| Case Report | ||
Open Vet J. 2022; 12(5): 728-734 Open Veterinary Journal, (2022), Vol. 12(5): 728–734 Case Report Acute severe pericarditis secondary to rodenticide intoxication in a dogMara Bagardi1*, Giulia Drago1, Paolo Luigi Ferrari1, Jacopo Riva2 and Melania Moioli1,31Anicura - Clinica veterinaria Orobica, via Isonzo n. 2/e, 24052 Azzano San Paolo – BG, Italy 2Ambulatorio veterinario Santa Maria, via Alla Chiesa n. 3, 24050 Calcinate – BG, Italy 3Antech Imaging Services, Irvine, CA, USA *Corresponding Author: Mara Bagardi. Anicura-Clinica Veterinaria Orobica, Bergamo, Italy. Email: mara.bagardi [at] anicura.it Submitted: 03/06/2022 Accepted: 27/08/2022 Published: 25/09/2022 © 2022 Open Veterinary Journal
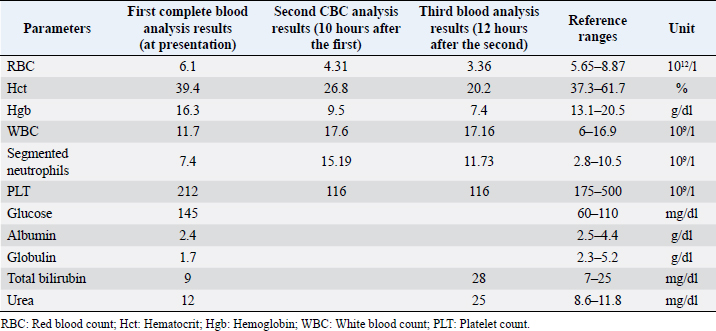
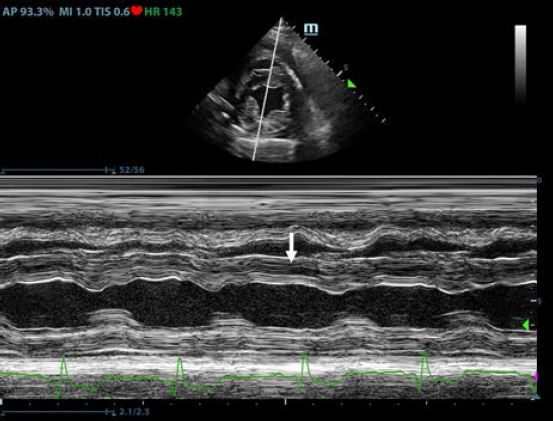
AbstractBackground: Pericardial effusions are well described in dogs; however, their association with rodenticide intoxication in the canine population is not widely described. Case Description: An adult mixed-breed dog was presented for 1-day history of anorexia and cough. Thoracic radiographs revealed moderate generalized cardiomegaly with globoid-shaped cardiac silhouette and mild bilateral pleural effusion. Echocardiography showed mild tamponating pericardial effusion and diffuse severe thickened pericardium. Compete blood count and blood chemistry at presentation were not specific. A coagulation profile was completed and showed severe prolongation of prothrombin time and partial thromboplastin time. Intravenous therapy with vitamin K was started at 5 mg/kg BID and on follow-up echocardiography performed 12 hours later there was evidence of complete regression of the pericardial thickening and pericardial effusion. Conclusion: To the authors’ knowledge, this is the first case report describing severe pericardial thickening, constrictive pericarditis, and cardiac tamponade secondary to spontaneous anticoagulant-induced hemopericardium in dogs. Keywords: Canine, Pericardial effusion, Rodenticide intoxication. IntroductionRodenticide intoxication is one of the most common poisonings reported; however, its cardiac effects have not been widely investigated in veterinary medicine (Petrus and Henik, 1999; Park et al., 2011). There are only two reported cases of pericardial effusion secondary to the ingestion of anticoagulant rodenticide in dogs (Petrus and Henik, 1999; Park et al., 2011). Anticoagulant rodenticides include first- and second-generation compounds. Coumarins such as warfarin, dicoumarol, and coumatetralyl, and indanediones such as valone and pindone belong to the first generation. The second-generation compounds include brodifacoum, diphacinone, and bromadiolone. The second-generation rodenticides are far more toxic and have a longer duration of action compared with the first generation (Mount et al., 1986; Murphy and Gerken, 1986; Murphy, 2002). Fatal internal bleeding can be caused by lethal doses of anticoagulants such as brodifacoum, coumatetralyl, or warfarin (Dorman, 1990; Murphy, 2007; Plumb, 2008). This case report describes a dog with severe pericardial thickening associated with mild pericardial and pleural effusion presumed to be secondary to rodenticide intoxication and successfully treated with standard vitamin K supplementation. Case DetailsA 2-year-old female indoor spayed mixed-breed dog was initially presented to her veterinarian for a 1-day history of anorexia and cough. The owners did not report history of exposure to rodenticides. The dog presented muffled heart sounds and thoracic radiographs revealed moderate globoid enlargement of the cardiac silhouette [vertebral heart score (VHS) of approx. 13], mild bilateral pleural effusion, and mild retraction of the lung lobes. Complete blood count (CBC) and chemistry panel at presentation were unremarkable [red blood count (RBC) 6.1 ×1012/l, reference range: 5.65–8.87 × 1012/l; Hct 39.4%, reference range: 37.3%–61.7%; Hgb 16.3 g/dl, reference range: 13.1–20.5 g/dl; white blood count (WBC) 11.7 × 109/l, reference range: 6–16.9 × 109/l; and segmented neutrophils 7.4 × 109/l, reference range: 2.8–10.5 × 109/l]. Platelet count (PLT) was within normal limits at 212 × 109/l (reference range: 175–500 × 109/l). The chemistry panel revealed mild hyperglycemia (145 mg/dl, reference range: 60–110 mg/dl), mild hypoalbuminemia 2.4 g/dl (reference range: 2.5–4.4 g/dl) and hypoglobulinemia 1.7 g/dl (reference range: 2.3–5.2 g/dl). CBC and chemistry data at presentation are reported in Table 1. The patient was referred to an emergency and specialty center for further evaluation. On presentation to the referral center that same day, the patient was reported to have a temperature of 37.4°C. On initial examination, the dog was tachycardic (heart rate 160 bpm), with cyanotic mucous membrane and prolonged refill time (>2 seconds). The patient had abnormal jugular pulses. Cardiac auscultation revealed mildly muffled heart sounds with regular rhythm. Respiratory rate was 34 breaths per minute. Oscillometric blood pressure evaluation was lower than normal limits (systolic pressure average of 100 mmHg). The QRS complexes were attenuated and electrical alternans was appreciated. Thoracic point of care ultrasound confirmed the presence of pericardial and pleural effusion. A complete echocardiographic examination demonstrated the presence of severe pericardial thickening, mild pericardial effusion, mild pleural effusion, and hemodynamic changes consistent with constrictive pericarditis (CP). Computed tomography (CT) was scheduled to further characterize cardiac abnormalities and for presurgical pericardiectomy planning. Table 1. CBC and chemistry panel at presentation, CBC 10 hours after the first, and CBC and chemistry analysis 12 hours after the second CBC.
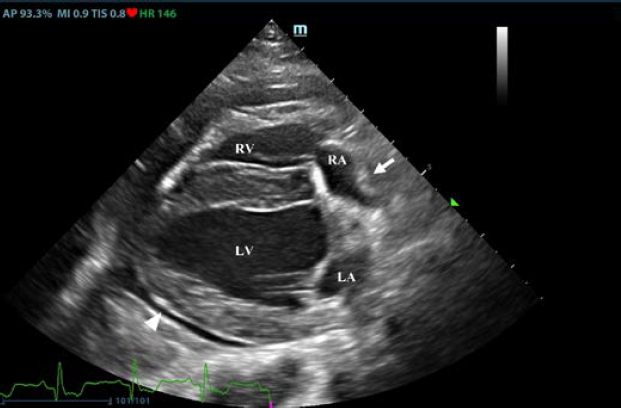
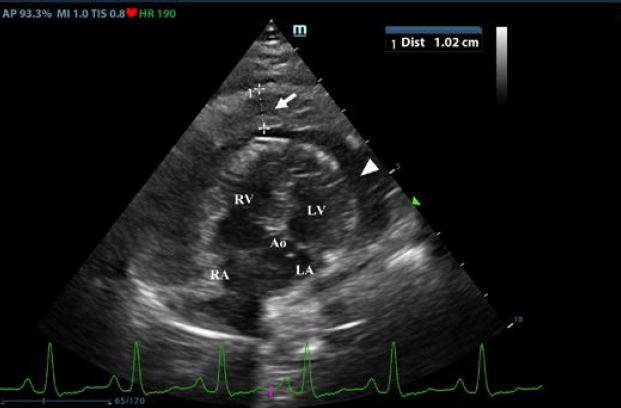
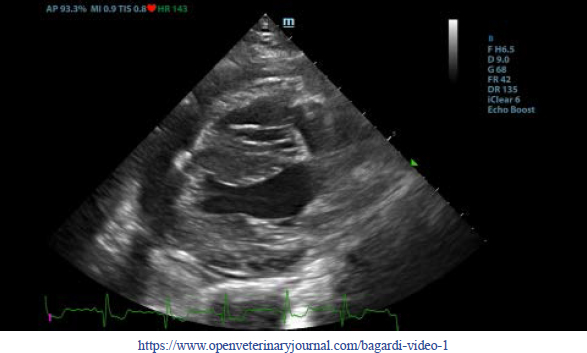
Thoracic radiographs interpretationThoracic radiographs at presentation showed elevation of the trachea which was parallel to the spine. The cardiac silhouette was moderately enlarged, with a broad sternal contact, a globoid shape and sharply defined margins (VHS approx. 13). There was mild bilateral pleural effusion and mild retraction of the lung lobes. The cranio-ventral lung lobes showed mild diffuse unstructured increase in opacity compatible with an interstitio-to-alveolar lung pattern, most likely related to retraction and mild atelectasis. Radiographic findings were compatible with pericardial effusion with signs of cardiac tamponade and right-sided heart failure. Echocardiogram interpretationFirst, transthoracic echocardiogram findings were a small amount of circumferential tamponating pericardial effusion (Fig. 1) and a severe hyperechoic pericardial thickening (10.2 mm) (Fig. 2). The right atrium was normal in size and there was collapse during systole. There was mild septal flattening seen on short axis view of the left ventricle. A short-axis left ventricular M-mode showed posterior displacement of the interventricular septum during late diastole at the time of atrial systole (Fig. 3). The epicardium and pericardium had a hyperechoic appearance with a thin, shaggy layer of heterogeneous echogenic material lining the surface of each (Fig. 1, Supplementary Video I). Pericardiocentesis was not recommended at this time given that the patient was minimally clinically and hemodynamically affected. Few hours later, a second echocardiographic examination, after stabilization of left ventricular volume with intravenous (IV) lactate ringer solution at maintenance rate, showed a worsening of pericardial thickness (13 mm) and an increase in pleural effusion. Furthermore, there was a >25% mitral inflow variation noted with respiration. In addition, there was >30% tricuspid inflow variation with respiration, signs of CP. During the second echocardiography, the patient appeared to be slightly dyspneic due to the increased thoracic effusion. CBC and blood chemistry interpretationA second CBC obtained 10 hours after the first one showed evidence of anemia (RBC 4.31 × 1012/l, reference range: 5.65–8.87 × 1012/l; Hct 26.8%, reference range: 37.3%–61.7%; and Hgb 9.5 g/dl, reference range: 13.1–20.5 g/dl), an increased WBC (17.6 × 109/l, reference range: 6–16.9 × 109/l), and segmented neutrophils (15.19 × 109/l, reference range: 2.8–10.5 × 109/l). PLT was lower than the previous one and lower compared to the reference ranges: 116 × 109/l (reference range: 175–500 × 109/l). A third CBC performed about 12 hours after the second one showed further decrease in the RBC (up to 3.36 × 1012/l reference range: 5.65–8.87 × 1012/l Hct 20.2%, reference range: 37.3%–61.7%; and Hgb 7.4 g/dl, reference range: 13.1–20.5 g/dl). PLT and WBC were unchanged compared to the previous blood work. Total bilirubin (28 mg/dl) and serum urea (25 mg/dl) increased (reference ranges: 7–25 mg/dl and 8.6–11.8 mg/dl, respectively). All data are reported in Table 1.
Fig. 1. Transthoracic echocardiogram – right parasternal long-axis four-chambered view. Note the pericardial effusion, the right atrial collapse (white arrow), the thickened, hyperechoic pericardium, and the scant pericardial effusion (white arrowhead). LA: left atrium; LV: left ventricle; RA: right atrium; and RV: right ventricle.
Fig. 2. Transthoracic echocardiogram – left apical five-chambered view. Note the pericardial effusion (white arrowhead) and the severe pericardial thickening (white arrow). Ao: aorta; LA: left atrium; LV: left ventricle; RA: right atrium; and RV: right ventricle.
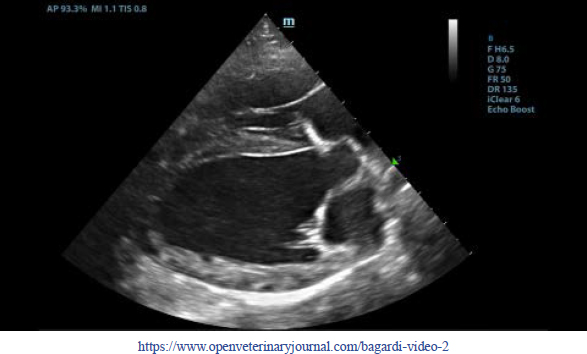
Fig. 3. Transthoracic echocardiogram – M-mode right parasternal short axis view of the left ventricle. Note the paradoxic septal motion (white arrow) characterized by diastolic posterior motion of the interventricular septum toward the left ventricular free wall. A sample of pleural effusion was collected for culture and susceptibility testing, as well as cytologic evaluation. Cytology of the pleural fluid and a concentrated sample prepared from submitted fluid were of low cellularity with marked hemodilution. Most nucleated cells were macrophages, and these were often vacuolated and contained phagocytized cellular material. Low numbers of reactive mesothelial cells were seen. No infectious organisms or overtly atypical cell populations were found. Neutrophils were compatible with the degree of hemodilution. There was evidence of prior hemorrhage (phagocytized erythrocytes), but significant inflammation (pericarditis) was not apparent in the cytology of the pleural fluid. A coagulation profile was completed and showed severe prolongation of prothrombin and partial thromboplastin time (PTT) PT (prothrombin time 100 seconds, reference range: 11–17 seconds; and PPT 128 seconds, reference range: 12–16 seconds) (Valchev et al., 2008). Cardiac troponin I was within normal ranges (0.01 ng/ml, reference range: <0.06 ng/ml). The patient was hospitalized overnight with IV fluid therapy and was started on amoxicillin-clavulanic acid (25 mg/kg every 12 hours PO – Synulox, Zoetis) and vitamin K (5 mg/kg every 12 hours IV; Vitamin K1 Laboratoire TVM, Domes Pharma). A CT examination, follow-up echocardiography, and possible pericardiocentesis in case of cardiac tamponade were planned for the next day. The next morning the patient was bright, alert, and responsive. She ate normally during the night. The mucous membrane refill time was within normal ranges and the jugular pulses were normal. Temperature was 39.7°C. The heart rate was 180 beats per minute and heart sounds were not muffled. Femoral pulses were strong. Pulmonary auscultation was unremarkable and the previous respiratory signs had resolved. Already after the first IV vitamin K supplementation, PT/PTT returned within normal limits. Oscillometric blood pressure evaluation was within normal limits (systolic pressure average of 143 mmHg). 24 hours later, the transthoracic echocardiogram revealed complete resolution of pericardial fluid and a significative thinning of the pericardial serosa. Two days after the beginning of the IV vitamin K supplementation, the pericardial serosa was completely normal (Supplementary Video II) and thoracic radiographs were within normal limits. The dog was discharged with oral dose of vitamin K (2.5 mg/kg every 12 hours for 3 weeks). The CBC and blood chemistry performed after 2 weeks were within the reference ranges. DiscussionPericardial effusion is a potentially life-threatening condition due to an increase in the intrapericardial pressure, resulting in varying degrees of hemodynamic compromise (Gidlewski and Petrie, 2005). Common causes of pericardial effusion in dogs include peritoneo-pericardial diaphragmatic hernia, congenital cysts, infectious pericarditis, right-sided heart failure, uremia, left atrial rupture, idiopathic pericardial effusion, cardiac neoplasia, and hemorrhage secondary to neoplasia (Kirsch et al., 2000). Pericardial effusion caused by rodenticide intoxication has been rarely reported in the veterinary literature (Petrus and Henik, 1999; Park et al., 2011). Petrus and Henik (1999) reported successful pericardiocentesis in a dog that ingested brodifacoum rodenticide; they drained the pericardial fluid into the pleural space without retrieving it for cytologic analysis. Similarly, Park et al. (2011) described hemorrhagic nonclotting pericardial fluid without clots removed from the pericardial space (Park et al., 2011). There are no reports about a spontaneous and immediate resolution of the pericardial thickening and pericardial effusion few hours after the administration of vitamin K without pericardiocentesis in dogs as in this case report. In human medicine, anticoagulants, such as warfarin, have been used for prevention and therapy of coronary artery diseases and previous reports describe the development of pericardial effusion associated with this anticoagulation therapy in humans (Miller, 1969; Granot and Shinar, 1982; Lee and Marwick, 1993). Despite strong evidence favoring a causative role of anticoagulation in the genesis of late cardiac tamponade from intrapericardial bleeding (Belic et al., 1978; Jones et al., 1979), there is no definitive evidence that anticoagulant treatment promotes development of pericardial effusion (Malouf et al., 1993). For this case report, CBC, blood chemistry, and coagulation profile have been essential for the precise and confident diagnosis of anticoagulant rodenticide intoxication because there was no known history of toxin exposure. Clinical examination showed a central cyanosis and an abnormal jugular pulse due to the reduction of the cardiac output and a venous congestion secondary to the pericarditis. Hematological exams showed hypochromic anemia, decreased hematocrit (Hct) values, leukocytosis with neutrophilia, thrombocytopenia, enhanced erythrocyte sedimentation rate, and decreased mean corpuscular volume. Biochemical profile showed mild hypoproteinemia and hypoalbuminemia, hyperglycemia, bilirubinemia, and increased urea concentration, signs of the toxic effect of anticoagulant rodenticides on liver parenchyma (Boermans, et al., 1991; James et al., 1998; Robben, et al., 1998; Kohn, et al., 2003; Binev et al., 2005). Coagulation profile abnormalities were prolonged PT and PTT (Kohn et al., 2003; Binev et al., 2005). The intoxications with anticoagulant rodenticides in animals are relatively frequent (Park et al., 2011), but this is the first case describing pericardial thickening and pericardial effusion responsive to vitamin K supplementation. Previous reports describe severe thickening of the urinary bladder wall and of the gastric wall secondary to a systemic bleeding disorder and the ultrasonographic appearance of the wall thickening reported in these cases is similar to the thickening of the pericardium identified in the patient of this case report (O’Brien and Wood, 1998; Marks et al., 2001; Londoño et al., 2015). It cannot be definitely proven that the pericardial changes were attributable to bleeding, and anticoagulant rodenticide intoxication was not confirmed; however, the complete resolution of both pericardial thickening and effusion following vitamin K therapy supports the presumptive diagnosis of pericardial hemorrhage and effusion secondary to rodenticide intoxication. Histologic analysis of the pericardium would have been required to confirm the diagnosis and rule out other differentials, but a biopsy was not obtained due to concerns regarding the coagulopathy and the quick resolution of both clinical and echocardiographic signs. For these reasons, in dogs with diffuse thickening of the pericardium and pericardial effusion, pericardial hemorrhage should be considered a possible differential diagnosis in addition to neoplasia and inflammation and testing for an underlying bleeding disorder is recommended. In conclusion, in veterinary medicine, if pericardial effusion is identified and suspected to be potentially related to rodenticide intoxication, it is important to confirm the presence of the pericardial effusion by using echocardiography, and to associate echocardiographic findings with CBC, blood chemistry, coagulation profile, and clinical findings to evaluate all the possible therapeutic options before performing a routine pericardiocentesis that could put the patient’s life at risk by generating further bleeding. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsAll authors contributed to making the completion of this manuscript possible. MB, GD, and MM were responsible for the bibliographic research and redaction of the manuscript; MB, JR, and MM were responsible for the figures, tables, clinical data analysis, and critical review of the manuscript; MB was responsible for the adaption of the tables and text; MM was responsible for the overall supervision and critical review of the manuscript. MB, GD, PF, JR, and MM analyzed the data and reviewed the paper. ReferencesBelic, L., Stafford, G., Allen, J.W. and Venkataraman, K. 1978. Cardiac tamponade during anticoagulation management with a pericardial drain and continued anticoagulation. J. Am. Med. Assoc. 240, 672. Binev, R., Petkov, P. and Rusenov, A. 2005. Intoxication with anticoagulant rodenticide bromadiolone in a dog – a case report. Vet. Arhiv. 75, 273–282. Boermans, H.J., Johnstone, I., Black, W.D. and Murphy, M. 1991. Clinical signs, laboratory changes and toxicokinetics of brodifacoum in the horse. Can. J. Vet. Res. 55, 21–27. Dorman, D.C. 1990. Anticoagulant, cholecalciferol and bromethalin-based rodenticides. Vet. Clin. North. Am. Small. Anim. Pract. 20(2), 339–352. Gidlewski, J. and Petrie, JP. 2005. Therapeutic pericardiocentesis in the dog and cat. Clin. Tech. Small. Anim. Pract. 20, 151–155. Granot, H. and Shinar, E. 1982. Spontaneous anticoagulant-induced hemopericardium with tamponade. Acta. Haemotol. 68, 339–340. James, S.B., Raphael, B.L. and Cook, R.A. 1998. Brodifacoum toxicity and treatment in a white-winged wood duck (Cairina scutulata). J. Zoo. Wildl. Med. 29, 324–327. Jones, M.R., Vine, D.L., Attas, M. and Todd, E.P. 1979. Late isolated left ventricular tamponade: clinical, hemodynamic, and echocardiographic manifestations of a previously unreported postoperative complication. J. Thorac. Cardiovasc. Surg. 77, 142–146. Kirsch, J.A., Dhupa, S. and Cornell, K.K. 2000. Pericardial effusion associated with metastatic disease from an unknown primary tumor in a dog. J. Am. Anim. Hosp. Assoc. 36, 121–124. Kohn, B., Weingart, C. and Giger, U. 2003. Haemorrhage in seven cats with suspected anticoagulant rodenticide intoxication. J. Feline. Med. Surg. 5, 295–304. Lee, K.S. and Marwick, T. 1993. Hemopericardium and cardiac tamponade associated with warfarin therapy. Cleve. Clin. J. Med. 60, 336–338. Londoño, L.A., Specht, A.J., VanderHart, D.J. and Bandt, C. 2015. What is your diagnosis? gastric wall hemorrhage secondary to anticoagulant rodenticide intoxication. J. Am. Vet. Med. Assoc. 247(3), 243–245. Malouf, J.F., Alam, S., Gharzeddine, W. and Stefadouros, M.A. 1993. The role of anticoagulation in the development of pericardial effusion and late tamponade after cardiac surgery. Eur. Heart. J. 14, 1451–1457. Marks, S.L., Tracy, L.G. and Jamie, W. 2001. Presumptive intramural gastric hemorrhage secondary to rodenticide intoxication in a dog. J. Vet. Emerg. Critical. Care. 11(1), 27–31. Miller, R.L. 1969. Hemopericardium with use of oral anticoagulant therapy. J. Am. Med. Assoc. 209, 1362–1364. Mount, M.E., Woody, B.J. and Murphy, M.J. 1986. The anticoagulant rodenticides. In Current veterinary therapy IX. Eds., Kirk, R.W. Philadelphia, PA: WB Saunders, pp: 156–165. Murphy, M.J. and Gerken, D.F. 1989. The anticoagulant rodenticides. In Current veterinary therapy X. Eds., Kirk, R.W. Philadelphia, PA: WB Saunders, pp: 143–146. Murphy, M.J. 2002. Rodenticides. Vet. Clin. North. Am. Small. Anim. Pract. 32, 469–484. Murphy, M.J. 2007. Anticoagulant rodenticides. In Veterinary toxicology. Basic and clinical priniples, 1st ed. Eds., Gupta, R.G. New York, NY: Academic Press; Amsterdam, The Netherlands: Elsevier. O'Brien, R.T. and Wood, E.F. 1998. Urinary bladder mural hemorrhage associated with systemic bleeding disorders in three dogs. Vet. Radiol. Ultrasound. 39(4), 354–356. Park, C., Lim, C.Y., Kim, J.H., Jang, J.I. and Park, H.M. 2011. Successful therapy of coumatetralyl rodenticide induced pericardial effusion with pericardiocentesis in a dog. Can. Vet. J. 52(2), 165–168. Petrus, D.J. and Henik, R.A. 1999. Pericardial effusion and cardiac tamponade secondary to brodifacoum toxicosis in a dog. J. Am. Vet. Med. Assoc. 215, 647–648. Plumb, D.C. 2008. Plumb’s veterinary drug handbook, 6th ed. Ames, IA: Blackwell Publishing Professional. Robben, J.H., Kuijpers, E.A. and Mout, H.C. 1998. Plasma superwarfarin levels and vitamin K1 treatment in dogs with anticoagulant rodenticide poisoning. Vet. Q. 20, 24–27. Valchev, I., Rumen, B., Veska, Y. and Yordan, N. 2008. Anticoagulant rodenticide intoxication in animals - a review. Turkish. J. Vet. Anim. Sci. 32, 237–243. Supplementary Material
Supplementary Video I. Transthoracic echocardiogram – right parasternal long-axis four-chambered view. Note the thickened, hyperechoic pericardium, the pericardial effusion, and the hyperechoic appearance of the epicardium and pericardium with a thin, shaggy layer of heterogeneous echogenic material lining the surface of both. The left ventricular chamber appears empty, and the walls are pseudohypertrophic.
Supplementary Video II. Transthoracic echocardiogram 2 days after discharge – right parasternal long-axis four-chambered view. Note the complete resolution of the pericardial effusion, the normal thickness of the pericardium, and normalization of the left ventricle volumes. | ||
| How to Cite this Article |
| Pubmed Style MB, GD, Ferrari PL, Riva J, Moioli M, . Acute severe pericarditis secondary to rodenticide intoxication in a dog. Open Vet J. 2022; 12(5): 728-734. doi:10.5455/OVJ.2022.v12.i5.18 Web Style MB, GD, Ferrari PL, Riva J, Moioli M, . Acute severe pericarditis secondary to rodenticide intoxication in a dog. https://www.openveterinaryjournal.com/?mno=38867 [Access: April 24, 2024]. doi:10.5455/OVJ.2022.v12.i5.18 AMA (American Medical Association) Style MB, GD, Ferrari PL, Riva J, Moioli M, . Acute severe pericarditis secondary to rodenticide intoxication in a dog. Open Vet J. 2022; 12(5): 728-734. doi:10.5455/OVJ.2022.v12.i5.18 Vancouver/ICMJE Style MB, GD, Ferrari PL, Riva J, Moioli M, . Acute severe pericarditis secondary to rodenticide intoxication in a dog. Open Vet J. (2022), [cited April 24, 2024]; 12(5): 728-734. doi:10.5455/OVJ.2022.v12.i5.18 Harvard Style , M. B., , G. D., Ferrari, P. L., Riva, J., Moioli, M. & (2022) Acute severe pericarditis secondary to rodenticide intoxication in a dog. Open Vet J, 12 (5), 728-734. doi:10.5455/OVJ.2022.v12.i5.18 Turabian Style , Mara Bagardi, Giulia Drago, Paolo Luigi Ferrari, Jacopo Riva, Melania Moioli, and . 2022. Acute severe pericarditis secondary to rodenticide intoxication in a dog. Open Veterinary Journal, 12 (5), 728-734. doi:10.5455/OVJ.2022.v12.i5.18 Chicago Style , Mara Bagardi, Giulia Drago, Paolo Luigi Ferrari, Jacopo Riva, Melania Moioli, and . "Acute severe pericarditis secondary to rodenticide intoxication in a dog." Open Veterinary Journal 12 (2022), 728-734. doi:10.5455/OVJ.2022.v12.i5.18 MLA (The Modern Language Association) Style , Mara Bagardi, Giulia Drago, Paolo Luigi Ferrari, Jacopo Riva, Melania Moioli, and . "Acute severe pericarditis secondary to rodenticide intoxication in a dog." Open Veterinary Journal 12.5 (2022), 728-734. Print. doi:10.5455/OVJ.2022.v12.i5.18 APA (American Psychological Association) Style , M. B., , G. D., Ferrari, P. L., Riva, J., Moioli, M. & (2022) Acute severe pericarditis secondary to rodenticide intoxication in a dog. Open Veterinary Journal, 12 (5), 728-734. doi:10.5455/OVJ.2022.v12.i5.18 |