| Original Article | ||
Open Vet J. 2022; 12(4): 584-594 Open Veterinary Journal, (2022), Vol. 12(4): 584–594 Original Research In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier and Levier (giant hogweed), Centaurea jacea L. (brown knapweed), and Chenopodium album L. (Pigweed): Three invasive plantsMbarga Manga Joseph Arsene1*, Podoprigora Irina Viktorovna1, Mefed Kirill Mikhaïlovitch1, Anyutoulou Kitio Linda Davares1, Kezimana Parfait2, Manar Rehailia2, Senyagin Alexander Nikolayevich1, Girich Valentina Stefanovna1, Souadkia Sarra1, Khabadze Zurab Sulikoevich3, Chernaia Zoya Anatolyevna4 and Das Shommiya51Department of Microbiology named after V.S. Kiktenko, Institute of Medicine, RUDN University, Moscow, Russia 2Department of Agrobiotechnology, Agrarian Institute, RUDN University, Moscow, Russia 3Department of Therapeutic Dentistry, RUDN University, Moscow, Russia 4Department of Microbiology and Virology, Pirogov Russian National Research Medical University, Moscow, Russia 5Department of Hospital Therapy with Endocrinology, Hematology and Clinical Laboratory Diagnostics courses, Institute of Medicine, RUDN University, Moscow, Russia *Corresponding Author:Mbarga Manga Joseph Arsene. Department of Microbiology named after V.S. Kiktenko, Institute of Medicine, RUDN University, Moscow, Russia. Email: josepharsenembarga [at] yahoo.fr Submitted: 09/05/2022 Accepted: 25/07/2022 Published: 25/08/2022 © 2022 Open Veterinary Journal
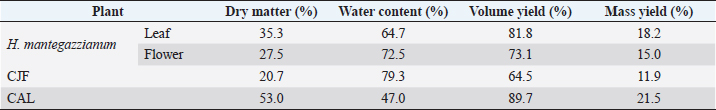
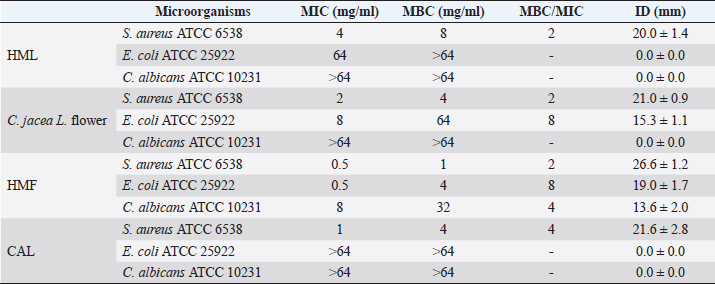
AbstractBackground: Plants, including invasive ones, can play a significant role in the fight against antibiotic resistance and the search for new antimicrobials. Aims: The present study aimed at assessing the antimicrobial activity, antibioresistance reversal properties, and toxicity of four samples from invasive plants, namely, Heracleum mantegazzianum (leaves and flowers), Chenopodium album (leaves), and Centaurea jacea (flowers). Methods: The extraction of active compounds was done with ethanol (80%, v/v) and the extraction yields were calculated. Antimicrobial activity was studied using the agar-well diffusion method against Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 6538, and Candida albicans ATCC 10231. Minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) were determined using the mircodilution method. The antibioresistance reversal properties were assessed using the checkerboard method and the toxicity of the extracts was studied using the larval form of the Greater Wax Moth (Galleria mellonella). Results: The mass yields were 11.9, 15.0, 18.2, and 21.5, respectively, for C. jacea flower (CJF), H. mantegazzianum flower (HMF), H. mantegazzianum leaf (HML), and C. album leaf (CAL). The highest inhibition diameters (ID) were found with HMF, CAL, CJF, and HML against S. aureus with 26.6, 21.6, 21.0, and 20.0 mm, respectively. Only CJF and HMF were active against E. coli with respective ID of 15.3 and 19.0 mm. Except HMF (ID=13.6 ± 2.0 mm), no other extract was active against C. albicans. Moreover, HMF exhibited the lowest MIC (0.5 mg/ml) and the lowest MBC (1 and 4 mg/ml) against both S. aureus and E. coli. Regarding the synergy test, an additional effect [0.5 ≤ fractional inhibitory concentration (FIC) ≤ 1] was found in almost all the combinations antibiotics + extracts excepted for HMF + (Kanamycin or Ampicillin) against S. aureus and CJF + Ampicillin against E. coli where we found synergy effect (FIC ≤0.5). The median lethal doses (LD50s) of HMF, HML, CAL, and CJF were 20.2, 0.58, 13.2, and 4.0 mg/ml, respectively. Conclusion: Only the ethanolic extract of HMFs showed noteworthy broad spectrum antimicrobial activity. Keywords: Invasive plants, Antimicrobial activity, Synergy, Toxicity. IntroductionAs the world gradually recovers from the health crisis caused by COVID-19, countries should pay more attention to other silent epidemics, such as resistance to antibiotics, which is constantly growing in medicine, agriculture, and animal breeding (Souadkia et al., 2021; Arsene et al., 2022). Recent estimates have shown that antibiotic resistance is responsible for 700,000 annual deaths worldwide, 230,000 of which have resulted from multidrug-resistant tuberculosis (WHO, 2019). The WHO estimates that if nothing is done to address this problem, drug-resistant diseases may cause 10 million deaths each year by 2050 and damage to the economy as catastrophic as the 2008–2009 global financial crisis (WHO, 2019). Antibiotic resistance is defined as the ability of bacteria to resist the inhibitory or destructive activity of an antibiotic to which it was initially sensitive (Su et al., 2020). It primarily results from the uncontrolled use of antibacterial drugs both in medicine and in agriculture, which leads to the recurrent exposure of bacteria to sub-lethal doses of antibiotics and results in their adaptation. This adaptation phenomenon results mainly from the enzymatic degradation of antibiotics by bacteria (Elekhnawy et al., 2020), the modification of the antibiotic target (Schaenzer and Wright, 2020), change in membrane permeability (Singh et al., 2020), and alternative metabolic pathways (Singh et al., 2020). Interbacterial transmission of antibiotic resistance through horizontal gene transfer (conjugation, transduction, and transformation) has made the situation critical worldwide (Su et al., 2020). To respond to this dangerously worrying situation, research teams from all over the world are permanently evaluating the use of potential alternatives to antibiotics such as nanoparticles (Amer and Awwad, 2021), probiotics (Arsene et al., 2021, 2021b), phage therapy (Ansald et al., 2020), antimicrobial, peptides (Andersson et al., 2016), and medicinal plants (Arsene et al., 2021c, 2021d; Mouafo et al., 2021). Among these alternatives, herbal remedies have various advantages because of their availability, fewer reported side effects, cost, high tolerance toward patients, and lack of bacterial resistance (Wojnicz et al., 2012). In this context, all plants, including invasive ones like Heracleum mantegazzianum Sommier and Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed) deserve to be investigated for their antimicrobial properties. Indeed, among terrestrial plants, species that are invasive tend to be larger and have higher growth rate and shoot allocation than do species that are not invasive (Havel et al., 2015). These characteristics could be an asset if the extracts of such plants prove to be antimicrobial since they are widespread and can be easily cultivable for an eventual large-scale exploitation. Therefore, the present study aimed at assessing the antimicrobial activity, antibioresistance reversal properties, and the toxicity of the pigweed’s and the brown knapweed’s leaves, and the giant hogweed’s leaves and flowers. Materials and MethodsVegetal materialThe vegetal materials used in this study were flowers of H. mantegazzianum Sommier and Levier and C. jacea L., and leaves of C. album L and H. mantegazzianum. The three plants were collected in June 2021 in the city of Mozhaysk (55°35ʹ02.6″N 35°51ʹ55.5″E, Mozhaysky District, Moscow Oblast, Russia) after identifying the plants using the mobile professional version of PictureThis-Plant Identifier (Glority LLC, 2021). After the harvest, the plant materials were conducted to the laboratory, dried at 37°C until constant mass and dry matter content was calculated. Thereafter, the plants were grinded and powders with a particle size of less than 1 mm were stored in a sterile airtight container until further use. Bacterial strainsThe microorganisms used for the screening of antimicrobial activity consisted of three standard strains. Staphylococcus aureus ATCC 6538 was used as Gram positive model, Escherichia coli ATCC 25922 as Gram negative model, and Candida albicans ATCC 10231 as fungi model. All the microorganisms were provided by the laboratory of microbiology of RUDN university. Galleria melonellaGalleria mellonella larvae were obtained commercially from https://ecobaits.ru/ (ECO BAITS, Moscow, Russia) and stored at 15°C prior to use. Dead larvae and those with dark spots or showing signs of melanisation were discarded. Chemicals and mediaDimethyl sulfoxide (DMSO) was purchased from BDH Laboratories, VWR International Ltd., USA. We also used BHIB (Brain Heart Infusion Broth, HiMedia™ Laboratories Pvt. Ltd., India), Muller Hinton Agar (MHA HiMedia™ Laboratories Pvt. Ltd., India), Sabouraud Dextrose Broth (SDB, HiMedia™ Laboratories Pvt. Ltd., India), and all other reagents and chemicals used were of analytical grade. Phytochemical extractionAs we described in our previous investigation ((Arsene et al., 2021b), 30 grams of each vegetal material was weighed and added to 270 ml of the ethanolic solution (80%, v/v) in a conical flask. The flasks were covered tightly and were shaken at 300 rpm for 24 hours and 25°C in a shaker incubator (Heidolph Inkubator 1,000 coupled with Heidolph Unimax 1,010, Germany). The mixtures were then filtered using Whatman filter paper No 1 then concentrated at 40°C in rotary evaporator (IKA RV8) equipped with a water bath IKA HB10 (IKA Werke, Staufen, Germany) and a vacuum pumping unit IKA MVP10 (IKA Werke, Staufen, Germany). The final dried crude extracts were weighed. Extraction volume and mass yield were determined using the following formulas: Preparation of antimicrobial solutionFor each plant extract, the crude extract was dissolved in the required volume of DMSO (5%, v/v) to achieve a concentration of 64 mg/ml. The extracts were sterilized by microfiltration (0.22 μm; Merck Millipore, Tullagreen, Cork, Ireland) and the solutions obtained was used to prepare the different concentrations used in the analytical process. Inoculum preparationBacteria were cultured for 24 hours, 0 rpm, and at 37°C in 10 ml of BHIB while the yeast (C. albicans ATCC 10231) was cultured in the same volume of SDB and the same conditions (0 rpm, 24 hours at 37°C in 10 ml). After incubation, the cells were collected by centrifugation (7,000 g, 4°C, 10 minutes), washed twice with sterile saline (0.9%), resuspended in 5 ml of sterile saline to achieve a concentration equivalent to McFarland 0.5 using DEN-1 McFarland Densitometer (Grant-bio, Grant instruments Ltd., Cambridge, UK). Screening of antibacterial activityAssessment of antimicrobial activity using well diffusion methodThe well-agar diffusion method described in our previous investigation (Arsene et al., 2021b) was used to assess the antimicrobial activity of the extracts. Briefly, 15 ml of sterile Muller-Hinton Agar (for bacteria) or Sabouraud Dextrose Agar (SDA) (For C. albicans) were poured into petri dishes and 100 μl of each microorganism were spread. Wells with a capacity of 20 µl were drilled on the culture medium and 20 µl (at 100 mg/ml) of each plant extracts were added. The sterile DMSO (5%, v/v) used to prepare the extracts was used as negative control and all the trials was done in triplicate. After incubation at 37°C for 24 hours, the inhibition diameters (ID) were measured. Determination of minimum inhibitory concentrations (MIC)MIC is the lowest concentration of antibacterial agent that completely inhibits the visible bacterial growth. The MIC of the extracts was determined using the microbroth dilution method as described in our previous published work (Arsene et al., 2021b). Briefly, 100 μl of broth (BHIB or SDB) was added to all the wells of sterile U-bottom 96-well microplates and extracts preparations (64 mg/ml) were subjected to serial twofold dilution. Each column represented one type of extract and a single strain. For each test well, 10 μl of the respective inoculum (with turbidity equivalent to a 0.5 McFarland scale) was added. Finally, the plates were covered and incubated at 37°C for 24 hours and after incubation, MIC was considered the lowest concentration of the tested material that inhibited the visible growth of the bacteria. Determination of minimum bactericidal concentration (MBC)MBCs were determined by subculturing the wells without visible growth (with concentrations ≥MIC) on MHA or SDA plates. Inoculated agar plates were incubated at 37°C for 48 hours and MBC was considered the lowest concentration that did not yield any microbial growth on agar. Tolerance levelTolerance level of tested bacterial strains against aqueous and ethanolic extract was determined using the following formula (Mondal et al., 2020): The characteristic of the antibacterial activity of extracts was determined by the tolerance level indicating the bactericidal or bacteriostatic action against the tested strains. When the ratio of MBC/MIC is ≥16, the antibacterial efficacy of the test agent is considered as bacteriostatic, whereas MBC/MIC ≤4 indicates bactericidal activity (Mondal et al., 2020). Modulation of common antibiotic using the checkboardThe checkerboard method, commonly used for the determination of synergy between the antibiotics and natural antibacterial compounds, was used for the antibiotic modulation assay (Arsene et al., 2021b). Modulations of ampicillin (AMP), nitrofurantoin (NIT), and kanamycin (KA) were performed with extracts whose MIC was successfully determined. The fractional inhibitory concentration (FIC) index was calculated, as previously described (Trabelsi et al., 2020; Arsene et al., 2021b). Briefly, the individual MICs of the antibiotics (MIC-ATB) and the extract (MIC-extr) on the two targeted strains (S. aureus and E. coli) were first determined using the microdilution method as described above. Then, the new MIC values (MIC′-ATB and MIC′-extr) were determined after combining the two substances. Combinations of antibiotics + extracts were prepared by mixing the two antimicrobial solutions in 50:50 (v:v) proportions with initial concentrations depending on each stock solutions. To assess the interaction between the antibiotic and the natural extract, the FIC was determined using the using the formula: . The FIC index was interpreted as follows FIC ≤0.5, synergy; 0.5 ≤ FIC ≤1, addition of effects; 1 ≤ FIC ≤4, indifference and for FIC >4, Antagonism (Trabelsi et al., 2020). Toxicity assayThe toxicity was evaluated as previously described (Mbarga et al., 2021a). First, each extract was dissolved in sterile phosphate-buffered saline (PBS) to obtain a concentration of 50 mg/ml which was then diluted to obtain concentrations of 40, 20, 10, 5, 2, 1, and 0.5 mg/ml. Furthermore, the larvae were weighed and only those from 0.2 to 0.5grams were retained. For each concentration of extract, 3 groups of 20 randomly selected G. mellonella larvae were used (Fig. 1-A). 20 µl of each dilution were injected using a 0.3 ml Terumo® Myjector® U-100 insulin syringe (VWR, Russia) through the base of the last left proleg as we described in our previous study (Mbarga et al., 2021a), and shown in Figure 1-B. Control groups of 10 larvae injected with 20 μl sterile PBS were also included. After 24 hours of incubation in the dark at 37°C, larvae were examined for mortality, and were considered dead if they were unmoving, failed to reorient themselves when placed on their backs, and failed to respond to stimuli. Percentage survival was plotted as a function of concentration for each of the plants extract using Spline cubic model in the statistical software XLSTAT 2020 (Addinsof Inc., New York, NY) and median lethal dose (LD50), 90% lethal dose (LD90) and the 100% lethal dose (LD100) values expressed in mg/ml were calculated using each specific spline cubic equation or spline cubic curves obtained. Spline curves were not used when the fit accuracy of the spline equation was less than 90%). The mean weight of each group of 20 larvae was used to extrapolate the LD50, LD90 and LD100 values for each plant extract in g/kg using the formulas: Ethical approvalNot applicable. ResultsDry matter and extraction yieldAs shown in Table 1, the highest dry matter content was observed on leaf of C. album (53.0%) and leaf of H. mantegazzianum (35.3%). Flowers of C. jacea and H. mantegazzianum consisted mainly of water with water contents of 79.3% and 72.5%, respectively. In addition, the volume yield was greater in leaves (89.7% and 81.8%) than in flowers (73.1% and 64.5%). The mass yields were 11.9%, 15.0%, 18.2%, and 21.5%, respectively, for C. jacea flower (CJF), H. mantegazzianum flower (HMF), H. mantegazzianum leaf (HML), and C. album leaf. Antimicrobial activityAntimicrobial activity was assessed both through ID using the agar well diffusion method and through determination of minimum MIC and MBC. The results of the antimicrobial activity of the extracts against S. aureus ATCC 6538, E. coli ATCC 25922, and C. albicans ATCC 10231 have been grouped in Table 2. All extracts were not active against all the microorganisms tested. Regarding the ID, we found that they ranged from 0.0 ± 0.0 to 26.6 ± 1.5 mm. The highest antimicrobial activity materialized with the highest IDs was observed against S. aureus with 26.6 ± 1.5, 21.6 ± 2.8, 21.0 ± 0.9 and 20.0 ± 1.4 mm, respectively, with HMF, C. album leaf (CAL), CJF and HML. Only CJF and HMFs were active against E. coli with respective ID of 15.3 ± 1.1 and 19.0 ± 1.7 mm. Except HMF (ID=13.6 ± 2.0 mm), no other extract was active against C. albicans. Moreover, MIC and MBC values varied from 0.5 to >64 mg/ml and 1 to >64 mg/ml, respectively. All the extracts were more active on S. aureus (0.5 < MIC <4 mg/ml) compared to their activity on other E. coli. Flowers of H. mantegazzianum exhibited the lowest MIC (0.5 mg/ml) and the lowest MBC (1 and 4 mg/ml) against both S. aureus and E. coli. Similarly, unlike other extracts which had indeterminate MIC (>64 mg/ml), only the MIC of HMF was successfully determined (8 mg/ml) against C. albicans. We were able to calculate the MBC/MIC ratio in 7/12 of the tests and this ratio varied from two to eight. The lowest MBC/MIC ratio (2) associated with the lowest MIC and MBC was observed with HMFs against S. aureus
Fig. 1. (A) Group of 20 larvae and (B) Injection of G. mellonella larvae with plant extract through the last left proleg. Table 1. Dry matter and extraction yield of the plant materials used.
Table 2. MIC, MBC, and ID (at 50 mg/ml) of plant extracts.
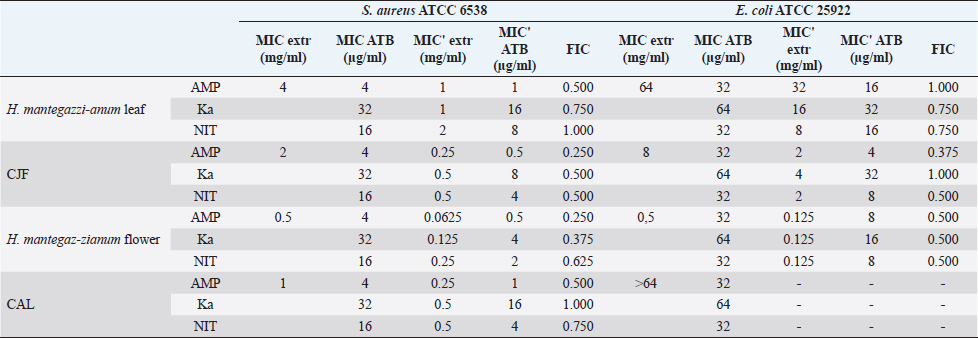
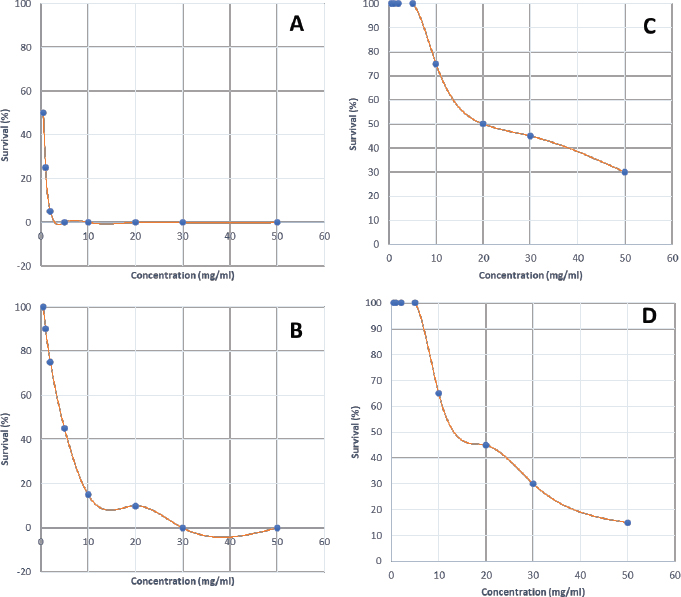
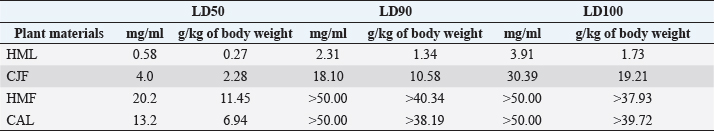
Synergistic effect between common antibiotics and plant extracts using checkboard methodTable 3 shows the results of modulation of AMP, KA, and NIT with H. mantegazzanum leaf (HML), CJF, HMF and CAL against S. aureus and E. coli. We noted that the FIC ranged from 0.250 to 1.000. The lowest FIC (0.250) associated with the highest fold decrease (8) of the concentration of antibiotic was found with the combinations AMP-CJF and AMP-HMF against S. aureus. FICs ≥1 were observed in KA-CAL and NIT-HML combinations against S. aureus and with KA-CJF and AMP-HML against E. coli. Toxicity assayThe median lethal doses (LD50), 90% (LD90) and 100% (LD100) were determined using the spline cubic survival curves (Fig. 2) from the data obtained on the survival rate of the Greater Wax Moth (G. mellonella) 24 hours after the injection with the extracts. As shown in Table 4, the LD50 values of HMF and CAL were 20.2 and 13.2 mg/ml, respectively. In addition, we found that leaves of H. mantegazzianum and flowers of C. jacea had the lowest LD. The LD50 (mg/ml) of HML was 0.58 (0.27 g/kg bw (body weight)) while that of CJF was 4.0 (2.28 g/kg bw). The concentrations of HMF and CAL capable of killing 100% of the larvae (LD100) were higher than the highest concentration tested (>50 mg/ml) while the LD100 (mg/ml) of the leaves of H. mantegazzianum and flowers of C. jacea were, respectively, 3.91 (1.73 g/kg bw) and 30.39 (19.21 g/kg bw). DiscussionInvasive plants are mainly known to cause economic losses in agriculture (Pimentel et al., 2005). This characteristic comes from the fact that they grow very quickly and sometimes with limited resources, even in hostile environments (Pintó-Marijuan and Munne-Bosch, 2013; Havel et al., 2015). Thus, research devoted to these plants is very often aimed at finding effective means to combat them, while very few studies investigate their biological properties (Máximo et al., 2020). Against the current backdrop of alarming growth in antibiotic resistance, invasive plants that demonstrate worthy antimicrobial activity can be of great use and their rapid growth could be an asset for their potential large-scale exploitation. Table 3. FIC of the combinations of extracts and antibiotics against S. aureus ATCC 6538 and E. coli ATCC 25922.
Fig. 2. Survival curves for G. mellonella larvae against ethanolic extract of (A) HML, (B) CJF, (C) HMF, and (D) CAL. Each data point represents the mean percentage survival of three groups of 20 larvae, following injection with 20 µl of specific concentrations of the selected plant extract and incubation for 24 hours at 37°C. Table 4. LD50, LD90, and LD100 values for the tested plants using G. mellonella model.
In the present study, we investigated the antimicrobial properties of four extracts from three plants recognized as invasive, namely, H. mantegazzianum (giant hogweed), C. jacea (brown knapweed), and C. album (Pigweed). First, as shown in Table 1, we found that the dry matter content and the extraction mass yields were positively and significantly correlated (Pearson correlation coefficient=0.974 and p=0.026). Indeed, plants with a high percentage of dry matter (e.g., 53.0% for CAL) had the highest mass yield (21.5% for CAL) while, plants with the lowest dry matter percentage (20.7%) like CJF had the lowest mass yield (11.9%). The same dynamic was observed with CJF and HMFs. This observation is quite strange because we used the same mass of each plant (30grams) after drying them all. However, we can explain it by the tendency of plants to try to reabsorb (during extraction) the amount of water that they lost during drying (Bewley, 1973). This hypothesis was confirmed by observing the volume yields (Table 1), where we found that the plants with the highest dry matter content were associated with the highest volume yields while the plants with the lowest dry matter content were associated with lower volume yields. Moreover, regarding the antimicrobial activity using well-agar diffusion method, HMF, CAL, CJF, and HML have demonstrated very good activity against S. aureus (with ID >20 mm), while only CJF and HMFs were active against E. coli with respective ID of 15.3 ± 1.1 and 19.0 ± 1.7 mm. On the one hand, the ID observed in this study showed that the active compounds involved in the antimicrobial activity of these plants can diffuse in aqueous media. Indeed, most of the time, one of the reasons why some plants do not show antibacterial activity is the relatively non-polar nature of the active compounds, which makes them very little diffuse in the aqueous agar matrix used in agar diffusion studies (Eloff, 2019). Moreover, this difference in the antimicrobial activity could be explained by the variability of phytochemical profile of the plants. In addition, the higher sensitivity of Gram-positive bacteria compared to Gram-negative bacteria could be explained by the differences in the composition of their cell wall (Acosta-Gutiérreza et al., 2020). Indeed, Gram-positive bacteria have an outer wall made essentially of peptidoglycan which is an ineffective permeability barrier (Acosta-Gutiérreza et al., 2020), while the outer phospholipid membrane of Gram-negative bacteria makes the outer layer impermeable to lipophilic solutes and constitutes a selective barrier to hydrophilic solutes. These observations were confirmed when determining MIC and MBC where the lowest MIC and MBC were observed on S. aureus compared to E. coli (Table 2). These findings are different to that stated by Etame et al. (2019) who found no significant difference in the MIC values of plant extracts against Gram positive and Gram-negative bacteria. Furthermore, Except HMF (ID=13.6 ± 2.0 mm), no other extract was active against C. albicans. This resistance of C. albicans compared to bacteria could be ascribed to their membrane composition which is different to those of bacteria. In fact, the higher number of anionic phospholipids in the membrane of bacteria ease their interaction with antimicrobial compounds and thus increase their sensitivity (Oren et al., 1997; Papo and Shai, 2003). Moreover, flowers of H. mantegazzianum exhibited the lowest MIC (0.5 mg/ml) and the lowest MBC (1 and 4 mg/ml) against both S. aureus and E. coli. The antimicrobial activity of H. mantegazzianum can be ascribe to compounds, such as octyl acetate hexyl 2-metylbutyrate, hexyl isobutyrate, and hexyl butyrate, which has been identified as it dominant constitute (Matoušková et al., 2019). Similarly, the activity of C. album leaves against S. aureus can be ascribed to flavonoid, saponin, cinnamic acid amide, alkaloid chenoalbicin, apocarotenoids, and phenols (Cutillo et al., 2004). The antimicrobial activity varies widely from plant to plant, and it is necessary to point out that, the antimicrobial activity observed in the present study may be higher (Akinsulire et al., 2018), lower (Romulo et al., 2018), or even equal to that of extracts from other plants, medicinal or not. However, the only advantage that these plants have over some medicinal plants is their invasive aspect which makes them easily available (Máximo et al., 2020) compared to certain seasonal medicinal plants such as Enantia chlorantha (Etame et al., 2020), Aesculus hippocastanum (Konstantinovitch et al., 2022), Anthonotha macrophylla (Essiet et al., 2019), and many others. Notwithstanding this, according to the classification established by Kuete (2010) and Kuete and Efferth (2010), except flowers of H. mantegazzianum, the other extracts could be considered as deserving a weak antimicrobial activity independently of the tested strain as they scored MIC value higher than 0.625 mg/ml. In addition, it is established in the literature that an antimicrobial compound is considered as bactericidal/fungicidal against a microbial strain when the ratio MBC/MIC or MFC/MIC is ≤4 (Oussou et al., 2008; Teke et al., 2011). Based on this classification, the ethanolic extract of the plants studied in the present work can be considered as bactericidal against S. aureus. The ethanolic extract of C. jacea L. flower and HMF can be considered as bactericidal against E. coli. Only the ethanolic extract of HMF can be considered as fungicidal against C. albicans. It is important to highlight that the parts of the plants tested were not chosen arbitrarily; the unavailability of the parts not tested (C. jacea leaves and C. album flowers) during the collection period led to their exclusion from the study. Thus, these other parts could also be tested and could potentially exhibit antimicrobial activity similar to the tested parts. Moreover, we evaluated the synergy between common antibiotics and the ethanolic extracts of the plants tested in this study. The use of combination therapy has been suggested as a new approach to improve the efficacy of antimicrobial agents by screening crude extracts from medicinal plants with good indications for use in combination with antibiotics (Ngongang et al., 2020). As observe in Table 3, no antagonism (FIC >4) or indifference (1 ≤ FIC ≤4) was noted between the extracts and the antibiotics. However, we found an additional effect (0.5 ≤ FIC ≤1) in almost all the combinations antibiotics + extracts excepted for HMF + (KA or AMP) against S. aureus ATCC 6538 and CJF + AMP against E. coli where we found synergy effect (FIC) (FIC ≤0.5). The lower is the FIC index, better is the synergy (Ngongang et al., 2020). The best synergies were found with HMF which well-modulated AMP(FIC=0.250 against S. aureus) and KA(FIC=0.375 against S. aureus). CJF also well-modulated AMP against E. coli with FIC=0.375. Finally, the larval form of G. mellonella was used to assess the acute toxicity of the plant extracts tested. This model has been suggested by several researchers as an ecofriendly in vivo approach for toxicity studies (Megaw et al., 2015; Ignasiak and Maxwell, 2017). The LD50s of HMF and leaves, CAL and CJF were 20.2, 0.58, 13.2 and 4.0 mg/ml respectively. The lower is the LD50, more toxic is the sample tested. Consequently, HML and CJF can be considered as the most toxic extracts to G. mellonella. In addition, according to the classification of Gosselin, Smith and Hodge (CCOHS et al., 2020), biologically active compounds can be super toxic (LD50 <5 mg/kg bw), extremely toxic [LD50 ∈ (5–50 mg/kg bw)], very toxic [LD50 ∈ (50–500 mg/kg bw)], moderately toxic [LD50 ∈ (0.5–5 g/kg bw)], slightly toxic [LD50 ∈ (5–15 g/kg bw)], and practically non-toxic (above 15 g/kg). Therefore, the data from our study indicate that ethanolic extract of H. mantegazzianum leaves (0.27 g/kg bw) is very toxic, the ethanolic extract from CJF (2.28 g/kg bw) is moderately toxic, and the ethanolic extract of HMFs (11.45 g/kg bw) and CAL (6.94 g/kg bw) are slightly toxic. Plants like HMFs may be recommended for further studies in the search for new antimicrobials because their toxic doses are far greater than the doses which have shown their optimal antimicrobial activity. ConclusionThe present study aimed to assess the antimicrobial activity of some invasive plants such as leaves and flowers of H. mantegazzianum Sommier and Levier, flowers of C. jacea L., and leaves of C. album L. All plants showed great activity when they were associated with antibiotics such as NIT, KA, and AMP. However, when used alone, only HMFs exhibited noteworthy antimicrobial activity against all the microorganisms tested. All other plants were only active against S. aureus and exhibited low antimicrobial activity against E. coli. It can be concluded that only HMFs have worthy antimicrobial effects, but further preclinical and clinical trials are required to evaluate the composition, the cytotoxicity and safety issues of these plant extracts and their combinations with common antibiotics before they can be recommended for antimicrobial therapy. Author’s contributionsMMJA designed the research study. MMJA, AKLD, KP, and MR performed the research. MMJA analyzed the data. SAN, MMJA, AKLD, MKM, GVS, KZS, CZA, DS, and PIV wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. " AcknowledgmentsThis study has been supported by the RUDN University Strategic Academic Leadership Program. FundingThis research received no external funding. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAcosta-Gutiérreza, S., Bodrenkoc, I. and Ceccarellia, M. 2020. Permeability through bacterial porins dictates whole cell compound accumulation. ChemRxiv. Preprint. doi:10.26434/chemrxiv.11877834.v1. Akinsulire, O.R., Aibin, I.E., Adenipekun, T., Adelowotan, T. and Odugbemi, T. 2007. In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. Afr. J. Tradit. Complement Altern. Med. 4(3), 338–344. Amer, M.W. and Awwad, A. 2021. Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization, and antibacterial activity. Chem. Int. 7, 1–8. Andersson, D.I., Hughes, D. and Kubicek-Sutherland, J.Z. 2016. Mechanisms, and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 26, 43–57. Ansald, M., Boulanger, P., Brives, C., Debarbieux, L., Dufour, N., Froissart, R., Gandon, S., Hénaff, C.L, Petit, M.A., Rocha, E., and Torres-Barceló, C. 2020. Les applications antibactériennes des bactériophages. Virologie 24(1), 23–36. Arsene, M.M.J., Davares, A.K.L., Andreevna, S.L., Vladimirovich, E.A., Carime, B.Z., Marouf, R. and Khelifi, I. 2021a. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World. 14(2), 319–328. Arsene, M.M.J., Podoprigora, I.V., Davares, A.K.L., Razan, M., Das, M.S. and Senyagin, A.N. 2021b. Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Vet. World. 14(5), 1330–1341. Arsene, M.M.J., Jorelle, A.B., Sarra, S., Viktorovna, P.I., Davares, A.K.L., Ingrid, N.K.C., Steve, A.A.F., Andreevna, S.L., Vyacheslavovna, Y.N. and Carime, B. Z. 2022. Short review on the potential alternatives to antibiotics in the era of antibiotic resistance. J. Appl. Pharm. Sci. 12(1), 29–40. Arsene, M.M.J., Viktorovna, P.I., Davares, A.K.L., Esther, N. and Nikolaevich, S.A. 2021c. Urinary tract infections: virulence factors, resistance to antibiotics, and management of uropathogenic bacteria with medicinal plants—a review. J. Appl. Pharm. Sci. 11(7), 1–12. Arsene, M.M.J., Viktorovna, P.I. and Davares, A.K.L. 2021d. Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open. Vet. J. 11(4), 651–661. Arsene, M.M.J., Viktorovna, P.I., Grigorievna, V.E., Davares, A.K.L., Sergeevna, D.M. and Nikolaevna, S.I. 2021e. Prolonged exposure to antimicrobials induces changes in susceptibility to antibiotics, biofilm formation and pathogenicity in Staphylococcus aureus. J. Pharm. Res. Int. 33(34B), 140–151. Bewley, J.D.1973. Desiccation and protein synthesis in the moss Tortilla ruralis. Can. J. Bot. 51(1), 203–206. CCOHS (Canadian Centre for Occupational Health and Safety Act). What is a LD50 and LC50? Available via https://www.ccohs.ca/oshanswers/chemicals/ld50.htmL (Accessed 2 September 2021). Cutillo, F., D'Abrosca, B., DellaGreca, M. and Zarrelli, A. 2004. Chenoalbicin, a novel cinnamic acid amide alkaloid from Chenopodium album. Chem. Biodivers. 1(10), 1579–1583. Elekhnawy, E., Sonbol, F., Abdelaziz, A. and Elbanna, T. 2020. Potential impact of biocide adaptation on selection of antibiotic resistance in bacterial isolates. Future J. Pharm. Sci. 6(1), 1–10. Eloff, J.N. 2019. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC. Complement. Altern. Med. 19(1), 1–8. Etame, R.M.E., Mouokeu, R.S., Poundeu, F.S.M., Voukeng, I.K., Cidjeu, C.L.P., Tiabou, A.T. Yaya A.J.G., Ngane R.A.N., Kuiate J.R., and Etoa, F.X. 2019. Effect of fractioning on antibacterial activity of n-butanol fraction from Enantia chlorantha stem bark methanol extract. BMC. Complement. Altern. Med. 19(1), 1–7. Essiet, G.A., Anwankwo, M.U., Akuodor, G.C., Ajoku, G.A., Offor, C.C., Megwas, A.U. and Aja, D.O.J. 2019. Antibacterial and toxicological evaluation of the ethanol leaf extract of Anthonotha macrophylla. J. Herb. Med. Pharmacol. 8(3), 205–211. Havel, J.E., Kovalenko, K.E., Thomaz, S.M., Amalfitano, S. and Kats, L.B. 2015. Aquatic invasive species: challenges for the future. Hydrobiologia 750(1), 147–170. Ignasiak, K. and Maxwell, A. 2017. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC. Res. Notes. 10(1), 1–8. Konstantinovitch, K.Y., Arsene, M.M.J., Aliya, M.V., Viktorovna, P.I., Elena, V.G., Azova, M.M., and Amira, A.A. 2022. Assessment of antimicrobial activity of ethanolic and aqueous extracts of Aesculus hippocastanum L. (Horse Chestnut) bark against bacteria isolated from urine of patients diagnosed positive to urinary tract infections. Front. Biosci. (Schol Ed), 14(2), 11. Kuete, V. 2010. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta. Med. 76, 1479–1491. Kuete, V. and Efferth, T. 2010. Cameroonian medicinal plants: pharmacology and derived natural products. Front. Pharmacol. 1, 123. Matoušková, M., Jurová, J., Gruľová, D., Wajs-Bonikowska, A., Renčo, M., Sedlák, V., Poráčová, J., Gogaľová, Z. and Kalemba, D. 2019. Phytotoxic effect of invasive Heracleum mantegazzianum essential oil on dicot and monocot species. Molecules 24(3), 425. Máximo, P., Ferreira, L.M., Branco, P.S. and Lourenço, A. 2020. Invasive plants: turning enemies into value. Molecules 25(15), 3529. Megaw, J., Thompson, T.P., Lafferty, R.A. and Gilmore, B.F. 2015. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chem 139, 197–201. Mbarga, M.J.A., Desobgo, S.C.Z., Tatsadjieu, L.N., Kavhiza, N. and Kalisa, L. 2021a. Antagonistic effects of raffia sap with probiotics against pathogenic microorganisms. Foods. Raw. Mater. 9(1), 24–31. Mondal, A.H., Yadav, D., Mitra, S. and Mukhopadhyay, K. 2020. Biosynthesis of silver nanoparticles using culture supernatant of shewanella sp. Ary1 and their antibacterial activity. Int. J. Nanomed. 15, 8295. Mouafo, H.T., Tchuenchieu, A.D.K., Nguedjo, M.W., Edoun, F.L.E., Tchuente, B.R.T. and Medoua, G.N. 2021. In vitro antimicrobial activity of Millettia laurentii De Wild and Lophira alata Banks ex CF Gaertn on selected foodborne pathogens associated to gastroenteritis. Heliyon 7(4), e06830. Ngongang, F.C., Fankam, A.G., Mbaveng, A.T., Wamba, B.E., Nayim, P., Beng, V.P. and Kuete, V. 2020. Methanol extracts from Manilkara zapota with moderate antibacterial activity displayed strong antibiotic-modulating effects against multidrug-resistant phenotypes. Pharmacology 3(1), 37. Oren, Z., Hong, J. and Shai, Y. 1997. A repertoire of novel antibacterial diastereomeric peptides with selective cytolytic activity. J. Biol. Chem. 23, 14643–14649. Oussou, K.R., Kanko, C., Guessend, N., Koukoua, G., Dosso, M., N'Guessan, Y.T., Figueredo, G., and Chalchat, J.C. 2008. Activites antibacteriennes des huiles essentielles de trois plantes aromatiques de Côte d’Ivoire. C. R. Chim. 7, 1081–1086. Papo, N. and Shai, Y. 2003. Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides 24(11), 1693–1703. Pimentel, D., Zuniga, R. and Morrison, D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52(3), 273–288. Pintó-Marijuan, M. and Munné-Bosch, S. 2013. Ecophysiology of invasive plants: osmotic adjustment and antioxidants. Trends. Plant. Sci. 18(12), 660–666. Romulo, A., Zuhud, E.A.M., Rondevaldova, J. and Kokoska, L. 2018. Screening of in vitro antimicrobial activity of plants used in traditional Indonesian medicine. Pharm. Biol. 56(1), 287–293. Schaenzer, A.J. and Wright, G.D. 2020. Antibiotic resistance by enzymatic modification of antibiotic targets. Trends. Mol. Med. 26(8), 768–782. Singh, A., Gautam, P.K., Verma, A., Singh, V., Shivapriya, P.M., Shivalkar, S., Sahoo, A.K. and Samanta, S.K. 2020. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: a review. Biotechnol. Rep. 25, e00427. Souadkia, S., Podoprigora, I.V., Yashina, N.V., Sarukhanova, L.E. and Kravtsov, E.G. 2020. Antibiotic-resistant uropathogenic Escherichia coli isolated from children with birth defects of the urinary system. Antibiot. Chemother. 65(7–8), 23–26. Su, T., Qiu, Y., Hua, X., Ye, B., Luo, H., Liu, D., Qu, P. and Qiu, Z. 2020. Novel opportunity to reverse antibiotic resistance: to explore traditional Chinese medicine with potential activity against antibiotics-resistance bacteria. Front. Microbiol. 11, 3372. Teke, G.N., Kuiate, J.R., Kuete, V., Teponno, R.B., Tapondjou, L.A., Tane, P., Giacinti, G. and Vilarem, G. 2011. Bio guided isolation of potential antimicrobial and antioxidant agents from the stem bark of Trilepisium madagascariense. South. Afr. J. Bot. 77, 319–327. Trabelsi, A., El Kaibi, M.A., Abbassi, A., Horchani, A., Chekir-Ghedira, L. and Ghedira, K. 2020. Phytochemical study and antibacterial and antibiotic modulation activity of Punica granatum (pomegranate) leaves. Scientifica 2020, 8271203. Wojnicz, D., Kucharska, A.Z., Sokół-Łętowska, A., Kicia, M. and Tichaczek-Goska, D. 2012. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 40(6), 683–697. World Health Organization (WHO). 2019. New report calls for urgent action to avert antimicrobial resistance crisis. Geneva, Switzerland: World Health Organization. Available via https://www.who.int/news/item/29-04-2019-new-reportcalls-for-urgent-action-to-avert-antimicrobial-resistancecrisis (Accessed 24 May 2021). | ||
| How to Cite this Article |
| Pubmed Style Arsene MMJ, Viktorovna PI, Mikhaïlovitch MK, Davares AKL, Parfait K, Rehailia M, Nikolayevich SA, Stefanovna GV, Sarra S, Sulikoevich KZ, Anatolyevna CZ, Shommiya D. In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants. Open Vet J. 2022; 12(4): 584-594. doi:10.5455/OVJ.2022.v12.i4.22 Web Style Arsene MMJ, Viktorovna PI, Mikhaïlovitch MK, Davares AKL, Parfait K, Rehailia M, Nikolayevich SA, Stefanovna GV, Sarra S, Sulikoevich KZ, Anatolyevna CZ, Shommiya D. In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants. https://www.openveterinaryjournal.com/?mno=34598 [Access: April 16, 2024]. doi:10.5455/OVJ.2022.v12.i4.22 AMA (American Medical Association) Style Arsene MMJ, Viktorovna PI, Mikhaïlovitch MK, Davares AKL, Parfait K, Rehailia M, Nikolayevich SA, Stefanovna GV, Sarra S, Sulikoevich KZ, Anatolyevna CZ, Shommiya D. In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants. Open Vet J. 2022; 12(4): 584-594. doi:10.5455/OVJ.2022.v12.i4.22 Vancouver/ICMJE Style Arsene MMJ, Viktorovna PI, Mikhaïlovitch MK, Davares AKL, Parfait K, Rehailia M, Nikolayevich SA, Stefanovna GV, Sarra S, Sulikoevich KZ, Anatolyevna CZ, Shommiya D. In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants. Open Vet J. (2022), [cited April 16, 2024]; 12(4): 584-594. doi:10.5455/OVJ.2022.v12.i4.22 Harvard Style Arsene, M. M. J., Viktorovna, . P. I., Mikhaïlovitch, . M. K., Davares, . A. K. L., Parfait, . K., Rehailia, . M., Nikolayevich, . S. A., Stefanovna, . G. V., Sarra, . S., Sulikoevich, . K. Z., Anatolyevna, . C. Z. & Shommiya, . D. (2022) In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants. Open Vet J, 12 (4), 584-594. doi:10.5455/OVJ.2022.v12.i4.22 Turabian Style Arsene, Mbarga Manga Joseph, Podoprigora Irina Viktorovna, Mefed Kirill Mikhaïlovitch, Anyutoulou Kitio Linda Davares, Kezimana Parfait, Manar Rehailia, Senyagin Alexander Nikolayevich, Girich Valentina Stefanovna, Souadkia Sarra, Khabadze Zurab Sulikoevich, Chernaia Zoya Anatolyevna, and Das Shommiya. 2022. In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants. Open Veterinary Journal, 12 (4), 584-594. doi:10.5455/OVJ.2022.v12.i4.22 Chicago Style Arsene, Mbarga Manga Joseph, Podoprigora Irina Viktorovna, Mefed Kirill Mikhaïlovitch, Anyutoulou Kitio Linda Davares, Kezimana Parfait, Manar Rehailia, Senyagin Alexander Nikolayevich, Girich Valentina Stefanovna, Souadkia Sarra, Khabadze Zurab Sulikoevich, Chernaia Zoya Anatolyevna, and Das Shommiya. "In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants." Open Veterinary Journal 12 (2022), 584-594. doi:10.5455/OVJ.2022.v12.i4.22 MLA (The Modern Language Association) Style Arsene, Mbarga Manga Joseph, Podoprigora Irina Viktorovna, Mefed Kirill Mikhaïlovitch, Anyutoulou Kitio Linda Davares, Kezimana Parfait, Manar Rehailia, Senyagin Alexander Nikolayevich, Girich Valentina Stefanovna, Souadkia Sarra, Khabadze Zurab Sulikoevich, Chernaia Zoya Anatolyevna, and Das Shommiya. "In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants." Open Veterinary Journal 12.4 (2022), 584-594. Print. doi:10.5455/OVJ.2022.v12.i4.22 APA (American Psychological Association) Style Arsene, M. M. J., Viktorovna, . P. I., Mikhaïlovitch, . M. K., Davares, . A. K. L., Parfait, . K., Rehailia, . M., Nikolayevich, . S. A., Stefanovna, . G. V., Sarra, . S., Sulikoevich, . K. Z., Anatolyevna, . C. Z. & Shommiya, . D. (2022) In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier & Levier (giant hogweed), Centaurea jacea L. (brown knapweed) and Chenopodium album L. (Pigweed): three invasive plants. Open Veterinary Journal, 12 (4), 584-594. doi:10.5455/OVJ.2022.v12.i4.22 |