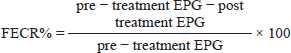| Original Article | ||
Open Vet J. 2021; 11(2): 238-250 Open Veterinary Journal, (2021), Vol. 11(2): 238–250 Original Research Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectinEnas Elmeligy1, Abdelbaset Abdelbaset2, Hanan K. Elsayed3, Sara A. Bayomi2, Ahmed Hafez4, Ashraf M. Abu-Seida5, Khaled A. S. El-Khabaz6, Dalia Hassan7, Rehab A. Ghandour8 and Arafat Khalphallah3*1Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 2Division of Clinical Laboratory Diagnosis, Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 3Division of Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 4Department of Pharmacology, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt 5Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 6Division of Infectious Diseases, Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 7Department of Animal & Poultry Hygiene and Environmental Sanitation, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 8Department of physiology, Faculty of Veterinary Medicine, Cairo University, P.O. Box 12211, Giza, Egypt *Corresponding Author: Arafat Khalphallah. Division of Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. Email: arafatvet2003 [at] yahoo.com Submitted: 04/02/2021 Accepted: 16/04/2021 Published: 06/05/2021 © 2021 Open Veterinary Journal
AbstractBackground: Parasitic infection is one of the main problems in equidae, particularly donkeys. Aim: This study evaluated the oxidative stress in donkeys infected with Strongylus spp by determining the correlation between antioxidants levels; malondialdehyde (MDA), total antioxidant capacity (TAC), and the severity of parasitic infection. It also compared the therapeutic efficacy of piperazine citrate as an oral anthelmintic drug and Doramectin as an injectable one. Methods: The study was conducted on 40 donkeys naturally infected with Strongylus spp. These donkeys were divided into two groups (20 donkeys each) according to treatment; One group was treated with piperazine citrate (PipTG) and the other with doramectin (DoraTG). Thorough clinical examination, hematological, biochemical, and parasitological assays were performed before (Day 0) and after treatment (Days 7, 14, 21, and 28). All data were statistically analyzed by independent-sample t-test or paired t-test. Results: In both groups, mean values of MDA were significantly reduced, while those of TAC were significantly elevated after treatment on days 7, 14, 21, and 28. These significant changes were reported after treatment between PipTG and DoraTG in favor of DoraTG. Serum concentrations of MDA were significantly reduced, while those of TAC were significantly elevated for DoraTG treatment group when their values were compared with those of PipTG either on days 7, 14, 21, or 28. Significant correlations were reported in PipTG and DoraTG. Negative significant correlations were reported between fecal egg count (FEC) and each of whole blood picture indices (RBCS, Hb, and PCV), serum TAC and faecal egg count reduction percentage FECR%. A positive correlation was seen between FEC and MDA. MDA exhibited a negative correlation with both blood picture and TAC; hence, TAC was positively correlated with these blood picture indices in both PipTG and DoraTG. In PipTG, anthelmintic resistance (R) was present on days 7 and 14, while it was suspected (S) at day 21 then it was absent (N) at day 28. In DoraTG, anthelmintic resistance was suspected (S) on day 7, then it became absent (N) on days 14, 21, and 28 post therapy. Conclusion: The immunological status of the infected donkeys had greatly improved after treatment. The therapeutic efficacy of injectable doramectin was more efficient than that of oral piperazine citrate in Strongylus spp. infected donkeys. Keywords: Donkeys, Doramectin, Malondialdehyde, Piperazine citrate, Strongylosis. IntroductionEquidae are a host of multiple parasites that are considered a risk factor in certain equine colic syndromes. Parasitic infection is one of the main health problems that hinder equine breeding and production and causes heavy losses. There are different kinds of damage associated with the migration of larvae, especially Strongylus vulgaris that may obstruct blood circulation, leading to colic, paralysis, heart diseases, or sudden death (Duncan, 1974; Gebrewold et al., 2004; Eckert et al., 2008). Donkeys are usually infected with a large number of parasites such as red worms (Strongylus), roundworms (Parascaris equorum and Parascaris univalens), liver flukes (Fasciola hepatica), pinworms (Oxyuris equi), lungworms (Dictyocaulus arnfieldi), the larva of botflies (Gasterophilus sp), and threadworms (Strongyloides westeri) (Eckert et al., 2008). Strongylosis is the most common parasitic infection in donkeys, usually found in the large intestine (Eysker et al., 1992; Nielsen et al., 2006). Strongylus of Equidae are classified into two subfamilies; large Strongylus (Strongylinae) and small Strongylus (Cyathostominae), and both of them are highly pathogenic parasites (Duncan and Pirie, 1985). The small Strongylus (Cyathostomins) is considered one of the most important gastrointestinal (GIT) nematodes in horses with an incidence of almost 100% (Reinemeyer et al., 1984), while the infection rate of large Strongylus infections among the GIT nematodes in horses is 58.5% (Saeed et al. 2010). Strongylus vulgaris has long been considered as one of the most common pathogenic parasites in Equidae with an incidence of 45%–90% in horses (McCraw and Slocombe 1976; Gasser et al., 2004; Nielsen et al., 2007; Toscan et al., 2012) and 73.02% in donkeys (Hamed et al., 2019). Infection with Strongylus spp. is complex and causes an inflammatory enteropathy consequently causing impairment in intestinal movement and microcirculation (Pilo et al., 2011). Clinically, small Strongylus (Cyathostominae) infection induces mild signs such as weight loss, anorexia, poor hair coat, intermittent diarrhea, lethargy, loss of body condition, peripheral edema, and disturbed intestinal motility (McCraw and Slocombe 1976; Hamed et al., 2019). Determining the number of Strongylus eggs per gram of feces (EPG) has been the most widely used method for diagnosing infection with adult Strongylus (Kaplan and Nielsen, 2010; Hamed et al., 2019). Thiabendazole has been widely used to treat adult Strongylus worms (Drudge et al., 1975). Other anthelmintics have also been developed, such as benzimidazole, tetra hydropyrimidines, and macrocyclic lactones (Bagchi et al.,1993; Oliveira and Cechini, 2000; Gasser et al., 2004; Gokbulut and McKellar, 2018). Inadequate dosage is the major cause of anthelmintic resistance, which is a serious problem faced during the control of intestinal nematodes in donkeys, particularly cyathostomins (Toscan et al., 2012). Lipid peroxidation is an ongoing physiological process that plays an important role in the pathogenesis of several parasitic diseases (Bagchi et al.,1993). Moreover, several studies have focused on the possible role of the highly reactive oxygen free radicals in the pathogenesis of parasitic infections (Oliveira and Cechini, 2000; Bildik et al., 2004). Free oxygen radicals cause lipid peroxidation, and the end product of lipid peroxidation is lipid peroxide [malondialdehyde (MDA)]. Estimation of MDA evaluates the degree of lipid peroxidation and the level of free oxygen radicals indirectly (Yagi, 1998). Estimating MDA level as an oxidant marker and serum antioxidants mainly, Total Antioxidant Capacity (TAC) is a major criterion concerning the severity of possible peroxidation occurring in the cell membrane (Bolfa et al., 2012). The correlation between Strongylus spp. infection and antioxidants levels in donkeys is not well understood, particularly its association with therapeutic strategies. Therefore, this study evaluated the oxidative stress in donkeys infected with Strongylus spp. by determining the correlation between oxidant/antioxidants levels, i.e., MDA and TAC, and the severity of parasitic infection. It also compared the therapeutic efficacy of piperazine citrate as an oral anthelmintic drug and doramectin as an injectable one. The current study focused on the effect of Strongylus spp. on clinical, hematological, and biochemical changes such as MDA and TAC in donkeys either pre or post-treatment with either oral piperazine citrate or injectable doramectin. Materials and MethodsAnimals and therapeutic strategyThe experiment was conducted on forty adult male donkeys, i.e., local breeds, used for educational purposes at the Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Egypt. The animals’ age ranged between 2 and 10 years, and their weight ranged between 170 kg and 250 kg. The donkeys were housed under hygienic and sanitary protocol in clean stables during the experiment. They were fed a balanced diet of mixed grain with hay and free access to water. Clinical and laboratory examinations were carried out in all donkeys at two main stages; pre-treatment (Day 0) and post-treatment stages (Days 7, 14, 21, and 28). According to fecal analysis, all examined donkeys were infected only with Strongylus spp. at day 0. No other nematodes were detected. Animals with negative presence of Strongylus spp. were excluded. According to the treatment, the donkeys were randomly distributed into two main groups (20 donkeys each); piperazine citrate treated group (PipTG), and doramectin treated group (DoraTG). PipTG received piperazine citrate powder (B.P. Vet 1977, UCCMA, Egypt) at a dose of 300 mg/kg by oral administration on day 0; meanwhile, DoraTG received doramectin (Dectomax®10 mg/ml injectable solution, Zoetis Co., USA) at a dose of 1 ml/50 kg by subcutaneous (s/c) injection on day 0 according to Hamed et al. (2019). The animals on day 0 either of PipTG or DoraTG were described as the control group as they had the highest levels of fecal egg count (FEC). SamplesWhole blood and serum samples were collected, and all precautions for collection and preparation of samples to achieve an accurate assessment of hematological and biochemical indices were taken into consideration as described before (Coles, 1986). Fecal samples were collected directly from the rectum of examined donkeys using arm-length plastic gloves and lubricant in clean plastic cup. The identification number of each animal was registered. The animals were sampled at day 0 before treatment and then again on days 7, 14, 21, and 28 post-treatment. The samples were protected in clean plastic cups and stored in a refrigerator at 4°C and labeled to be processed as soon as possible, either immediately or within 24 hours of collection. Each sample was divided into two parts; the first part was examined on the same day of collection by a direct fecal smear using microscopic examination and floatation sedimentation test for detection of helminths eggs. The second part was used for FEC using the McMaster technique (Duncan and Pirie, 1985; Kaplan and Nielsen, 2010; Hamed et al., 2019). For floatation fluid, saturated sucrose solution (sugar solution) was prepared by completely dissolving 500 g ordinary sugar and 6.5 g phenol in 330 ml distilled water and stirring over low heat. This solution was used after cooling it to room temperature for both the floatation and McMaster techniques (Blagburn and Butler, 2006). Clinical examinationBody temperature, pulse rate, respiratory rate, lymph nodes, mucous membranes, signs of dehydration, and Capillary Refill Time (CRT) were examined in all donkeys as mentioned before (Garba et al., 2015) on day 0 (before treatment) and on days 7, 14, 21, and 28 following treatment. Complete blood picture indicesComplete blood picture indices including red blood corpuscles (RBCs), total leukocytic count (TLC), differential leukocytic count (DLC), hemoglobin (Hb), packed cell volume (PCV), and mean corpuscular values (MCVs) were manually estimated. Mean corpuscular values included; mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). The DLC was determined by using the four-field meander method (Coles, 1986; Harvey, 2001; Latimer et al., 2011). Serum biochemical assaysSerum concentrations of MDA and TAC were measured for all donkeys by using colorimetric method by SPECTRO UV-Vis RS spectrophotometer (Labomed, Inc., USA) with commercial kits (Biodiagnostic Company, Egypt) according to Satoh (1978); Koracevic et al. (2001), respectively. Fecal analysis and detection of egg sheddingDirect smear and fecal sedimentation flotation were carried out to quantify the egg output by McMaster technique (Gasser et al., 2004). The counted eggs of the parasite were calculated in two chambers, summed together, and the total number was multiplied by 50 to give EPG. The Strongylus spp. eggs were identified based on the morphological characteristics, envelope shape, and number of blastomeres. Other parasites,eggs were determined according to the manual of veterinary parasitological laboratory techniques (Urquhart et al., 2003). The degree of infection was obtained from number of eggs per gram of feces according to Soulsby (1986) as follows: mild infection: less than 500 EPG, moderate infection: 500–1,000 EPG, and severe infection: > 1,000 EPG. Fecal egg count reduction test was used for detection of anthelmintic resistance by counting the worm eggs present in fecal samples of the infected donkeys shortly before treatment and 7–14 days after treatment. It is the method recommended by WAAVP guidelines (Coles et al., 1992). The quantitative McMaster technique is used to count the eggs in the fecal sample. To calculate the percentage reduction within each group, the following equation has been used (Kaplan, 2002; Coles et al., 2006):
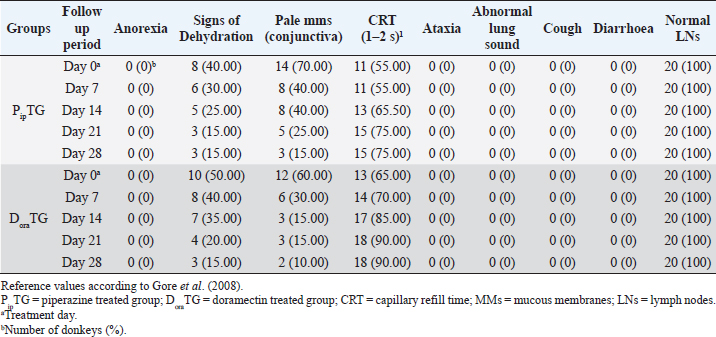
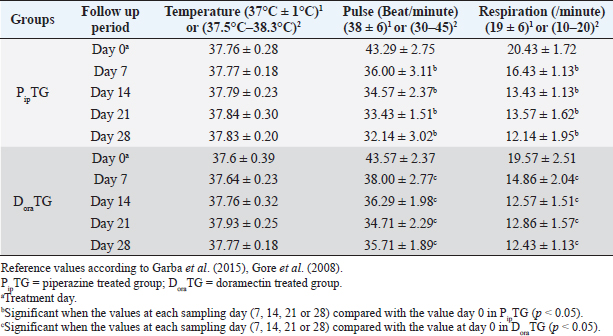
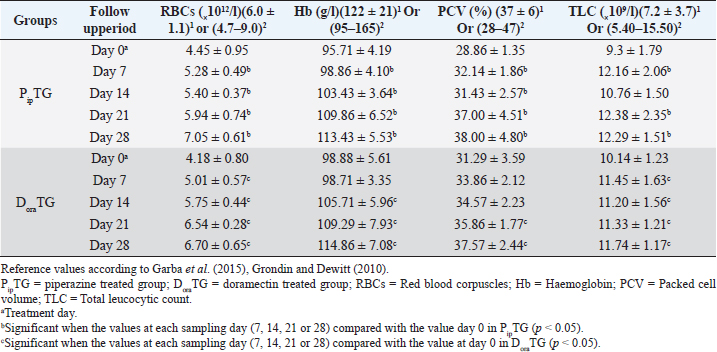
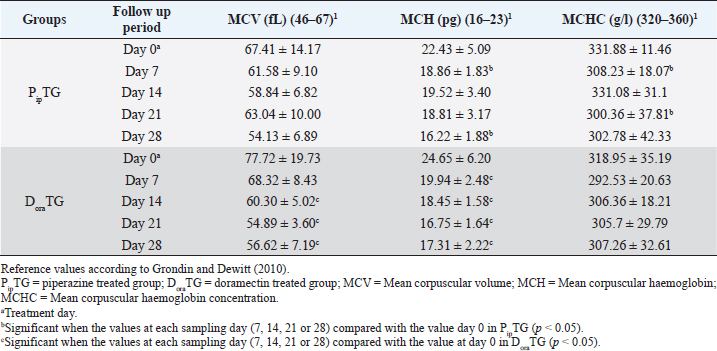
Resistance is present (R) if FECR is less than 90%, resistance is suspected (S) if FECR equals or more than 90%, and no resistance (N) if FECR equals or more than 95%. In the event when FEC for an individual animal increased after treatment, the FECR% was considered to be zero. According to Coles et al. (1992) the resistance of nematodes in domestic animals can be declared when the percentage reduction in egg count is less than 95% and/or the lower 95% confidence intervals (95% CI) is less than 90%. Statistical analysisAll statistical analyses were performed using computer software (Statistical Package for the Social Sciences version 17.0, Chicago, IL). The data obtained were analyzed by independent-sample t-test or paired t-test. The significance of differences between the means of PipTG and DoraTG either in days 0, 7, 14, 21, and 28 or between days 0 and each of days 7, 14, 21, or 28 either of PipTG or DoraTG was evaluated by Dunnett’s test at p < 0.05. Correlation coefficient was calculated using Pearson Correlation at p < 0.05 or p < 0.01. Ethical approvalAll experimental protocols were approved by Institutional Animal Care and Use Committee guidelines of Assiut University, Egypt that was in agreement with the Guide for Laboratory Animals Care and Use of the National Institutes of Health in the USA (NIH publication No. 86-23, revised 1996). ResultsClinical findingsAll donkeys of both groups exhibited normal body temperature, appetite, and lymph nodes with absence of ataxia, diarrhea, cough, and abnormal lung sounds either before or after treatment. Gradual disappearance of dehydration signs and mucous membranes paleness as well as clear improvement of CRT were reported in PipTG and DoraTG due to therapy (Table 1). The pulse and respiration were significantly (p < 0.05) reduced after therapy either in PipTG or DoraTG at days 7, 14, 21, and 28 after treatment when compared with their values at day 0 within the same group. The body temperature, pulse rate, and respiration showed no significant changes (p > 0.05) between PipTG and DoraTG at each sampling day (0, 7, 14, 21, and 28) as shown in Table 2. Whole blood picture indicesIn both groups, mean values of each of RBCs, Hb, and PCV showed a significant improvement (p < 0.05) on days 7, 14, 21, and 28 post treatment when their values were compared with those on days 0 within the same diseased group (Table 3). Mean values of MCV were relatively reduced post treatment in both groups (Days 7, 14, 21, and 28) when compared with those at day 0; however, the values were within the physiological levels. Both groups showed a significant decrease (p < 0.05) in mean values of MCH and MCHC on days 7, 14, 21, and 28 after treatment when compared with those on days 0 (Before treatment), however they were within the physiological values (Table 4). Mean values of TLC in both groups showed a significant increase (p < 0.05) on days 7, 14, 21, and 28 post treatment when their values were compared with those of days 0 (Table 3). Table 1. The most common clinical findings in piperazine treated group (n=20) and DoraTG (n=20).
Table 2. Mean values (M ± SD) of temperature, pulse and respiration in piperazine treated group (n=20) and DoraTG (n=20).
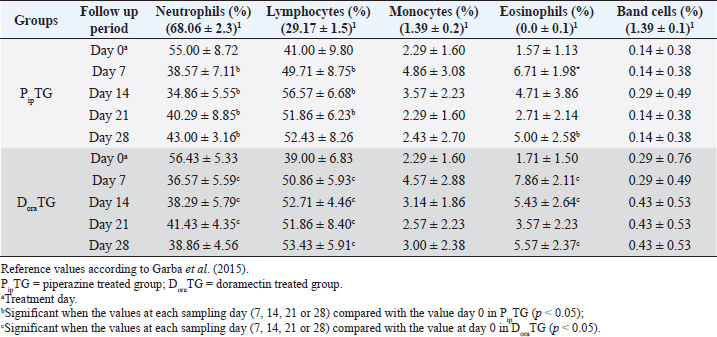
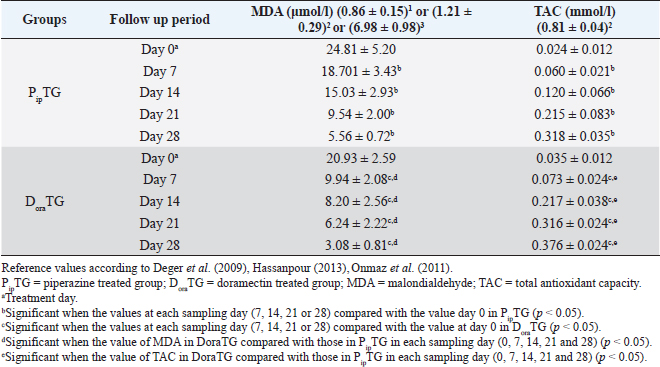
In both groups, DLC showed a significant increase (p < 0.05) in lymphocytes and eosinophils; meanwhile, neutrophils showed a remarkable decrease on days 7, 14, 21, and 28 after treatment upon comparison with those of days 0 within each group. (Table 5). There were no significant changes between PipTG and DoraTG when the values in DoraTG were compared with the values in PipTG of each sampling day (0, 7, 14, 21, and 28) either for RBCs, Hb, PCV, MCV, MCH, MCHC, TLC, or DLC (Tables 3–5). Serum biochemical assaysIn both groups, mean values of MDA was significantly reduced (p < 0.05) while those of TAC was significantly elevated (p < 0.05) post treatment at days 7, 14, 21, and 28 upon comparing the values with those of day 0 within each group (Table 6). These significant changes (p < 0.05) were reported after treatment between PipTG and DoraTG with favor of DoraTG either at days 7, 14, 21, or 28 (Table 6). Serum concentrations of MDA were significantly reduced while those of TAC were significantly elevated for DoraTG when a detailed comparison was made with those of PipTG either on days 7, 14, 21, or 28. Table 3. Mean values (M ± SD) of blood picture indices in piperazine treated group (n=20) and DoraTG (n=20).
Table 4. Mean values (M ± SD) of corpuscular values in piperazine treated group (n=20) and DoraTG (n=20).
Table 5. Mean values (M ± SD) of neutrophils, lymphocytes, monocytes, eosinophils and band cells in piperazine treated group (n=20) and DoraTG (n=20).
Table 6. Mean values (M ± SD) of malondialdehyde and TAC in piperazine treated group (n=20) and DoraTG (n=20).
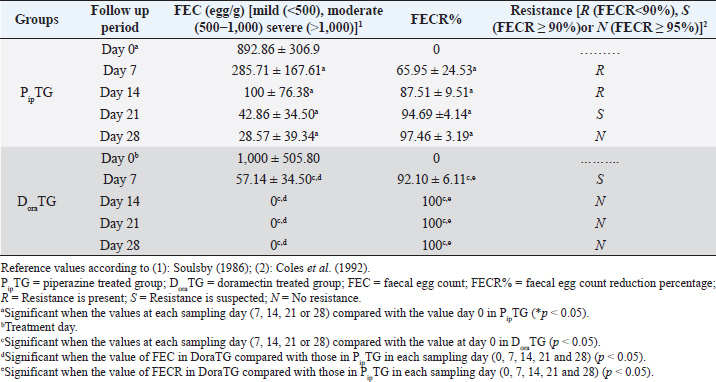
Fecal egg count PipTGThe degree of Strongylus spp. infection was moderate to severe on day 0 (FEC=892.86 ± 306.9 egg/g) and changed post-therapy to a mild degree as represented by the following values: 285.71 ± 167.61, 100 ± 76.38, 42.86 ± 34.50, and 28.57 ± 39.34 at days 7, 14, 21, and 28, respectively. The reduction in FEC increased gradually on day 7 till it reached the highest FECR on day 28 (Table 7). Compared with FEC on day 0, the diseased donkeys in PipTG showed a significant (p < 0.05) reduction in FEC (egg/g) of Strongylus spp. post-therapy on days 7, 14, 21, and 28. Also, a remarkable (p < 0.05) elevation of FECR of Strongylus spp. was detected post treatment for days 7, 14, and 21, as shown in Table 7. Table 7. Mean values (M ± SD) of FEC and FECR% in piperazine treated group (n=20) and DoraTG (n=20).
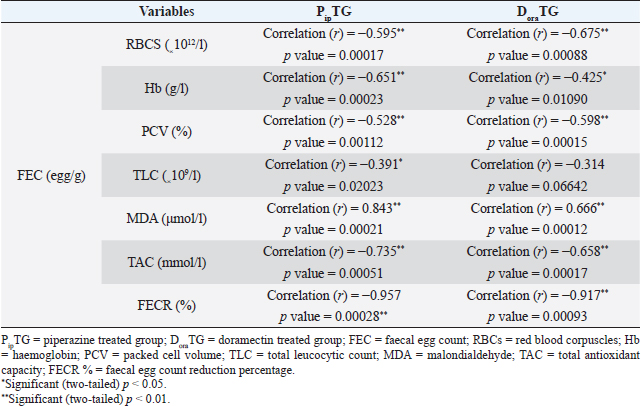
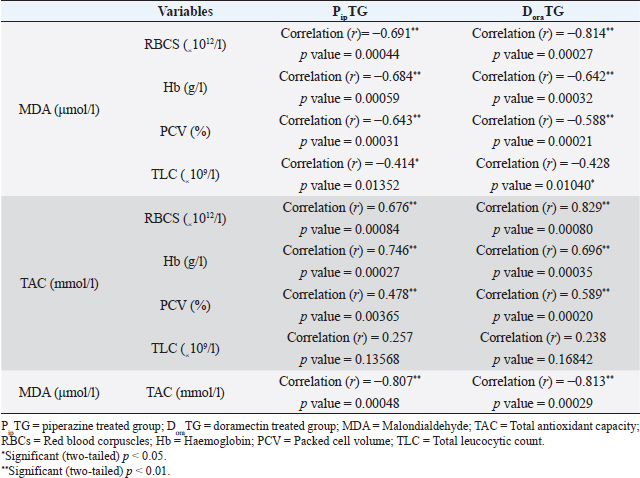
As shown in Table 7, anthelmintic resistance of piperazine citrate was present (R) for days 7 and 14, suspected (S) on day 21, and absent (N) on day 28 of post-therapy DoraTGThe degree of parasitic infection was moderate to severe for day 0 (FEC=1,000 ± 505.80) and changed post-therapy to a mild degree as follows: 57.14 ± 34.50, 0, 0, and 0 at days 7, 14, 21, and 28, respectively. The reduction in FEC increased gradually on the 7th day till it reached the highest FECR from day 14 to day 28 (Table 7). Mean values of FEC (egg/g) of Strongylus spp. were significantly (p < 0.05) decreased for days 7, 14, 21, and 28, while a remarkable (p < 0.05) increase of FECR was detected after 7, 14, 21, and 28 days of treatment. Anthelmintic resistance was suspected (S) for day 7 and absent (N) on days 14, 21, and 28 post therapy (Table 7). The efficacy of doramectin as an anthelmintic against Strongylus spp. in donkeys was higher than that of piperazine citrate as DoraTG showed a significantly (p < 0.05) higher FECR% than the PipTG on days 7, 14, 21, and 28 after treatment (Table 7). Correlation relationships for PipTG and DoraTGIn both treatment groups, the FEC before and after treatment was positively correlated with MDA and negatively correlated with RBCs, Hb, PCV, TLC, TAC, and FECR%) as shown in Table 8. As shown in Table 9, serum MDA concentrations before and after treatment were negatively correlated with RBCs, Hb, PCV, and TLC in both groups. A positive correlation was obtained between TAC and all blood picture indices (RBCs, Hb, PCV, and TLC) in PipTG and between TAC and RBCs Hb and PCV in DoraTG for either pre or post-therapy. In both treatment groups, the MDA, either pre or post-therapy was negatively correlated with TAC, where the reduction in MDA due to therapy was associated with a significant increase in TAC. DiscussionClinical findingsStrongylosis is the main problem in donkeys and constitutes a potential threat to donkey’s health and performance (McCraw and Slocombe 1976; Pilo et al., 2011; Hamed et al., 2019). The present data revealed strong correlations before and after treatment in each of PipTG and DoraTG. Moreover, the present study revealed that the efficacy of doramectin as an injectable anthelmintic therapy had higher efficacy than piperazine citrate as oral anthelmintic therapy in the case of Strongylus spp. infection in donkeys In the present study, the clinical findings were variable either in severity or in their response to therapy. The body temperature, appetite, lymph nodes, lung sound, and defecation were normal. At the same time, signs of dehydration, paleness of MMs, delayed CRT, increased pulse and increased respiration were clearly observed in donkeys infected with Strongylus spp (large strongyles). After treatment either with piperazine citrate or doramectin, these signs gradually vanished and almost disappeared on day 28 after treatment. The present results of CRT, body temperature, pulse, and respiration were in agreements with the reference values mentioned by Garba et al. (2015) and Gore et al. (2008). In contrast, diarrhea, loss of body weight, subcutaneous limb edema, and/or ventral abdomen edema were the more common signs in small strongylidae infection than for large strongylidae with a mortality rate of >50% (Love et al., 1999). Also, in contrast to our finding of normal body temperature in the infected donkeys, Pilo et al. (2011) stated that fever in Strongylus vulgaris infection is attributed to tissue damage or a toxic substance elaborated by larvae. Table 8. Pearson correlation coefficient between FEC and each of blood picture indices, biochemical parameters and FECR% in piperazine treated group (n=20) and DoraTG (n=20).
Table 9. Pearson correlation coefficient between malondialdehyde, TAC and blood picture indices in piperazine treated group (n=20) and DoraTG (n=20).
Whole blood picture indicesThe infected donkeys suffered from anemia as RBCs on day 0 were lower than the reference values reported by Grondin and Dewitt (2010). This may be attributed to the blood-sucking nature of Strongylidae that causes several changes in the hematological values like reduced cell survival. Both treatment groups showed a significant improvement as a result of treatment. In the present study, the low number of RBCs explained the clinical signs of dehydration, pale MMs, and delayed CRT. These findings agree with those reported by earlier workers as Waqas et al. (2015) reported a reduction in the blood values in strongylosis infected donkeys that are suggestive of anemia. In the current work, mean corpuscular values were reduced after treatment; however, they were within the physiological values reported before (Grondin and Dewitt, 2010). This could be explained by the significant elevation in blood picture items such as RBCs, Hb, and PCV that indicates the success of both therapies. Saleem et al. (2000) and Abd Ellah et al. (2011) also noticed that in both moderate and severely infected donkeys with Strongylus spp., anemia was evident because Strongylidae is a voracious blood sucker. Both PipTG and DoraTG showed a significant increase in TLC post-therapy that was not highly different from reference values reported by previous workers (Grondin and Dewitt, 2010; Garba et al., 2015). Moreover, there was no significant difference between PipTG and DoraTG in each sampling day regarding the increase in TLC. In the present study, DLC showed a significant increase in lymphocytes and eosinophils after treatment and a significant decrease in neutrophils in both PipTG and DoraTG for days 7, 14, 21, and 28, with no significant difference between both treatment groups. These findings were due to IgE's extremely elevated levels in parasitized animals that mediate mast cell degranulation, thereby stimulating the release of eosinophil chemotactic factor of anaphylaxis. This material, in turn, mobilizes the body’s eosinophil pool resulting in the release of a large number of eosinophil into the circulation (Tizzard, 1982). No significant changes were reported between PipTG and DoraTG in each sampling day (0, 7, 14, 21, and 28) for the blood picture indices. The other literature mentioned that change in the blood picture associated with Strongylus vulgaris was not unlike that seen in bacterial infections (Drudge et al., 1984). Alterations in blood’s biochemical and hematological parameters were detected in a proportion of infected horses (Dowdall et al., 2004). Serum biochemical assayIn both treatment groups, the mean values of MDA were significantly reduced, while those of TAC were significantly elevated after treatment. Moreover, there was a significant difference between DoraTG and PipTG regarding the changes in MDA and TAC levels after days 7, 14, 21, or 28 of treatment. The decrease of MDA level is due to a reduction in the amount of reactive oxygen radicals that cause lipid peroxidation; meanwhile, the increase of TAC level is due to improvement of antioxidant capacity of the host. Although the MDA and TAC levels did not reach their reference values that were reported by Deger et al. (2009) and Hassanpour (2013), even after 28 days of post-treatment, the reported levels at day 28 after treatment are in agreement with those reported by Mudgal et al. (2013). MDA values on day 28 agreed with those reported by Onmaz et al. (2011). The increase in the MDA level in donkeys infected with Strongylus spp. might be due to the combined effect of chronic intestinal inflammation, immunostimulation, resultant local secondary bacterial infection, and reduced absorption caused by the intestinal parasites (Moreau and Chauvin, 2010). Mudgal et al. (2013) observed that blood MDA levels in helminths positive horses before treatment were significantly higher than those in healthy control horses. MDA levels decreased significantly after treatment, and levels reached equal to that of normal healthy horses after 15 days of treatment. The other reports explained the trend of changes in blood MDA via that helminths infected horses suffer from oxidative stress (El-Moghazy, 2011). This might be due to the combined effect of chronic intestinal inflammation (Wang et al., 2008), immunostimulation (Moreau & Chauvin, 2010), resultant local secondary bacterial infection (Drudge et al., 1966), and reduced absorption of feed or fodder caused by the helminths parasites (Ramakrishna et al., 2006). Here, these significant changes in MDA and TAC were reported between the two groups where mean values of MDA were significantly reduced. At the same time, those of TAC were significantly elevated for DoraTG due to treatment when the values in DoraTG compared with those in PipTG either on days 0, 7, 14, 21, or 28. On the other side, several reports indicate that infection with various parasites is associated with a marked elevation in lipid peroxidation (MDA) (Bagchi et al., 1993; Oliveira and Cechini, 2000; Bildik et al., 2004). Deger et al. (2009), Ambawat et al. (1999) reported a significantly increased level of lipid peroxidation product (MDA), which was detected in both horses and mules infected with T. equi and B. caballi, and in donkeys infected with B. equi. This increase in lipid peroxidation in equids may indicate oxidative stress and erythrocytic injury caused by babesiosis or theileriosis. FEC and FECR% PipTGThe diseased donkeys in PipTG showed a significant decline in FEC (egg/g), hence, a remarkable elevation of FECR% of Strongylus spp. post-therapy was detected when their values were compared with the values obtained on day 0. Khan et al. (2015) added that the seasonal use of anthelmintic is the key to suppress the disease and avoid anthelmintic resistance. The present results, according to Soulsby )57(, revealed that the degree of parasitic infection, i.e. Strongylus spp. was moderate to severe on day 0; meanwhile, it changed to a mild degree post-therapy (Days 7 and 14) as FEC reduced gradually till reached its lowest value at day 28 even though FECR was increased gradually from day 7 (65.95% ± 24.53%) till reached its highest value at day 28 (97.46% ± 3.19%). Poynter (1955) said that Strongylus spp. FEC reduced from 500 EPG to 21 EPG after piperazine citrate administration in bran mash. The current study mentioned that FEC was reduced from 892.86 ± 306.9 EPG (Day 0) to 28.57 ± 39.34 EPG (Day 28) in PipTG. Hence, according to Coles et al. (1992), Kaplan (2002), and Coles et al. (2006), the present study reported that the anthelmintic resistance of piperazine citrate was present (R) as FECR was <90% on days 7 (65.95% ± 24.53%) and 14 (87.51 ± 9.51), while it was suspected (S) as FECR was ≥90% (94.69 ± 4.14) at day 21 then it was absent (N) as FECR (97.46% ± 3.19%) was ≥ 95% at day 28. Pook et al. (2002), Lyons et al. (2016) reported anthelmintic resistance of piperazine citrate against Ascarids, Strongyles, and Strongyloides in horse foals. Saleem et al. (2000) studied the efficacy of various anthelmintic drugs for control of horse strongylosis; the efficacy of anthelmintic was evaluated on the reduction of EPG on 7th, 14th, and 21st-day post medication, he noticed that the efficacy of albendazole was 64.2%, 53.1%, and 42.1%, respectively, for oxfendazole was 100%, 98.7%, and 94%, respectively, and for piperazine was 52.3%, 38.8%, and 28%, respectively, on 7th, 14th, and 21st day post medication. In contrast, DiPietro et al. (1985) observed that horses infected with strongylosis when treated with febantel and piperazine at all dosage (25 or 55 mg base/kg) had significantly lower mean Strongylidae EPG counts and greater percentage reduction in mean Strongylidae EPG counts (99.7% to 99.9%) 7 days after treatment, respectively. DoraTGMean values of FEC (egg/g) of Strongylus spp. were found to be significantly decreased on days 7, 14, 21, and 28, while a remarkable increase of FECR was detected after treatment when their values were compared with those of day 0. Based on the results reported by Soulsby (1986), the present results revealed that the degree of parasitic infection with Strongylus spp. in examined donkeys was moderate to severe on day 0; meanwhile, it changed to a mild degree post-therapy as FEC reduced gradually till it reached its lowest value on day 28 even though FECR was increasing gradually from day 7 (92.10% ± 6.11%) till it reached its highest value on days 14, 21, and 28 (100%). Seri et al. (2005) stated that doramectin induced 100% efficacy (FECR=100%) for 28 days after treatment in donkeys suffering from gastrointestinal nematodes in donkeys (Equus asinus). It may be due to seldom use of doramectin in the treatment of equine parasite. The current study mentioned that FEC was reduced from 1,000 ± 505.80 EPG (Day 0) to 0 EPG (Days 14, 21, and 28) in DoraTG. Gokbulut et al. (2005) stated that doramectin has effective plasma concentration for at least 30 days in donkeys. The present study, based on the results reported by Coles et al. (1992), demonstrated that the anthelmintic resistance of doramectin in infected donkeys was suspected (S) as FECR was ≥90% (92.10% ± 6.11%) on day 7, then it became absent (N) as FECR was ≥ 95% (100%) at days 14, 21, and 28 post therapy. Davies and Schwalbach (2000) mentioned that a 100% reduction in FECs (FEC=0) was reported in horses treated with intramuscular injectable doramectin (Day 14 post-therapy). These findings indicate the high efficacy of doramectin against adult Cyathostomes, Strongylus spp., and Trichostrongylus axei in horses. Bauer et al. (1986) added that resistance was defined as a FEC reduction of less than 90% after anthelmintic treatment. The present article revealed that the efficacy of doramectin as an injectable anthelmintic therapy had higher efficacy than piperazine citrate as oral anthelmintic therapy in the case of Strongylus spp. in donkeys where FEC was significantly reduced for DoraTG when their values after treatment for days 7, 14, 21, and 28 were compared with those of PipTG. DoraTG also showed a significant elevation in FECR% after treatment (Days 7, 14, 21, and 28) when their values were compared with those of PipTG. Davies and Schwalbach (2000) and Prichard (1990) supported the current results. They mentioned that intramuscular injection of doramectin against adult Cyathostomes and Strongylus spp equine ensures that no anthelmintic is dissipated. Therefore no under-dosing occurs, provided the animals’ mass is accurately calculated. Under-dosing has been proposed as an essential factor in accelerating resistance development, which is often a risk associated with oral dosing, as spillage frequently occurs. In contrast, Eysker et al. (1992) said that equine treated with oral anthelmintic (non-ivermectin) drugs had lower EPG values of Cyathostomes than those treated with doramectin, probably due to the effect of macrocyclic lactones on late larval stages in the bowel lumen that give a longer inhibition of fecal egg output compared with other anthelmintics. Campbell et al. (1989) added that parenteral injection of anthelmintics, including endectocides, is not utilized in equine species. After parenteral administration, rare adverse reactions such as Clostridium spp. infections and anaphylaxis have been observed in the animals. These undesirable effects were responsible for the withdrawal of the parenteral preparation of macrocyclic lactones for use on horses in 1984. The present data revealed strong correlations in each of PipTG and DoraTG. A strong correlation was reported between the reduction in FEC and improvements of each blood picture indices serum TAC and FECR%. FEC was positively correlated with MDA, where the reduction in FEC was associated with a reduction in serum levels of MDA. MDA was negatively correlated. Meanwhile, TAC was positively correlated with blood picture parameters (RBCS, Hb, and PCV). MDA was negatively correlated with TAC, where the reduction in MDA due to therapy was associated with a significant increase in TAC. Saes et al. (2016) also studied the time required by different anthelmintic agent (ivermectin, moxidectin, febendazole, piperazine, or no treatment) to reduce Strongylidae egg shedding in horses according to fecal examinations that were performed 4, 8, 12, 18, 24, 36, and 48 hours after anthelmintic treatment. FEC revealed the absence of eggs from the fecal analysis starting from 72 hours and 4 days, respectively, following treatment with moxidectin or ivermectin. After treatment with piperazine 48 hours up to 9 days, FECR was greater than 95%, with its highest FECR value (98.1%) reported after 7 days of post-treatment. However, the FECR was below 90% in the last two samplings (26th and 28th day of post-treatment). The lowest efficacy was stated in the febendazole group presented as FECR and was below 90% in all samplings. ConclusionThe immunological status of the infected donkeys was greatly improved after treatment as the levels of MDA was highly decreased, and those of TAC were highly increased in PipTG and DoraTG. This improvement was greater in DoraTG as compared to PipTG. Consequently, the resulting imbalance between oxidant and antioxidant processes might play a central role in the pathologic conditions associated with Strongylus spp. infection in donkeys. Significant correlations were reported in each of PipTG and DoraTG. A remarkable correlation was demonstrated between the reduction in FEC and improvements of the blood pictures, serum TAC and FECR%. FEC was positively correlated with MDA. MDA was negatively correlated; meanwhile, TAC was positively correlated with the whole blood picture (RBCS, Hb, and PCV). MDA was negatively correlated with TAC. The therapeutic efficacy of doramectin as an injectable anthelmintic therapy was more efficient than that of piperazine citrate as oral anthelmintic therapy in the case of Strongylus spp. in donkeys. AcknowledgmentsThe authors are very grateful and thankful for Dr. Mahmoud Rushdi Abdellah, Professor of clinical laboratory diagnosis, and for Dr. Maha Hamed, Professor of infectious diseases, Faculty of Veterinary Medicine, Assiut University, Egypt, for their great help and kind support during conducting this study. Authors' contributionAK, EE, AA, and HKE conceived, designed, and supervised the project. AK, AA, DH, KASE and EE conducted the practical part of the study. AMA, AH, RAG and KASE collected laboratory samples and conducted biochemical analyses. AK, RAG, DH and EE conducted the statistical analyses. AK, SAB, RAG and HKE performed Analysis and/ or interpretation of data. AK, EE, SAB and HKE drafted the manuscript. AK, EE, SAB and AMA carried out final writing and critical review/revision of the manuscript. AK and EE had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conflict of interestsThe authors declare that they have no conflicts of interest. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors ReferencesAbd Ellah, M.R., Al-Hosary, A.T., Sayed, M.B., Oraby, M.S. and Hussein, AM. 2011. Effect of strongylosis on some blood constituents in donkeys. In the Proceedings of the XVth International Congress on Animal Hygiene and Sustainable Livestock Production, Vienna, Austria, pp: 883–885. Ambawat, H.K., Malhotra, D.V., Kumar, S. and Dha, Sr.S. 1999. Erythrocyte associated haemato-biochemical changes in Babesia equi infection experimentally produced in donkeys. Vet. Parasitol. 85, 319–324. Bagchi, M., Mukherjee, S. and Basu, M.K. 1993. Lipid peroxidation in hepatic microsomal membranes isolated from mice in health and in experimental leishmaniasis. Indian J. Biochem. Biophs. 30, 277–281. Bauer, C., Merkt, J.C., Janke-Grimm, G. and Burger, H.J. 1986. Prevalence and control of benzimidazole resistant small strongyles in German thoroughbred studs. Vet. Parasitol. 21(3), 189–203. Bildik, A., Kargın, F., Seyrek, K., Pasa, S. and Ozensoy, S. 2004. Oxidative stress and non-enzymatic antioxidative status in dogs with visceral Leishmaniasis. Res. Vet. Sci 77, 63–66. Blagburn, B.L. and Butler, J.M. 2006. Optimize intestinal parasite detection with centrifugal fecal flotation. Vet. Med. 101, 455–464. Bolfa, P.F., Leroex, C., Pintea, A., Anderi, S., Catori, C., Taulescu, M., Tabaran, F. and Spinu, M. 2012. Oxidant-antioxidant imbalance in horses infected with equine infectious anaemia virus. Vet. J. 192, 449–454. Campbell, W.C., Leaning, W.H.D. and Seward, R.L. 1989. Use of ivermectin in horses. In: Ivermectin and Abmectin. Eds., Campbell, W.C. New York, NY: New York Inc, Springer Verlag, pp: 234–244. Coles, E.H. 1986. Veterinary clinical pathology, 4th ed. Philadelphia, PA: W.B., Saunders Company, pp: 132–139. Coles, G.C., Bauer, C., Borgsteede, F.H., Geerts, S., Klei, T.R., Taylor, M.A. and Waller, P.J. 1992. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 44(1–2), 35–44. Coles, G.C., Jackson, F., Pomroy, W.E., Prichard, R.K., von Samson-Himmelstjerna, G., Silvestre, A., Taylor, M.A and Vercruysse, J. 2006. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136(3–4), 167–185. Davies, J.A. and Schwalbach, L.M.J. 2000. A study to evaluate the field efficacy of ivermectin, fenbendazole and pyrantel pamoate, with preliminary observations on the efficacy of doramectin, as anthelmintics in horses. J. S. Afr. Vet. Assoc. 71(3), 144–147. Deger, S., Deger, Y., Bicek, B., Ozdal, N. and Gul, A. 2009. Status of lipid peroxidation, antioxidants, and oxidation products of nitric oxide in equine babesiosis: status of antioxidant and oxidant in equine babesiosis. J. Equine Vet. Sci. 29, 743–747. DiPietro, J.A, Todd, K.S. Jr., Lock, T.F. and Reuter-Dallman, V. 1985. Evaluation of febantel used currently piperazine citrate in horses. Am. Vet. Med. Assoc. 186(3), 262–264. Dowdall, S.M.J., Proudman, C.J., Klei, T.R., Mair, T. and Matthews, J.B. 2004. Characterisation of IgG (T) serum antibody responses to two larval antigen complexes in horses naturally - or experimentally-infected with cyathostomins. Int. J. Parasitol. 34(1), 101–108. Drudge, J.H., Lyons, E.T. and Tolliver, S.C. 1975. Critical tests of suspension, paste and pellet formulations of cambendazole in the horse. Am. J. Vet. Res. 36, 435–439. Drudge, J.H., Lyons, E.T. and Tolliver, S.C. 1984. Critical tests of morantel-trichlorfon paste formulation against external parasites of the horse. Vet. Parasitol. 14, 55–64. Drudge, J.H., Lyons, E.T., Szanto, J. and Soulsby, E.J.I. 1966. Biology of parasites. New York, NY: Academic Press, pp: 199–214. Duncan, J.L. 1974. Strongylus vulgaris infection in the horse. Vet. Rec. 95, 34–37. Duncan, J.L. and Pirie, H.M. 1985. The pathogenesis of single experimental infections with Strongylus vulgaris in foals. Res. Vet. Sci. 18, 82–93. Eckert, J., Friedhoff, K.T., Zahner, H. and Doplazes, P. 2008. Textbook of parasitology for the animal medicine, 3rd ed. Stuttgart, Germany: Enke, pp: 575. El-Moghazy, F.M 2011. Impact of parasitic infection on ovarian activity in Buffaloes-heifers with emphasis on ascariasis. World J. Zool. 6(2), 196–203. Eysker, M., Boersema, J.H. and Kooyman, F.N. 1992. The effect of Ivermectin against inhibited early third stage, late third stage and fourth stage larva and adult stage of the cyathostomes in Shetland ponies and spontaneous explusion of these helminths. Vet. Parasitol. 42, 295–302. Garba, U.M., Sackey, A.K., Idris, L.A. and Esievo, K.A. 2015. Baseline vital, haematological and serum biochemical parameters of donkeys. J. Vet. Med. Anim. Health. 7, 94–98. Gasser, R.B., Hung, G.C., Chilton, N.B. and Beveridge, I. 2004. Advances in developing molecular diagnostic tool for stronglyoid nematodes of equids: fundamentals and applied implications. Mol. Cell Probes. 18, 3–16. Gebrewold, A., Tegegne, A. and Yami, A. 2004. Research need of donkey utilization in Africa. In: Donkeys, people and development. A resource book of the animal traction network for Eastern and Southern Africa (ATNESA), Eds., Fielding, D. and Starkey, P. Wageningen, The Netherlands: Technical Center for Agriculture and Rural Cooperation (CTA), pp: 77–81. Gokbulut, C. and McKellar, Q.A. 2018. Anthelmintic drugs used in equine species. Vet. Parasitol. 261, 27–52. Gokbulut, C., Boyacioglu, M. and Karademir, U. 2005. Plasma pharmacokinetics and faecal excretion of ivermectin and doramectin following oral administration in donkeys. Res. Vet. Sci. 79, 233–238. Gore, T., Gore, P. and Giffin, J.M. 2008. Normal physiological data. In: Horse Owner’s Veterinary Handbook, 3rd ed. Hoboken, NJ: Wiley Publishing Inc., pp: 619–624. Grondin, T.M. and Dewitt, S.F. 2010. Normal hematology of the horse and donkey. In: Schalm’s veterinary hematology, 6th edition. Eds., Weiss, D.J. and Wardrop, K.J.. Ames, IA: Blackwell Publishing Ltd, Wiley-Blackwell, pp: 821–828. Hamed, M.I., El-Allawy, T.A. and Hassnein, E.A. 2019. Prevalence and anthelmintic resistance of strongyle infection of donkeys in El-Wadi El-Gadid, Egypt. J. Adv. Vet. Res. 9, 144–150. Harvey, J.H. 2001. Atlas of veterinary hematology. Philadelphia, PA: WB Saunders Company, Elsevier, pp: 3–74. Hassanpour, A. 2013. Correlation of malondialdehyde and antioxidants of serum in the horses with strangles. Indian J. Fund Appl. Life Sci. 3, 327–334. Kaplan, R.M. 2002. Anthelmintic resistance in nematodes of horses. Vet. Res. 33(5), 491–507. Kaplan, R.M. and Nielsen, M.K. 2010. An evidence based approach to equine parasite control. Equine Vet. Edu. 22, 306–316. Khan, M.A., Roohi, N. and Rana, M.A.A. 2015. Strongylosis in equines: a review. J. Anim. Plant Sci. 25, 1–9. Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S. and Cosic, V. 2001. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 54, 356–361. Latimer, K.S., Mahaffey, E.A. and Prasse, K.W. 2011. Duncan and Prasse's veterinary laboratory medicine: clinical pathology, 5th ed. Ames, IA: Wiley-Blackwell, Blackwell Publishing Ltd, pp: 3–82. Love, S., Murphy, D. and Mellor, D. 1999. Pathogenicity of cyathostome infections. Vet. Parasitol. 85, 113–122. Lyons, E.T., Dorton, A.R. and Tolliver, S.C., 2016. Evaluation of activity of fenbendazole, oxibendazole, piperazine, and pyrantel pamoate alone and combinations against ascarids, Strongylus, and strongyloides in horse foals in field tests on two farms in Central Kentucky in 2014 and 2015. Vet. Parasitol. 3, 23–26. McCraw, B.W. and Slocombe, J.O.D. 1976. Strongylus vulgaris in the horse: a review. Can. Vet. J. 17, 150–157. Moreau, E. and Chauvin, A. 2010. Immunity against helminths: interactions with the host and the intercurrent infections. J. Biomed. Biotech. 2010, 1–9. Mudgal, N.K., Singh, A.P., Dedar, R.K., Anil Ahuja, Kachhawa, J.P. and Kachhawaha, S. 2013. Biomarkers of oxidative stress in helminthes infected horses. Vet. Pract. 14, 26–27. Nielsen, M.K., Kaplan, R.M., Thamsborg, S.M., Monrad, J. and Olsen, S.N. 2007. Climatic influences on development and survival of free-living stages of equine Strongylus. Vet. J. 174, 23–32. Nielsen, M.K., Monard, J. and Olsen, S.N. 2006. Prescription -only anthelmintics- a questionnaire surveillance and control of equine Strongylus in Denmark. Vet. Parasitol. 135, 47–55. Oliveira, F.J.A. and Cechini, R. 2000. Oxidative stress of liver in hamsters infected with Leishmania (L.) chagasi. J. Parasitol. 86, 1067–1072. Onmaz, A.C., Van den hoven, R., Gunes, V., Cinar, M. and Kucuk, O. 2011. Oxidative stress in horses after a 12-hours transport period. Rev. Med. Vet. 162(4), 213–217. Pilo, C., Alteaa, A., Pirino, S., Nicolussi, P., Varcasia, A., Genchi, M. and Scala, A. 2011. Strongylus vulgaris (Looss, 1900) in horses in Italy: is it still a problem? Vet. Parasitol. 184, 161–167. Pook, J.F., Power, M.L., Sangster, N.C., Hodgson, J.L. and Hodgson, D.R. 2002. Evaluation of tests for anthelmintic resistance in cyathostomes. Vet. Parasitol. 106(4), 331–343. Poynter, D. 1955. The efficiency of piperazine adipate administered in bran mash to horses. Vet. Record. 67(34), 625–626. Prichard, R.K. 1990. Anthelmintic resistance in nematodes: extent, recent understanding and future directions for control and research. Int. J. Parasitol. 20, 515–523. Ramakrishna, B.S., Venkataraman, S. and Mukhopadhya, A. 2006. Tropical malabsorption. Postgrad. Med. J. 82(974), 779–787. Reinemeyer, C.R., Smith, S.A., Gabel, A.A. and Herd, R.P. 1984. The prevalence and intensity of internal parasites of horses in the USA. Vet. Parasitol. 15, 75–83. Saeed, K., Qadir, Z., Ashraf, K. and Ahmad, N. 2010. Role of intrinsic and extrinsic epidemiological factors on strongylosis in horses. J. Anim. Plant Sci. 20, 277–280. Saes, I.L., Vera, J.H.S., Fachiolli, D.F., Yamada, P.H., Dellaqua, J.V.T., Saes, R.L., Amarante, A.F.T. and Soutello, R.V.G. 2016. Time required by different anthelmintics to reach expected efficacy levels in horses infected by strongyles. Vet. Parasitol. 229, 90–92. Saleem, A., Pervez, K, Khan, M.S. and Hashmi, H.A. 2000. Prevalence and chemotherapy of strongylosis and its effect on various blood parameters in horse. Pak. J. Med. Sci. 52(3–4), 41–43. Satoh, K. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 90, 37–43. Seri, H.I., Ababakr, A.D., Ismail, A.A. and Tigani, T.A. 2005. Efficacy of ivermectin in an injectable formulation against gastrointestinal nematodes of donkeys (Equus asinus). Vet. Arch. 75, 369–374. Soulsby, E.J.I. 1986. Helminths, arthropods and protozoa of domesticated animals, 7th ed. London, UK: Tindall, Bailliere, pp: 809. Tizzard, I. 1982. An introduction to veterinary immunology, 2nd ed. Philadelphia, PA: W.B. Sanders Co., pp: 236–237. Toscan, G., Cezar, A.S., Pereir, R.C.F., Silva, G.B., Sangioni, L.A., Oliveir, L.S. and Vogel, F.S.F. 2012. Comparative performance of macrocyclic lactones against large Strongylus in horses. Parasitol. Int. 61, 550–553. Urquhart, G.M., Armour, J., Duncan, A.M. and Jennings, F.W. 2003. Veterinary parasitology, 3rd ed. Ames, IA: Blackwell Science Ltd, pp: 4–78. Wang, L.J., Cao, Y. and Shi, H.N. 2008. Helminth infections and intestinal inflammation. World J. Gastroenterol. 14(33), 5125–5132. Waqas, M., Nawaz, M., Sajid, S.M., Ahmad, Z., Mushtaq, A., Abdul Jabbar and Zubair, M. 2015. Strongylosis (red worm infection); a potential threat to donkey's health and performance. Global. Vet. 14(3), 355–360. Yagi, K. 1998. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol 108, 101–106. | ||
| How to Cite this Article |
| Pubmed Style Elmeligy E, Abdelbaset A, Elsayed HK, Bayoumi SA, Hafez A, Abu-Seida AM, El-Khabaz KA, Ghandour RA, Khalphallah A. Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. Open Vet J. 2021; 11(2): 238-250. doi:10.5455/OVJ.2021.v11.i2.8 Web Style Elmeligy E, Abdelbaset A, Elsayed HK, Bayoumi SA, Hafez A, Abu-Seida AM, El-Khabaz KA, Ghandour RA, Khalphallah A. Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. https://www.openveterinaryjournal.com/?mno=43861 [Access: April 25, 2024]. doi:10.5455/OVJ.2021.v11.i2.8 AMA (American Medical Association) Style Elmeligy E, Abdelbaset A, Elsayed HK, Bayoumi SA, Hafez A, Abu-Seida AM, El-Khabaz KA, Ghandour RA, Khalphallah A. Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. Open Vet J. 2021; 11(2): 238-250. doi:10.5455/OVJ.2021.v11.i2.8 Vancouver/ICMJE Style Elmeligy E, Abdelbaset A, Elsayed HK, Bayoumi SA, Hafez A, Abu-Seida AM, El-Khabaz KA, Ghandour RA, Khalphallah A. Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. Open Vet J. (2021), [cited April 25, 2024]; 11(2): 238-250. doi:10.5455/OVJ.2021.v11.i2.8 Harvard Style Elmeligy, E., Abdelbaset, . A., Elsayed, . H. K., Bayoumi, . S. A., Hafez, . A., Abu-Seida, . A. M., El-Khabaz, . K. A., Ghandour, . R. A. & Khalphallah, . A. (2021) Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. Open Vet J, 11 (2), 238-250. doi:10.5455/OVJ.2021.v11.i2.8 Turabian Style Elmeligy, Enas, Abdelbaset Abdelbaset, Hanan K. Elsayed, Sara A. Bayoumi, Ahmed Hafez, Ashraf M. Abu-Seida, Khaled A.S. El-Khabaz, Rehab A. Ghandour, and Arafat Khalphallah. 2021. Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. Open Veterinary Journal, 11 (2), 238-250. doi:10.5455/OVJ.2021.v11.i2.8 Chicago Style Elmeligy, Enas, Abdelbaset Abdelbaset, Hanan K. Elsayed, Sara A. Bayoumi, Ahmed Hafez, Ashraf M. Abu-Seida, Khaled A.S. El-Khabaz, Rehab A. Ghandour, and Arafat Khalphallah. "Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin." Open Veterinary Journal 11 (2021), 238-250. doi:10.5455/OVJ.2021.v11.i2.8 MLA (The Modern Language Association) Style Elmeligy, Enas, Abdelbaset Abdelbaset, Hanan K. Elsayed, Sara A. Bayoumi, Ahmed Hafez, Ashraf M. Abu-Seida, Khaled A.S. El-Khabaz, Rehab A. Ghandour, and Arafat Khalphallah. "Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin." Open Veterinary Journal 11.2 (2021), 238-250. Print. doi:10.5455/OVJ.2021.v11.i2.8 APA (American Psychological Association) Style Elmeligy, E., Abdelbaset, . A., Elsayed, . H. K., Bayoumi, . S. A., Hafez, . A., Abu-Seida, . A. M., El-Khabaz, . K. A., Ghandour, . R. A. & Khalphallah, . A. (2021) Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. Open Veterinary Journal, 11 (2), 238-250. doi:10.5455/OVJ.2021.v11.i2.8 |