinoma in canine mammary gland with metastasis in the abdominal cavity. Braz. J. Vet. Pathol. 13, 26–32. Sharma, V., Anuragi, G., Singh, S., Patel, P., Jindal, A. and Sharma, R.G. 2015. Secretory carcinoma of the breast: report of two cases and review of the literature. Case Rep. Oncol. Med. 2015, 1–5. Silva, H.C., de Oliveira, A.R., Horta, R.S., Merísio, A.C.R., de Sena, B.V., de Souza, M.C.C. and Flecher, M.C. 2019. Epidemiology of canine mammary gland tumors in espírito santo. Acta. Sci. Vet. 47, 1–9. Sorenmo, K.U., Worley, D.R. and Goldschmidt, M.H. 2013. Tumors of the mammary gland. In Small animal clinical oncology, 5th ed. Eds., Withrow, S.J. and Vail, D.M. St. Louis, MO: Saunder Elsevier, pp: 538–551. Suzuki, F., Saito, A., Ishi, K., Okazaki, T., Kina, K., Koyatsu, J. and Sugiyama, K. 1999. Secretory carcinoma of the breast: an immunohistochemical and ultrastructural study. Med. Electron. Microsc. 32, 50–56. Tahara, R.K., Brewer, T.M., Theriault, R.L. and Ueno, N.T. 2019. Bones metastasis of breast cancer. In Breast cancer metastasis and drug resistance, 2nd ed. Ed., Ahmad, A. Cham, Switzerland: Springer, pp: 117–129. Tei, M., Uchida, K., Chambers, J.K., Harada, H., Takahashi, M., Nishimura, R., Watanabe, M. and Nakayama, H. 2012. Mammary lipid-rich carcinoma with extensive amyloid deposition in a dog. J. Vet. Med. Sci. 74, 809–811. Theriault, R.L. and Theriault, R.L. 2012. Biology of bone metastases. Cancer Control 19, 91–101. Trost, M.E., Inkelmann, M.A., Galiza, G.J.N., Silva, T.M. and Kommers, G.D. 2014. Occurrence of tumors metastatic to bones and multicentric tumors with skeletal involvement in dogs. J. Comp. Pathol. 150, 8–17. Vasudev, P. and Onuma, K. 2011. Secretory breast carcinoma: unique, triple-negative carcinoma with a favorable prognosis and characteristic molecular expression. Arch. Pathol. Lab. Med. 135, 1606–1610. | ||
| How to Cite this Article |
| Pubmed Style Silva H, MR, Cassali G, Souza F, Horta R, BS, AB, AP, Souza T, Flecher M, . Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report. Open Vet J. 2022; 12(4): 502-507. doi:10.5455/OVJ.2022.v12.i4.12 Web Style Silva H, MR, Cassali G, Souza F, Horta R, BS, AB, AP, Souza T, Flecher M, . Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report. https://www.openveterinaryjournal.com/?mno=49314 [Access: April 18, 2024]. doi:10.5455/OVJ.2022.v12.i4.12 AMA (American Medical Association) Style Silva H, MR, Cassali G, Souza F, Horta R, BS, AB, AP, Souza T, Flecher M, . Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report. Open Vet J. 2022; 12(4): 502-507. doi:10.5455/OVJ.2022.v12.i4.12 Vancouver/ICMJE Style Silva H, MR, Cassali G, Souza F, Horta R, BS, AB, AP, Souza T, Flecher M, . Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report. Open Vet J. (2022), [cited April 18, 2024]; 12(4): 502-507. doi:10.5455/OVJ.2022.v12.i4.12 Harvard Style Silva, H., , M. R., Cassali, G., Souza, F., Horta, R., , B. S., , A. B., , A. P., Souza, T., Flecher, M. & (2022) Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report. Open Vet J, 12 (4), 502-507. doi:10.5455/OVJ.2022.v12.i4.12 Turabian Style Silva, Halana, Marina Reys, Geovanni Cassali, Fernanda Souza, Rodrigo Horta, Bruna Sena, Adriano Bindaco, Ana Pinto, Tayse Souza, Mayra Flecher, and . 2022. Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report. Open Veterinary Journal, 12 (4), 502-507. doi:10.5455/OVJ.2022.v12.i4.12 Chicago Style Silva, Halana, Marina Reys, Geovanni Cassali, Fernanda Souza, Rodrigo Horta, Bruna Sena, Adriano Bindaco, Ana Pinto, Tayse Souza, Mayra Flecher, and . "Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report." Open Veterinary Journal 12 (2022), 502-507. doi:10.5455/OVJ.2022.v12.i4.12 MLA (The Modern Language Association) Style Silva, Halana, Marina Reys, Geovanni Cassali, Fernanda Souza, Rodrigo Horta, Bruna Sena, Adriano Bindaco, Ana Pinto, Tayse Souza, Mayra Flecher, and . "Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report." Open Veterinary Journal 12.4 (2022), 502-507. Print. doi:10.5455/OVJ.2022.v12.i4.12 APA (American Psychological Association) Style Silva, H., , M. R., Cassali, G., Souza, F., Horta, R., , B. S., , A. B., , A. P., Souza, T., Flecher, M. & (2022) Secretory carcinoma of the canine mammary gland with nodal and bone metastases: case report. Open Veterinary Journal, 12 (4), 502-507. doi:10.5455/OVJ.2022.v12.i4.12 |