| Original Article | ||
Open Vet J. 2022; 12(3): 370-374 Open Veterinary Journal, (2022), Vol. 12(3): 370–374 Original Research Species diversity of ticks (Acari: Ixodidae) in Tarhuna, LibyaAsmaa M. Abdulsalam1, Walid K. Saadawi2, Hoda I. Kharwat3 and Taher Shaibi4*1Biology Department, Faculty of Science, Azzaytuna University, Tarhuna, Libya 2National Centre for Disease Control, Ministry of Health, Libya 3Department of Zoology, Faculty of Science, University of Gharyan, Gharyan, Libya 4Department of Zoology, Faculty of Science, University of Tripoli, Tripoli, Libya *Corresponding Author: Taher Shaibi. Department of Zoology, Faculty of Science, University of Tripoli, P.O. Box: 13793, Tripoli, Libya. Email: t.shaibi [at] uot.edu.ly Submitted: 14/11/2021 Accepted: 03/05/2022 Published: 04/06/2022 © 2022 Open Veterinary Journal
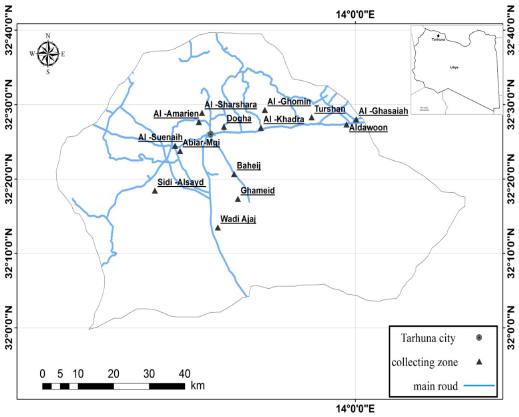
AbstractBackground: Twenty-five species of ticks have been recorded in Libya. No systematic studies have been conducted in Tarhuna region regarding tick species that infest livestock. Aim: To identify the species of ticks that infest livestock in Tarhuna, Libya. Methods: The study was carried out from August 2015 to May 2016 in Tarhuna, which is located in northwestern Libya. A total of 634 ticks (349 males, 280 females, and 5 nymphs) were collected from 145 randomly selected animals (camels, sheep, and goats). Samples were collected seasonally from different locations. Results: Four species of ticks were recorded: Hyalomma dromedarii (83.12%), Rhipicephalus bursa (6.94%), Hyalomma excavatum (6.63%), and Rhipicephalus camicasi (3.31%). The mean prevalence of all species was 4.37 ticks/host. All of the tick species were collected from sheep and goats, except H. dromedarii which was collected from camels only. With respect to the season of collection, the number of species varied among seasons; the highest prevalence was in summer (6.53 tickshost) and the lowest was in spring (2.18 tickshost). The highest average number of tick species was observed in H. dromedarii (10.13 ticks/host), followed by 1.21 ticks/host in R. camicasi, 1.07 ticks/host in H. excavatum, and the lowest in R. bursa (1.02 tick/host). Conclusions: These findings reveal that the abundance of ticks varied among species and seasons. Keywords: Ticks, Livestock, Prevalence, Seasons, Libya. IntroductionTicks are parasitic animals having a wide animal host range, including mammals, birds, reptiles, and amphibians (Hoogstraal, 1956; Burgdorfer et al., 1982). During the blood feeding, ticks can transmit bacteria, viruses, protozoa, and fungi to wildlife, livestock, and humans around the world (Tonbak et al., 2006; M’ghirbi et al., 2010; Mashebe et al., 2014). They are considered the second most important vector after mosquitoes in regard to disease transmitting agents (Parola and Raoult, 2001; Crowder et al., 2010) and they cause severe economic losses in livestock (Furman and Loomis, 1984). Tick species are distributed widely around the world. They are found in most, if not all, the countries of the world, especially in tropical and subtropical zones with appropriate humid and hot climate (Magnarelli, 2009). Climate plays an important role in the spread of ticks through its effect on vegetation (Belozerov, 1982). The occurrence records of vectors are important to assess the risk of associated vector-borne disease agents (Hutchings et al., 2016). Few studies have been, recently, published on the acarine parasites of farm livestock in Libya (Gabaj et al., 1992; Elsaid et al., 2013; Hador, 2015). Italian entomologists, physicians, and public health workers had generated reports based on small collections or single species (Gabaj et al., 1992). Twenty-five species of ticks have been recorded in Libya; 21 of them belong to family Ixodidae and the rest belong to family Argasidae (Hoogstraal and Kaiser, 1960; Gabaj et al., 1992; Hador, 2015) The apparition ectoparasites in sheep and goats’ flocks were poorly reported in Libya (Elsaid et al., 2013). No comprehensive studies have been carried out in this area regarding tick species that infest livestock in Tarhuna, where farm livestock is considered a part of the economic resources. It is important to have a knowledge of ticks infesting those animals to clarify the natural cycles of tick-borne pathogens for any control programs. This study aims to identify the species of ticks infesting livestock in Tarhuna. Materials and MethodsStudy areaThe study was carried out from August 2015 to May 2016 in Tarhuna which is located in northwestern Libya (32°26ʹ2″N, 13°38ʹ4″E), about 80 km southeast to Tripoli, and 398 meters above sea level (Fig. 1). The area is characterized by semidry climate, where semidesert plants are grown widely, such as thyme, rosemary, and white wormwood. The mean temperature, in general, varies between 10.8°C and 26°C, with an average annual rainfall of 237.6 mm.
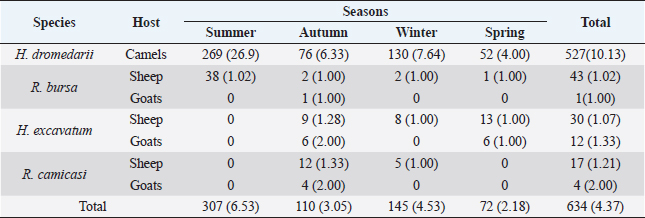
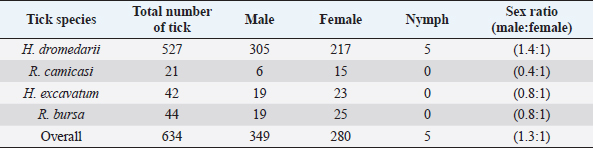
Fig. 1. Map of Tarhuna municipality showing the sampling locations. Collection and identification of ticksSamples were collected seasonally; in the summer (2–19 August 2015), autumn (9–11 November, 2015), winter (13–23 February 2016), and spring (12–24 May, 2016). Ticks were collected from a total of 145 animals (52 camels, 81 sheep, and 12 goats) from different locations (Fig. 1). The examined animals were local breeds of camels (4–12 years), sheep (1–6 years), and goats (1.5–4 years). Ticks were removed manually by forceps from each of the randomly selected hosts. The body sites where the ticks were collected from included ears, neck, tail, fore limb, udder, scrotum, and around eyes. The collected ticks were placed in vials containing ethanol 70% and preserved at room temperature. Data were taken for each sample, such as host type, the number of hosts, region, and date of collection. For identification, samples were transferred to the National Center for Disease Control’s Research Lab for Parasites and Vectors of Diseases in Tripoli. The ticks were placed on petri plates and morphologically identified using a tick taxonomy key under a dissecting microscope (Walker et al., 2003). Data analysisThe data were classified according to location, species, host, and season of collection. The average number of ticks per host was calculated by dividing the number of ticks (all species) collected from a particular type of hosts by the total number of individuals of hosts from which the ticks were collected. The average numbers of collected ticks per host were tested for normality by the one-sample Kolmogorov–Smirnov Z test. Thereafter, the data, which were not normally distributed, were compared using nonparametric tests, considering P=0.05 to be significant. All statistical analyses were carried out on SPSS 20.0 statistical software (SPSS, 2011). Ethical approvalNot required for this study. ResultsA total of 634 ticks (349 males, 280 females, and 5 nymphs) were collected from 145 animals (camels, sheep, and goats); the differences between collected stages were significant (χ2, p < 0.007). Four species of ticks were recorded (Table 1): Hyalomma dromedarii (83.12%), Rhipicephalus bursa (6.94%), Hyalomma excavatum (6.63%), and Rhipicephalus camicasi (3.31%). Each species collected from one or more hosts, but no species was collected from all hosts; the mean prevalence of all species was 4.37 tickshost. Significant differences were seen between the number of individuals of collected species (Kruskal–Wallis test, χ2=8.45, df=3, p=0.029); H. dromedarii differed significantly from all the three species using Mann–Whitney U test (p ≤ 0.021). Numbers of individuals of collected species varied among seasons (Table 1), but the difference was not significant (Kruskal–Wallis test, χ2=1.54, df=3, p=0.67). All species were recorded in autumn and winter, while only three species were recorded in spring and two species in summer. Tick prevalence increased in summer (6.53 tickshost), while the lowest prevalence was in spring (2.18 tickshost). The highest average number of H. dromedarii per host was in summer (Table 1), which was significantly higher than that of all other seasons ( χ2, p < 0.0005), while the other seasons were not different from each other (χ2, p > 0.4). Nymphs were found only in summer. R. camicasi was collected from sheep in autumn and winter, and from goats only in autumn. The highest average number of ticks per sheep was in autumn. No significant differences were found between seasons (χ2, p > 0.16). In H. excavatum, the highest average number of ticks per sheep was in autumn and then declined slightly in winter and spring; no ticks were collected from sheep in summer. While the highest average of ticks per goat was in autumn, followed by spring, no ticks were collected from goats in summer and winter. No significant differences were found between seasons (χ2, p > 0.15). Rhipicephalus bursa was collected in all seasons, with the highest average (1.02 tickssheep) in summer then gradually declined to one ticksheep in the other seasons, while it was collected from goats only in autumn with average one tick per goat. No significant differences were found between seasons (χ2, p > 0.32). Regarding sex ratio for tick species which were collected from the study area (Table 2), the differences between male and female was significant (χ2=14.835, df=1, p=0.0001). DiscussionThis is the first study that investigated ticks associated with livestock in Tarhuna. Four species were collected in Tarhuna. They were among the 25 species of ticks that were recorded in Libya (Hoogstraal and Kaiser, 1960; Gabaj et al., 1992; Hador, 2015). In this study, all individuals of H. dromedarii were collected from camels. Camels are the preferred host for this species (Elghali and Hassan, 2009; El Tigani and Mohammed, 2010; Biu and Konto, 2011; Fard et al., 2012). Hyalomma dromedarii was found in all seasons with the highest prevalence in summer; this may indicate that H. dromedarii adapts to extreme dryness (Salim-Abadi et al., 2010). Gharbi et al. (2013) found a positive correlation between temperature and tick burden and a negative correlation with relative humidity. H. dromedarii can transmit pathogens such as Theileria annulata and spotted fever group Rickettsia, which can cause mortality in camel populations (Al-Deeb et al., 2015). Rhipicephalus camicasi was recorded for the first time in Libya, by Al-Baida by Hador (2015), on sheep more than goats in autumn and winter. In this study, most of these species were collected from sheep and little from goats in autumn and winter. The preferred hosts of the adults of this species are sheep and goats (Walker et al., 2003). Table 1. Number of ticks collected during the study seasons between August 2015 and May 2016. The numbers in brackets indicate the average number of ticks per host.
Table 2. Sex ratio for each tick species in Tarhuna between August 2015 and May 2016.
Hyalomma excavatum, in this study, was collected from sheep and goats. Mohammad (2015) collected H. excavatum from sheep more than goats. The highest average number of ticks per host was in autumn and spring; no ticks were collected in summer. Hador (2015) reported that the peak of infestation of H. excavatum extends from early spring to late autumn. Adults of H. excavatum were found on livestock throughout the year with a peak in spring and a reduction in the numbers in winter (Walker et al., 2003). It is poorly known whether H. excavatum has a role in transmitting diseases. The immature stages of this species may be a vector of pathogens, such as Theileria (Walker et al., 2003), Rickettsia (Chochlakis et al., 2012), and Anaplasma (M’ghirbi et al., 2012). All individuals of R. bursa were collected from sheep, except one specimen was collected from a goat; these results are similar to studies previously conducted (Yakhchali and Hosseine, 2006; Chaligiannis et al., 2014), which collected R. bursa from sheep more than goats. This may be explained by the differences in the goat’s skin and its physiology. Rhipicephalus bursa was the lowest abundant species of tick species (9%) that was identified in this study. It was collected in all seasons. This can be attributed to its ability to complete its life cycle at various temperatures (Papadopoulos et al., 1996). The highest average was in summer; this result agrees with Hasson’s (2012) study, which found the highest seasonal prevalence of R. bursa on sheep in summer. The important determinant affecting Rhipicephalus tick distribution is vegetation type, which is linked with the rainy season length and relative humidity (Hoogstraal, 1956). It was found that R. bursa has been involved in the transmission of Babesia spp. (Estrada-Pena et al. 2004; Tavassoli et al., 2013). So, the identification of distribution and abundance of R. bursa has high importance of tick-borne diseases epidemiology (Hurtado et al., 2015). Our results provide important information that contributes to completing the picture about the ticks species present in Libya, and also contributes to providing essential information for any program to control tick-borne diseases in Tarhuna. Authors’ contributionsAMA and TS: conception, design, and organization of the study. AMA, TS, WKS, and HIK: conducted the study; AMA: acquisition of data; AMA and TS: analysis and interpretation of data; AMA and TS: drafting of the manuscript and critiquing the output for important intellectual content. All authors discussed the results and commented on the manuscript. FundingThis work was funded by the National Centre for Disease Control, Ministry of Health, Libya. Conflict of interestThe authors have no conflict of interest to declare. AcknowledgmentThe authors express their deepest appreciation to the farmers who permitted them to collect ticks from the animals. ReferencesAl-Deeb, M.A., Bin Muzaffar, S., Abu-Zeid, Y.A., Enan, M.R. and Karim, S. 2015. First record of a spotted fever group Rickettsia sp. and Theileria annulata in Hyalomma dromedarii (Acari: Ixodidae) ticks in the United Arab Emirates. Florida Entomologist 98, 135-139. Belozerov, V.N. 1982. CHAPTER 13 - diapause and biological rhythms in ticks. In: Obenchain, F.D.andGalun, R. (Eds.), Physiology of Ticks Pergamon, pp: 469-500. Biu, A.A. and Konto, M. 2011. Survey of tick species infesting the one humped camel (Camelus dromedarius) in Borno State, Nigeria. J. Agric. Vet. Sci. 4, 1-6. Burgdorfer, W., Barbour, A.G., Hayes, S.F., Benach, J.L., Grunwaldt, E. and Davis, J.P.J.S. 1982. Lyme disease-a tick-borne spirochetosis? Science 216(4552), 1317-1319. Chaligiannis, I., Papa, A. and Sotiraki, S. 2014. Ticks feeding on ruminants and humans in Greece. Parasites Vectors 7, O1. https://doi.org/10.1186/1756-3305-7-S1-O1. Chochlakis, D., Ioannou, I., Sandalakis, V., Dimitriou, T., Kassinis, N., Papadopoulos, B., Tselentis, Y. and Psaroulaki, A. 2012. Spotted fever group rickettsiae in ticks in Cyprus. Microb. Ecol. 63, 314–323. Crowder, C.D., Rounds, M.A., Phillipson, C.A., Picuri, J.M., Matthews, H.E., Halverson, J., Schutzer, S.E., Ecker, D.J. and Eshoo, M.W. 2010. Extraction of total nucleic acids from ticks for the detection of bacterial and viral pathogens. J. Med. Entomol. 47, 89-94. El Tigani, M.A. and Mohammed, A.S. 2010. Ticks (Acari: Ixodidae) infesting camels in El Butana area, mid-central Sudan. Sudan J. Vet. Res. 25, 51-54. Elghali, A. and Hassan, S.M. 2009. Ticks (Acari: Ixodidae) infesting camels (Camelus dromedarius) in Northern Sudan. Onderstepoort J. Vet. Res. 76(2), 177-185. Elsaid, M.M.A., El-Arifi, E.O. and El-Buni, A.A. 2013. The prevalence of ectoparasites on sheep and goats at El Khoms region, Libya. J. Am. Sci. 9, 359-363. Estrada-Pena, A., Bouattaur, A., Camicas, J.L. and Walker, A.R. 2004. Ticks of Domestic Animals in the Mediterranean Region. University of Zaragoza, Spain. Fard, S.R.N., Fathi, S., Asl, E.N., Nazhad, H.A. and Kazeroni, S.S. 2012. Hard ticks on one-humped camel (Camelus dromedarius) and their seasonal population dynamics in southeast, Iran. Trop. Anim. Health Prod. 44(1), 197-200. Furman, D.P. and Loomis, E.C. 1984. The Ticks of California (Acari: Ixodida). California: University of California Press. Gabaj, M.M., Awan, M.A.Q. and Beesley, W.N. 1992. A survey of ticks on farm animals in Libya. Ann. Trop. Med. Parasitol. 86(5), 543-548. Gharbi, M., Moussi, N., Jedidi, M., Mhadhbi, M., Sassi, S. and Darghouth, M.A. 2013. Population dynamics of ticks infesting the one-humped camel (Camelus dromedarius) in central Tunisia. Tick Tick Borne Dis. 4(6), 488-491. Hador, A.B. 2015. A survey on ticks in some surrounding farms of Al-Baida, Al-Jabel Al-Akhdar, Libya, M.Sc, Omar AL-Mukhtar University, Al-Baida, Libya Hasson, R.H. 2012. Tick distribution and infestation among sheep and cattle in Baghdad’s south suburb. Kufa J. Vet. Med. Sci. 3(1), 77-90. Hoogstraal, H. 1956. African Ixodoidea. I. Ticks of Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera: Boophilus, Margaropus and Hyalomma). Washington DC: U.S. Department of Navy. Hoogstraal, H. and Kaiser, M.N. 1960. Observations on ticks (Ixodoidea) of Libya. Ann. Entomol. Soc. Am. 53, 445-457. Hutchings, R.S.G., Hutchings, R.W., Menezes, I.S., Motta, M.d.A. and Sallum, M.A.M. 2016. Mosquitoes (Diptera: Culicidae) from the northwestern Brazilian Amazon: Padauari river. J. Med. Entomol. 53, 1330-1347. Hurtado, A., Barandika, J.F., Oporto, B., Minguijón, E., Povedano, I. and García-Pérez, A.L. 2015. Risks of suffering tick-borne diseases in sheep translocated to a tick infested area: a laboratory approach for the investigation of an outbreak. Ticks Tick Borne Dis. 6(1), 31-37. M’ghirbi, Y., Hurtado, A. and Bouattour, A. 2010. Theileria and Babesia Parasites in Ticks in Tunisia. Transbound. Emerg. Dis. 57, 49–51. Magnarelli, L.A. 2009. Global importance of ticks and associated infectious disease agents. Clin. Microbiol. Newsletter 31, 33-37. Mashebe, P., Lyaku, J.R. and Mausse, F. 2014. Occurrence of ticks and tick-borne diseases of livestock in Zambezi region: a review. J. Agric. Sci. 6, 142-149. Mohammad, M.K. 2015. Distribution of ixodid ticks among domestic and wild animals in central Iraq. Bull. Iraq Nat. Hist. Mus. 13, 23-30. Papadopoulos, B., Morel, P.C. and Aeschlimann, A. 1996. Ticks of domestic animals in the Macedonia region of Greece. Vet. Parasitol. 63(1–2), 25-40. Parola, P. and Raoult, D. 2001. Tick-borne bacterial diseases emerging in Europe. Clin. Microbiol. Infect. 7, 80-83. Salim-Abadi, Y., Telmadarraiy, Z., Vatandoost, H., Chinikar, S., Oshaghi, M., Moradi, M., Mirabzadeh-Ardakan, E., Hekmat, S. and Nasiri, A. 2010. Hard ticks on domestic ruminants and their seasonal population dynamics in Yazd Province, Iran. Iranian J. Arthropod-Borne Dis. 4, 66-71. SPSS. 2011. SPSS for Windows, release 20. Inc., Chicago, USA. Tavassoli, M., Tabatabaei, M., Mohammadi, M., Esmaeilnejad, B. and Mohamadpour, H. 2013. PCR-based Detection of Babesia spp. Infection in Collected Ticks from Cattle in West and North-West of Iran. J. Arthropod Borne Dis. 7(2), 132-138. Tonbak, S., Aktas, M., Altay, K., Azkur, A.K., Kalkan, A., Bolat, Y., Dumanli, N. and Ozdarendeli, A. 2006. Crimean-Congo hemorrhagic fever virus: genetic analysis and tick survey in Turkey. J. Clin. Microbiol. 44, 4120–4124. Walker, A.R., Bouattour, A., Camicas, J.L., Estrada-pena, A., Horak, I.G., Latif, A.A., Pegram, R.G. and Preston, P.M. 2003. Ticks of domestic animals in Africa: A guide to identification of species. Bioscience Reports, Edinburgh, UK. Yakhchali, M. and Hosseine, A. 2006. Prevalence and ectoparasites fauna of sheep and goats flocks in Urmia suburb, Iran. Veterinarski Arhiv. 76(5), 431–442. | ||
| How to Cite this Article |
| Pubmed Style Abdulsalam AM, Saadawi WK, Kharwat HI, Shaibi T, . Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya. Open Vet J. 2022; 12(3): 370-374. doi:10.5455/OVJ.2022.v12.i3.10 Web Style Abdulsalam AM, Saadawi WK, Kharwat HI, Shaibi T, . Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya. https://www.openveterinaryjournal.com/?mno=3938 [Access: April 24, 2024]. doi:10.5455/OVJ.2022.v12.i3.10 AMA (American Medical Association) Style Abdulsalam AM, Saadawi WK, Kharwat HI, Shaibi T, . Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya. Open Vet J. 2022; 12(3): 370-374. doi:10.5455/OVJ.2022.v12.i3.10 Vancouver/ICMJE Style Abdulsalam AM, Saadawi WK, Kharwat HI, Shaibi T, . Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya. Open Vet J. (2022), [cited April 24, 2024]; 12(3): 370-374. doi:10.5455/OVJ.2022.v12.i3.10 Harvard Style Abdulsalam, A. M., Saadawi, W. K., Kharwat, H. I., Shaibi, T. & (2022) Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya. Open Vet J, 12 (3), 370-374. doi:10.5455/OVJ.2022.v12.i3.10 Turabian Style Abdulsalam, Asmaa M., Walid K. Saadawi, Hoda I. Kharwat, Taher Shaibi, and . 2022. Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya. Open Veterinary Journal, 12 (3), 370-374. doi:10.5455/OVJ.2022.v12.i3.10 Chicago Style Abdulsalam, Asmaa M., Walid K. Saadawi, Hoda I. Kharwat, Taher Shaibi, and . "Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya." Open Veterinary Journal 12 (2022), 370-374. doi:10.5455/OVJ.2022.v12.i3.10 MLA (The Modern Language Association) Style Abdulsalam, Asmaa M., Walid K. Saadawi, Hoda I. Kharwat, Taher Shaibi, and . "Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya." Open Veterinary Journal 12.3 (2022), 370-374. Print. doi:10.5455/OVJ.2022.v12.i3.10 APA (American Psychological Association) Style Abdulsalam, A. M., Saadawi, W. K., Kharwat, H. I., Shaibi, T. & (2022) Species diversity of ticks (Acari: Ixodidae) in Tarhuna, Libya. Open Veterinary Journal, 12 (3), 370-374. doi:10.5455/OVJ.2022.v12.i3.10 |