| Original Article | ||
Open Vet J. 2023; 13(4): 400-406 Open Veterinary Journal, (2023), Vol. 13(4): 400–406 Original Research Serological survey on bovine viral diarrhea virus in man and evaluation of relation with Zika virus-associated microcephalyMassimo Giangaspero1* and Tamaki Okabayashi2,31Faculty of Veterinary Medicine, University of Teramo, Teramo, Italy 2Center for Animal Disease Control, University of Miyazaki, Miyazaki, Japan 3Laboratory of Veterinary Microbiology, Department of Veterinary Medicine, Faculty of Agriculture, University of Miyazaki, Miyazaki, Japan *Corresponding Author: Massimo Giangaspero. Faculty of Veterinary Medicine, University of Teramo, Teramo, Italy. Email: giangasp [at] gmail.com Submitted: 01/12/2022 Accepted: 09/03/2023 Published: 04/04/2023 © 2023 Open Veterinary Journal
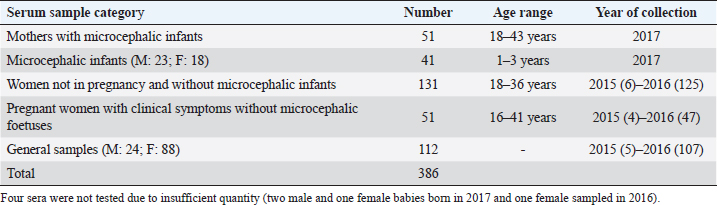
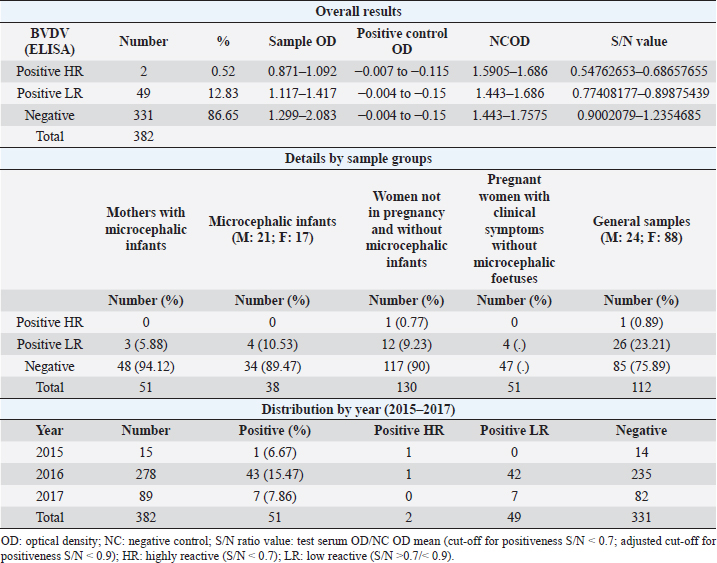
AbstractBackground: In 2015, an unprecedented epidemic of microcephaly occurred in Brazil. Preliminary observations suggested the involvement of cofactors in the etiopathology of Zika virus-associated microcephaly. Bovine viral diarrhea virus (BVDV) was identified in fetal samples with microcephaly, originating in the state of Paraíba, and two virus sequences, obtained from the amniotic fluid collected from mothers with babies affected by Zika and microcephaly, have been characterized as two different species of BVDV, types 1 and 2. Aim: The involvement of BVDV as a co-factor in the etiopathogenesis of Zika virus-associated microcephaly was explored. Methods: A serological screening using an ELISA test was undertaken to detect antibodies against BVDV among patients referred to the Central Laboratory of Natal, Rio Grande do Norte, encompassing microcephalic babies and their mothers, mothers and pregnants not associated with microcephaly and general patients as a control group. Results: Two samples were positive out of 382 tested (0.52%). No specific relation with birth defects could be established. Conclusions: The study might suggest serological evidence of BVDV in humans. Further studies and the application of improved diagnostic tests adapted to humans are necessary to clarify the epidemiological extent and impact of BVDV. Keywords: Bovine viral diarrhea virus, Brazil, Cofactor, Microcephaly, Zika virus. IntroductionZika virus is the agent of a disease of African origin, first reported in the late 1940s in Eastern Africa, in Uganda in 1947 (Dick et al., 1952), characterized for a long time as a mild arthropod-borne infection, causing small outbreaks in Western Africa and Asia (1960s, 1970s). Zika virus disease is transmitted primarily by Aedes mosquitoes, the same vector carrying dengue, yellow fever, chikungunya, and Rift Valley viruses. The transmission is vectorial and assured by Aedes aegypti and Aedes albopictus. However, also sexual transmission occurs, and the virus may persist in semen for about 6 months (Foy et al., 2011). Symptoms are resumed as a simile-influenza clinical course, with various symptoms such as fever, muscular and articular pain, and cutaneous rash. Neurologic complications are mainly Guillain-Barré syndrome (higher incidence in Brazil and French Polynesia), meningoencephalitis, transverse myelitis, and facial paralysis. However, the major complication is microcephaly which affects fetuses. The increased diffusion of the Zika virus seems to be linked to the expansion of A. albopictus, commonly known as the Asian tiger mosquito, originally restricted to some Asian regions and Madagascar until 1980, then spread throughout the world, from Australasia, Europe, and to the Americas. This allowed a geographic extension of arboviruses. Zika virus reached naïve human populations, causing an epidemic in Micronesia in 2007 (Duffy et al., 2009). The spread was accompanied by the occurrence of more severe clinical forms. Similarly, this was also observed for the Chikungunya virus, until 2005, considered a self-limiting and non-fatal disease, responsible in 2005–2006 in La Réunion for a serious outbreak affecting 35% of the population (265,000 ill persons) and causing 254 deaths due to meningoencephalitis (Vazeille et al., 2007). From Micronesia, the current strain of Zika virus diffused to Nouvelle Calédonie, French Polynesia, and other islands in the Pacific Ocean in 2013 and 2014 (Cao-Lormeau et al., 2016). In 2014, the virus was reported in Chile (Easter Islands), then in Brazil in 2015, and subsequently registered in numerous other countries of the Americas (Musso, 2015). All the isolates were genetically more closely related to that of Polynesia than the African strains (Calvez et al., 2018). In 2015 and 2016, Brazil lived an unprecedented epidemic of microcephaly that has not yet been fully explained (Heymann et al., 2016). Also, cases of Guillain-Barré syndrome increased dramatically. However, after 2 years, a radical decreasing trend occurred throughout Brazil. Similar decreasing trends of Zika infection and associated health complications, including Guillain-Barré syndrome, have been observed elsewhere, as in French Polynesia during 2013–2014 (Cao Lormeau et al., 2016). Previous to Zika occurrence, microcephaly was endemic in Brazil at a low rate among the population, as demonstrated by a study conducted by the Faculty of Medicine of Ribeirão Preto and the Federal University of Maranhão (Grota, 2018). Therefore, the Zika virus represented an apparent risk factor determining the increase in cases. Furthermore, a cross-sectional study on the epidemic of microcephaly due to Zika virus in Brazil (Medeiros Figueiredo et al., 2021) indicated a higher risk of microcephaly linked to the infection. It provided evidence that cases of the Zika virus occurred well before the initially suspected introduction of the virus in the country, linked with the Olympic games during the summer of 2016. In reality, epidemiological evidence suggested the entry of a new virus, indicating the presence of Zika virus in Brazil between the second quarter of 2013 and the beginning of 2014, considerably increasing the risk of microcephaly. This also corresponds to observations made in the studied area. The first case of microcephaly was retraced in June 2014 in Lucrécia, a tiny municipality of Rio Grande do Norte, well documented by typical skin rash in mother and compatible neuro-images (de Andrade and Giangaspero, 2017). However, the epidemiology of microcephaly associated with the Zika virus in Brazil suggested the occurrence of possible cofactors, suspecting an association with farmed animals. Dr. Marinho, director of information and health analysis at Brazil’s Health Ministry, raised the question of something more than the Zika virus was causing the high intensity and severity of cases, suspecting that the cattle-farming industry may indeed be involved, and looking into the question of whether Bovine viral diarrhea (BVD) has combined with Zika to cause birth-defects (Butler, 2016; Mountain, 2016). Livestock is not known to be susceptible to the Zika virus. Still, livestock is kept in peri-urban and rural areas, where their need for water also increases opportunities for mosquitoes to breed and thrive. Nevertheless, recent research undertaken in 2018 by the Biological Institute, linked to the São Paulo Department of Agriculture, identified the presence of Zika virus in healthy cattle and deer in the interior of the State of São Paulo, suggesting that cattle can contribute to the spread of Zika virus (Okuda, 2019), but this did not explain the increased pathogenicity of Zika virus. On the other side, BVD affects cows worldwide, capable of interfering with central nervous system development in fetuses, causing cerebellar hypoplasia, hydranencephaly, internal hydrocephalus, microencephaly, or porencephaly, and it is known to express synergy with other pathogens in animal diseases. Research teams from the Federal University of Rio de Janeiro and the research Institute Professor Joaquim Amorim Neto, Campina Grande, identified BVD virus (BVDV) in fetal samples with microcephaly, originating in the state of Paraíba (Nogueira et al., 2016). Two virus sequences obtained from the amniotic fluid collected from mothers with babies affected by Zika and microcephaly have been characterized as BVDV, according to secondary structure analysis (Giangaspero and Apicella, 2018). The two sequences were clustered among sub-genotype BVDV-1b1, of cosmopolitan diffusion, and BVDV-2b, a typical genotype circulating in South America, showing unreported variants in the Internal Ribosome Entry Site. The only strain previously reported in humans belonged to genotype BVDV-1d, another cluster with cosmopolitan diffusion (Giangaspero et al., 1997). Aiming to explore the involvement of BVDV as a co-factor in the etiopathogenesis of Zika virus-associated microcephaly, a serological screening was undertaken to detect antibodies against BVDV in the human population in northeastern Brazil, an area severely affected by the outbreak of microcephaly. Material and MethodsSamplesThree hundred eighty-two serum samples were available from the Central Laboratory, Natal, Rio Grande do Norte, Brazil. The samples included sera collected from 2015 to 2017 (15 collected in 2015, 278 in 2016, and 89 in 2017) from patients (337 females and 45 males) of age ranging from 16 to 43, referred to the laboratory from the province of Natal and all other provinces of the State, also for serological investigations on arboviruses (Zika, Dengue, and Chikungunya viruses). Some women were mothers of microcephalic babies (n 51). In addition, babies (21 males and 17 females, 1–3 years of age) suffering from microcephaly were also tested. Mothers with microcephalic infants were generally favorable for Zika virus antibodies (87.5%), while only 24.39% of microcephalic babies were Zika virus antibody positive. Samples included pregnant women (1–3 months to 6–9 months of gestation). Most of them showed clinical symptoms associated with active Zika virus infection during sampling: fever, cutaneous rash, itch, headache, myalgia, intercostal pain, arthralgia, periarticular edema, asthenia, nausea, vomiting, and diarrhea. The selected sera have been organized into different groups: mothers with microcephalic infants and microcephalic infants (n 89, all collected in 2017), pregnant women with clinical symptoms without microcephalic fetuses (n 51), women not in pregnancy and without microcephalic infants (n 130) and general samples (n 112) (control group) (Table 1). Serological testAn ELISA commercial kit (Median, South Korea) to screen anti-BVDV antibodies in bovine animals was used in the present study. The test included bovine hyperimmune positive and negative control (NC) and was executed following the manufacturer’s recommendations. Plates were read by an ELISA reader at 450 nm. Test sera and NC optical density (OD) values were used to compute the S/N ratio value (sample OD/ NC OD mean) for cut-off determination. Sera with S/N < 0.7 were considered positive. Statistical analysisAccording to their association with microcephaly, pregnancy, and general samples, human sera were compared for BVDV infection rate proportions by computing chi-square statistics based on the deviations of the observed ratios from equality. These chi-square tests were based on one degree of freedom, using Chi-Square Test Calculator, Social Science Statistics (Stangroom, 2022). Ethical approvalThis research was approved by the Brazilian National Council of Ethics in Research, Brasilia (opinion number 2.590.914, April 10, 2018). ResultsHuman samples collected from Rio Grande do Norte, Brazil, for serological screening for antibodies to BVDV (ELISA test) showed an overall positiveness of 0.52% (n=2) out of 382 tested (Table 2). One positive serum was from a woman who was not pregnant and without microcephalic infants, collected in 2015, and the other was from a woman in the group of general samples collected in 2016. Based on the cut-off value of S/N < 0.7, valid for cattle, five sera were positive. These sera were retested, and three did not score positive SN value. Thus they were considered negative. Forty-nine sera (12.83%) showed low reactivity against BVDV antigen, just under the cut-off value (S/N >0.7/<0.9). Retesting did not confirm additional eight sera as low reactive, thus considered negative. Adjusting the cut-off for positiveness to S/N < 0.9 for the application on human sera, taking into account the low antibody titers that humans develop against BVDV, especially cytopathic (CP) strains, generally used as antigenic substrate in tests designed for cattle (Giangaspero et al., 1988, 1993a, 1993b), and also considering these “low reactive” sera as positive, and negative the sera with S/N ratio values ≥0.9, the overall positiveness could be 13.35%. Based on the cut-off value of S/N < 0.9, the group of mothers with microcephalic infants scored the lowest level of seropositive (5.88%). 10.53% of microcephalic infants were positive. A similar rate (10%) resulted in the group of women, not in pregnancy and not associated with microcephalic infants. In the group of pregnant women with clinical symptoms without microcephalic fetuses, the positiveness was 7.84%. Higher positive rates were observed in the group of general samples (control group) (24.11%). Concerning the collection year, 2016 accounted for the highest number of BVDV-positive sera (15.47%). Samples collected in 2015 and 2017 showed lower seropositiveness, 6.67 and 7.86 %, respectively. The statistical tests applied to compare the groups associated with microcephaly (mothers and infants) with other groups demonstrated that there was no difference between the groups (p-value > 0.1). DiscussionBVDV in humans was preliminary explored by a serological survey in Rio Grande do Norte. The serological reaction observed in the present study might suggest the circulation of BVDV in humans. Furthermore, the results corroborated the previous isolation of BVDV in microcephalic babies (Nogueira et al., 2016). Considering that Zika and dengue viruses belong to the family Flaviviridae and BVDV was previously classified in the same family, in the present study, the occurrence of an eventual cross-reaction was excluded by the preliminary screening for Zika virus, dengue made on the tested sera. Concerning the origin of the detected antibodies against BVDV in human sera, no evidence of transmission between humans was revealed, and the immune reaction was probably related to the circulation of the infection in domestic animals. However, it cannot be excluded that another source might be the contamination of biological products with BVDV. Contamination of live virus vaccines for human use by adventitious BVDV was previously reported (Giangaspero et al., 2001). Nevertheless, the Pestivirus sequences from amniotic fluid collected from mothers with babies affected by Zika virus and microcephaly and characterized as BVDV-1 and BVDV-2 (Giangaspero and Apicella, 2018), were different from Asian BVDV strains detected in measles, mumps, and rubella vaccine samples used in Brazil (Prof Tanuri, Federal university, Rio de Janeiro, personal communication). In Central and South America, the serological positiveness for BVD in a human was previously reported only in one study undertaken in 1988, using indirect immunofluorescence and ELISA tests with non-cytopathic (NCP) A19 and CP C24V and NADL strains (Giangaspero et al., 1988). Potential cross-reactivity due to genetically related viruses such as classical swine fever virus, rubella, and yellow fever was considered and excluded. Table 1. Human samples collected from Rio Grande do Norte, Brazil, for serological screening for antibodies to BVDV (ELISA test).
Table 2. Results of serological screening for antibodies to BVDV (ELISA test) in human samples from Rio Grande do Norte, Brazil.
The role of pestiviruses in human pathology remains unknown, and data on epidemiology in humans are still fragmentary. BVDV has been suspected as a co-factor in human immunodeficiency virus (HIV) infection. Seropositivity to BVDV was found in HIV-positive patients in a study undertaken in Zambia and Europe (Giangaspero et al., 1993a, 1993b). Furthermore, considering the tropism for nerve cells in animal pathology, pestiviruses have been suspected to be related to neurological disorders and birth defects in humans by different authors. In Italy, high anti-BVDV antibody titers were detected for 4 years in a farmer suffering from a rare form of post-viral mononeuritis of unknown etiology, the Personnage Turner syndrome (Di Bella, 1986), which arose at the same time as a fatal outbreak of BVDV in his cattle farm (Giangaspero and Cominardi, 2006). In the USA, antibodies against BVDV were detected in mothers with microcephalic infants (Potts et al., 1987) and in 40% of sera from identical twins discordant for schizophrenia (Yolken et al., 1993). Infection during pregnancy and cerebral white matter damage in preterm neonates have also been explored (Dammann and Leviton, 1998, 2004; Leviton et al., 2005; Dammann et al., 2006; Rennie and Peebles, 2006). Most of the studies were based on serological screening, and some have been conducted to reveal antigens. However, these studies remained experimental, and no definite conclusions could be achieved. Similarly, despite the suspicion of co-factor concurrence in microcephaly, no relation could be established in the present study between BVDV and Zika virus-associated birth defects. Even taking into account results of sera close to positivity (n 49), while some of these “low reactive” sera were also observed among infants affected with microcephaly and their mothers, seroconversion in this group associated with microcephaly was low. Only in one case were the mother and the respective child positive for Zika virus antibodies and low reactive to BVDV. In addition, low reactive sera were also distributed independently in all other patient groups unrelated to microcephaly. Concerning serology, the main problem with conducting surveys on the zoonotic potential of pestiviruses is related to the lack of specific tests to be applied to human samples. In the present study, due to diagnosis limitations, there is a remote chance that the two positive cases were false positives. However, the sera were retested, and only those showing high OD values were retained as positive. Nowadays, no commercial test for BVDV in humans is available. Therefore, diagnostic tools validated for cattle were considered an alternative to being possibly applied as routine studies as serological surveys on human samples instead of more complex and expensive homemade tests. Using kits designed for bovines represents a major problem regarding antigen substrate and different immune responses seen in humans and cattle. According to previous studies, the immune reactivity of human sera against BVDV strains showed considerable variation. Human sera showed much lower titers against BVDV compared to those obtained with bovines, and this difference was enhanced when using CP strains. Human sera scored higher antibody titers with NCP strains and low titers or negative with CP strains as NADL, commonly used as antigen substrate in commercial ELISA kits for cattle screening (Giangaspero et al., 1988, 1993a, 1993b). Of course, this aspect represented a limitation of the present study, as the optimal should have been the application of a validated test for humans, implying the use of NCP BVDV strains as antigenic substrate, reported more appropriate to reveal immune reactions against pestiviruses in man. The not negligible number of sera-scoring results just behind the S/N cut-off value valid for cattle (about 12.8%) recall this need for future studies. ConclusionAlthough the study could not contribute to elucidating causes and co-factors that determined the severe epidemic of microcephaly in Brazil, the serological results obtained on human sera screened for anti-BVDV antibodies represented a preliminary activity in the framework of investigations on Pestivirus diffusion in the human population. They might suggest a circulation of BVDV in men in northeastern Brazil. More sensitive and confirmation methods have to be applied in future studies. Antigenic evidence will be fundamental through isolation and sequencing. Progress has been made in clarifying the pathogenesis of Zika virus infection (Wen et al., 2017). However, many unanswered questions remain regarding risk factors and epidemiology (Dufort and White, 2018). The epidemic of microcephaly in Brazil has to be fully understood to allow efficient prevention and control measures against the reoccurrence of the phenomenon, also elsewhere. Other pathogens might be considered in further studies focusing on potential co-factors in the etiopathogenesis of Zika virus-associated microcephaly. Among pathogens of animal origin, the Schmallemberg virus, transmitted by mosquitoes, causing abortion and birth defects in cattle and small ruminants, was considered and ruled out since no evidence could be observed among domestic animals in Brazil (de Souza Nunes Martins et al., 2022). Furthermore, other host species with the potential to play an epidemiological role in Zika virus disease should be investigated. An extensive serological survey among horses from New Caledonia and French Polynesia demonstrated that horses could be infected with the Zika virus (Beck et al., 2019). Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionM. Giangaspero and T. Okabayashi contributed equally to the present study. ReferencesBeck, C., Leparc-Goffart, I., Desoutter, D., Debergé, E., Bichet, H., Lowenski, S., Dumarest, M., Gonzalez, G., Migné, C., Vanhomwegen, J., Zientara, S., Durand, B. and Lecollinet, S. 2019. Serological evidence of infection with dengue and Zika viruses in horses on French Pacific Islands. PLoS Negl. Trop. Dis. 13(2), e0007162. Butler, D. 2016. Brazil’s birth-defects puzzle. Nature 535, 475–476. Calvez, E., O’Connor, O., Pol, M., Rousset, D., Faye, O., Richard, V., Tarantola, A. and Dupont-Rouzeyrol, M. 2018. Differential transmission of Asian and African Zika virus lineages by Aedes aegypti from New Caledonia. Emerg. Microbes Infect. 7(1), 159. Cao-Lormeau, V.M., Blake, A., Mons, S., Lastère, S., Roche, C., Vanhomwegen, J., Dub, T., Baudouin, L., Teissier, A., Larre, P., Vial, A.L., Decam, C., Choumet, V., Halstead, S.K., Willison, H.J., Musset, L., Manuguerra, J.C., Despres, P., Fournier, E., Mallet, H.P., Musso, D., Fontanet, A., Neil, J. and Ghawché, F. 2016. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387, 1531–1539. Dammann, O. and Leviton, A. 1998. Is some white matter damage in preterm neonates induced by a human Pestivirus? Arch. Dis. Child Fetal Neonatal. Ed. 78, F230. Dammann, O. and Leviton, A. 2004. Inflammatory brain damage in preterm newborns dry numbers, wet lab, and causal inferences. Early Hum. Dev. 79, 1. Dammann, O., Hori, A., Szentiks, C. and Hewicker-Trautwein, M. 2006. Absence of Pestivirus antigen in brains with white matter damage. Dev. Med. Child Neurol. 48, 290. de Andrade, I. and Giangaspero, M. 2017. Epidemiology of Zika virus associated microcephaly in Brazil suggesting cofactor involvement. Sci. Fed. Virol. Res. J. 1(2), 1–6. de Souza Nunes Martins, M., Pituco, E.M., Taniwaki, S.A., Okuda, L.H. and Richtzenhain, L.J. 2022. Schmallenberg virus: research on viral circulation in Brazil. Braz. J. Microbiol. 53, 377–383. Di Bella, P. 1986. Mononeuropatie e lesioni dei plessi. In Guida alla neurologia clinica 1986, 2nd ed. Eds., Angeleri, F., Ferrari, E. and Muratorio, A. Bologna, Italy: Monduzzi, vols. 2, 4, pp: Ji3. Dick, G.W., Kitchen, S.F. and Haddow, A.J. 1952. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 46, 509–520. Duffy, M.R., Chen, T.H., Hancock, W.T., Powers, A.M., Kool, J.L., Lanciotti, R.S., Pretrick, M., Marfel, M., Holzbauer, S., Dubray, C., Guillaumot, L., Griggs, A., Bel, M., Lambert, A.J., Laven, J., Kosoy, O., Panella, A., Biggerstaff, B.J., Fischer, M. and Hayes, E.B. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 360, 2536–2543. Dufort, E. and White, J. 2018. Pre-Zika microcephaly in Brazil: closer to the elusive baseline and new questions raised. Pediatrics 141(2), e20173811. Foy, B.D., Kobylinski, K.C., Chilson Foy, J.L., Blitvich, B.J., Travassos da Rosa, A., Haddow, A.D., Lanciotti, R.S. and Tesh, R.B. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 17, 880–882. Giangaspero, M. and Apicella, C. 2018. Bovine viral diarrhea virus type 1 current taxonomy according to palindromic nucleotide substitutions method. J. Virol. Methods 256, 37–76. Giangaspero, M. and Cominardi, P.F. 2006. Parsonage turner syndrome associated with anti-bovine viral diarrhea virus antibodies. Vet. Ital. 42(3), 249–259. Giangaspero, M., Harasawa, R. and Verhulst, A. 1997. Genotypic characteristics of the 5’- untranslated region of a Pestivirus strain isolated from human leukocytes. Microbiol. Immunol. 40(10), 829–834. Giangaspero, M., Vacirca, G., Büttner, M., Wolf, G., Vanopdenbosch, E. and Muyldermans, G. 1993a. Serological and antigenical findings indicating Pestivirus in man. Arch. Virol. Suppl. 7, 53–62. Giangaspero, M., Vacirca, G., Morgan, D., Baboo, K.S., Luo, A., DuPont, H.L. and Zumla, A. 1993b. Anti-bovine viral diarrhoea virus antibodies in adult Zambian patients infected with the human immunodeficiency virus. Int. J. STD AIDS 4(5), 300–302. Giangaspero, M., Vacirca, G., Harasawa, R., Büttner, M., Panuccio, A., De Giuli Morghen, C., Zanetti, A., Belloli, A. and Verhulst, A. 2001. Genotypes of Pestivirus RNA detected in live virus vaccines for human use. J. Vet. Med. Sci. 63(7), 723–733. Giangaspero, M., Wellemans, G., Vanopdenbosch, E., Belloli, A. and Verhulst, A. 1988. Bovine virale diarrhoea. Lancet 2, 110. Grota, R. 2018. Pesquisa mostra que a microcefalia já era endêmica no Brasil antes do surto de Zika. Portal PEBMED. Heymann, D.L., Hodgson, A., Sall, A.A., Freedman, D.O., Staples, J.E., Althabe, F., Baruah, K., Mahmud, G., Kandun, N., Vasconcelos, P.F., Bino, S. and Menon, K.U. 2016. Zika virus and microcephaly: why is this situation a PHEIC? Lancet 387, 719–721. Leviton, A., Dammann, O. and Durum, S.K. 2005. The adaptive immune response in neonatal celebral white matter damage. Ann. Neurol. 58, 821. Medeiros Figueiredo, A., Sanchez-Villegas, P., Cristina Moreira Marculino Figueiredo, D., Sousa Soares de Araujo, J. and Daponte-Codina, A. 2021. Microcephaly epidemic in Brazil: an earlier chapter. Infect. Dis. Now. 51(3), 260–265. Mountain, M. 2016. Zika related to cattle farming? Available via https://www.all-creatures.org/articles/mdi-zika-cattle-farming.html Musso, D. 2015. Zika virus transmission from French Polynesia to Brazil. Emerg. Infect. Dis. 21, 1887. Nogueira, F.C.S., Velasquez, E., Melo, A.S.O. and Domont, G.B. 2016. Zika virus may not be alone: proteomics associates a bovine-like viral diarrhea virus to microcephaly. bioRxiv preprint. 15, 062596; doi: 10.1101/062596. Okuda, L.H. 2019. Bois podem ser hospedeiros do zika vírus, aponta Instituto Biológico. BeefPoint. Available via www.beefpoint.com.br Potts, B.J., Sever, J.L., Tzan, N.R., Huddleston, D. and Elder, G.A. 1987. Possible role of pestiviruses in microcephaly. Lancet 1, 972–973. Rennie, J. and Peebles, D. 2006. Pestivirus as a cause of white matter damage—down but not out. Dev. Med. Child Neurol. 48, 243. Stangroom, J. 2022. Chi-Square Test Calculator. Social Science Statistics. Available via https://www.socscistatistics.com (Accessed January, 2023). Vazeille, M., Moutailler, S., Coudrier, D., Rousseaux, C., Khun, H., Huerre, M., Thiria, J., Dehecq, J-S., Fontenille, D., Schuffenecker, I., Despres, P. and Failloux, A-B. 2007. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One 2(11), e1168. Wen, Z., Song, H. and Ming, G. 2017. How does Zika virus cause microcephaly? Genes Dev. 31, 849–861. Yolken, R.H., Petric, M., Collett, M. and Fuller, T.E. Pestivirus infection in identical twins discordant for schizophrenia. Proceedings of the IXth Congress of Virology, Glasgow, England, 1993, pp 148. | ||
| How to Cite this Article |
| Pubmed Style Giangaspero M, Okabayashi T. Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly. Open Vet J. 2023; 13(4): 400-406. doi:10.5455/OVJ.2023.v13.i4.1 Web Style Giangaspero M, Okabayashi T. Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly. https://www.openveterinaryjournal.com/?mno=17125 [Access: April 20, 2024]. doi:10.5455/OVJ.2023.v13.i4.1 AMA (American Medical Association) Style Giangaspero M, Okabayashi T. Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly. Open Vet J. 2023; 13(4): 400-406. doi:10.5455/OVJ.2023.v13.i4.1 Vancouver/ICMJE Style Giangaspero M, Okabayashi T. Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly. Open Vet J. (2023), [cited April 20, 2024]; 13(4): 400-406. doi:10.5455/OVJ.2023.v13.i4.1 Harvard Style Giangaspero, M. & Okabayashi, . T. (2023) Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly. Open Vet J, 13 (4), 400-406. doi:10.5455/OVJ.2023.v13.i4.1 Turabian Style Giangaspero, Massimo, and Tamaki Okabayashi. 2023. Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly. Open Veterinary Journal, 13 (4), 400-406. doi:10.5455/OVJ.2023.v13.i4.1 Chicago Style Giangaspero, Massimo, and Tamaki Okabayashi. "Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly." Open Veterinary Journal 13 (2023), 400-406. doi:10.5455/OVJ.2023.v13.i4.1 MLA (The Modern Language Association) Style Giangaspero, Massimo, and Tamaki Okabayashi. "Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly." Open Veterinary Journal 13.4 (2023), 400-406. Print. doi:10.5455/OVJ.2023.v13.i4.1 APA (American Psychological Association) Style Giangaspero, M. & Okabayashi, . T. (2023) Serological survey on Bovine viral diarrhea virus in man and evaluation of relation with Zika virus associated microcephaly. Open Veterinary Journal, 13 (4), 400-406. doi:10.5455/OVJ.2023.v13.i4.1 |