| Case Report | ||
Open Vet J. 2022; 12(1): 138-147 Open Veterinary Journal, (2022), Vol. 12(1): 138–147 Case Report Urrets-Zavalia syndrome following cataract surgery in dogs: A case seriesFrancisco Cantero1,2, Marta Leiva1,2*, Laura Gaztelu1,2, Irene Cerrada1,2, Rita Vilao Cardoso1,2, and Teresa Peña1,21Servei d’Oftalmologia, Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain 2Departament de Medicina i Cirurgia Animals, Facultat de Veterinària, Universitat Autònoma de Barcelona, Bellaterra, Spain *Corresponding Author: Marta Leiva. Servei d’Oftalmologia, Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain. Email: marta.leiva [at] uab.cat Submitted: 21/10/2021 Accepted: 23/01/2022 Published: 26/02/2022 © 2022 Open Veterinary Journal
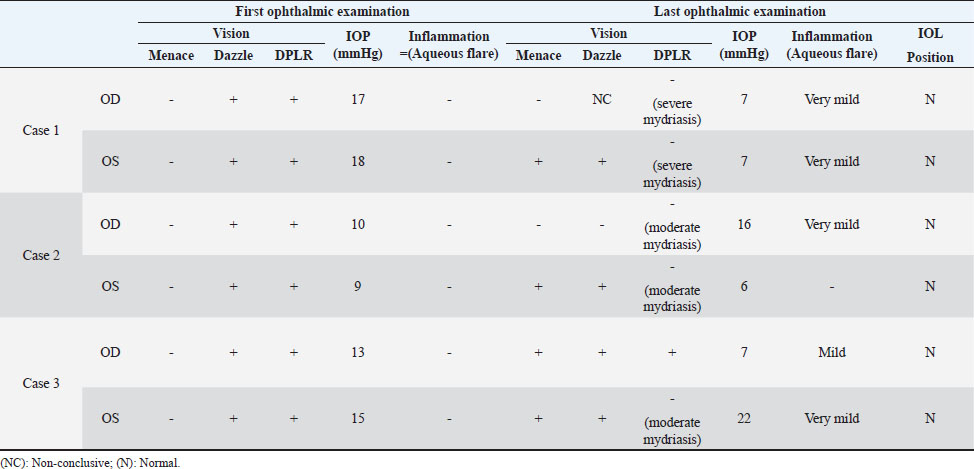
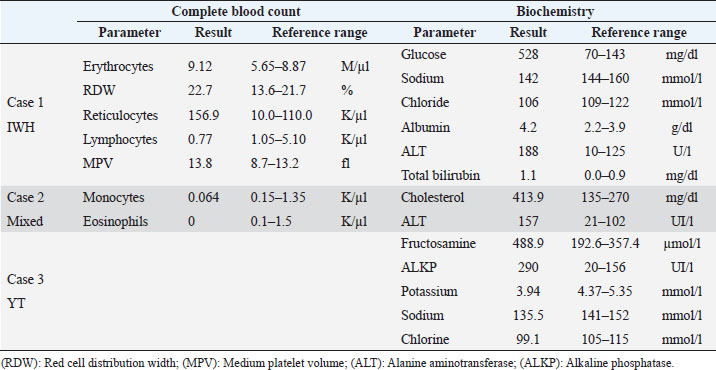
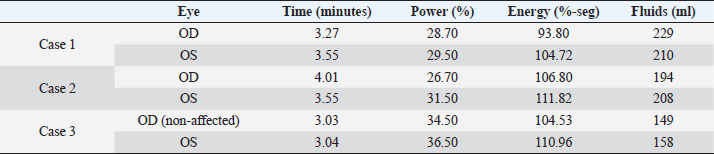
AbstractBackground: In human medicine, Urrets-Zavalia syndrome (UZS) is a well-recognized but uncommon postoperative complication characterized by a fixed dilated pupil, accompanied by iris atrophy and glaucoma. Although it was originally reported in 1963 after penetrating keratoplasty surgery for keratoconus, it has been associated with various ophthalmic procedures such as cataract surgery. The condition has not been previously published in the veterinary literature. Case Description: Three client-owned diabetic dogs that developed UZS´s triad after cataract surgery are described. Despite uneventful phacoemulsification in the six eyes, five developed moderate-to-severe postoperative ocular hypertension. Although intraocular pressure (IOP) spikes were initially controlled, fixed dilated pupils accompanied by iris atrophy and chronic ocular hypertension were seen in the five affected eyes. Aggressive medical and surgical management maintained vision in three of those eyes. In one eye, uncontrolled IOP led to blindness. Conclusion: This is the first published description of UZS in dogs, occurring after phacoemulsification. Although no exact, demonstrable causative element could be determined, we believe that should be considered a triggering condition for this syndrome, as it directly affects the ocular blood flow autoregulation and intrinsic uveal tissue integrity. Until the contrary is proved, diabetes mellitus might be considered as a risk factor for developing this syndrome after cataract surgery in dogs. Keywords: Iris atrophy, Mydriasis, Ocular hypertension, Phacoemulsification, Postoperative complication. IntroductionIn 1963, an Argentinian ophthalmologist named Alberto Urrets-Zavalia described a fixed and dilated pupil syndrome, accompanied by iris atrophy and glaucoma, as a complication of penetrating keratoplasty for keratoconus (Urrets-Zavalia, 1963). At that time, the author associated the uncommon postoperative complication with the use of topical mydriatic agents (atropine). Later on, this syndrome was described in association with other surgical procedures, such as different methods of keratoplasty (Maurino et al., 2002; Minasian and Ayliffe, 2002; Srinivasan and Patnaik, 2004; Fournié et al., 2009; Anwar et al., 2012; Bozkurt et al., 2012; Foroutan et al., 2016), intracameral gas injection for the treatment of corneal hydrops (Aralikatti et al. 2008), trabeculectomy (Jain et al., 2000), goniotomy (Chelnis et al., 2012; Walton, 2013), intraocular lens implantation (Yuzbasioglu et al., 2006; Park et al., 2008; Pérez-Cambrodí et al., 2013; Narang et al. 2017), cataract surgery (Monson et al., 1992), argon laser retinal photocoagulation (Lifshitz and Yassur, 1988), 360-degree peripheral iridoplasty (Espana et al., 2007), and anterior chamber foreign body removal (Totuk et al., 2018). The exact mechanism of Urrets-Zavalia syndrome (UZS) is still unknown. The dilated pupils do not seem to respond to pilocarpine, sympatholytic agents, or to alpha-adrenergic blockers. Hence, the treatment in human patients is directed toward the symptomatic control of glare, intraocular pressure (IOP), and photophobia with concealment of anisocoria with cosmetic contact lenses (Narang et al., 2017) or corneal micro pigmentation (Reed, 1994; Alio et al., 2012). In the veterinary literature, apart from a case report recently presented at the European College of Veterinary Ophthalmologists meeting (Bilotta and Busse, 2021), no descriptions of UZS have been published, so far. Herein, we describe three cases of UZS in diabetic dogs occurring after cataract surgery and discuss the etiological mechanisms for this syndrome. Case DetailsThe medical records of three client-owned dogs that developed clinical signs compatible with UZS after bilateral cataract surgery at the Veterinary Teaching Hospital of the Autonomous University of Barcelona (VTH-UAB) from 2020 to 2021 were retrospectively reviewed. In the short-term postoperative period of uneventful phacoemulsification, the syndrome was suspected when an acute, fixed and dilated pupil, accompanied with clinical iris atrophy and glaucoma were detected. Signalment, clinical findings, and preoperative evaluationThere were two spayed females [a mix breed dog (case 1) and an Ibizan Warren Hound (IWH; case 2)] and a neutered male [Yorkshire Terrier (YT; case 3)], of ages between 9 and 10 years and 4–15 kg. All the animals were diagnosed with diabetes mellitus 2–5 months prior to presentation. Owners reported that the dogs have gone blind within a very short period after the diagnosis of diabetes (1–3 months). Apart from hypothyroidism in case 2, no other concurrent systemic diseases were detected, and complete physical examinations at presentation were unremarkable. Ocular findings at the first presentation are summarized in Table 1. In three dogs, initial ophthalmic examination revealed absent menace response in both eyes (OU) with no other relevant findings in the neuro-ophthalmological examination. Schirmer Tear Test 1 (MSD Animal Health, Madison, NJ) and tonometric values (TonoVet®, Icare Finland Oy, Helsinki, Finland) were within normal limits. Biomicroscopic examination (Kowa SL17®, Kowa Company Ltd., Tokyo, Japan) revealed intumescent cataracts OU, impairing fundus examination. Although mild senile iris atrophy was bilaterally seen in cases 1 and 2, pupillary light reflexes (PLRs) were not affected. Fluorescein test was negative in all the eyes. Abnormalities of the preoperative complete blood count and serum biochemistry studies for each dog are summarized in Table 2. In all the eyes, the preoperative gonioscopic evaluation revealed an open iridocorneal angle (ICA) with no pectinate ligament dysplasia. In addition, ocular ultrasound examination was unremarkable, with biometric measurements of the crystalline lens diameter varying between 12.9 and 14.3 mm, and short-protocol electroretinography revealed regular retinal activity in all the eyes. Preoperative treatment and surgical procedureAll the dogs were treated 5–6 hours before surgery with flunixin-meglumine (0.5 mg/kg IV; Nixyvet 50mg/ml®, Divasa-Farmavic, S.A. Group, Barcelona, Spain), and at anesthesia induction with cefazolin (25 mg/kg IV; Cefazolina Normon, Laboratorios Normon S.A., Madrid, Spain). In addition, topical tropicamide (Colircusí Tropicamida®, Alcon Healthcare, Barcelona, Spain), 1% phenylephrine (Colircusí Fenilefrina®, Alcon Healthcare, Barcelona, Spain), 0.1% nepafenac (Nevanac 1 mg/ml®, Alcon Healthcare, Barcelona, Spain), and ciprofloxacin (Oftacilox®, Alcon Healthcare, Barcelona, Spain), were applied every 30 minutes for 2 hours. Cataract surgery consisted of a routine one-handed divided-and-conquer phacoemulsification procedure using a peristaltic pump (Alcon Infinity Vision System®, Alcon Healthcare, Barcelona, Spain). Intracameral fluids used along the surgery included: balanced salt solution, refrigerated Ringer´s solution, 2.2% sodium hyaluronate viscoelastic (An-bfh 2.2%®, an-vision, Hennigsdorf, Germany), and intracameral tissue plasminogen activator (0.25 μg/0.1 ml). In all the eyes, and acrylic lens of the appropriate size was inserted in the capsular bag (MD8®, an-vision, Hennigsdorf, Germany). All surgeries were uneventful, and sodium hyaluronate was removed using the automated irrigation/aspiration system following placement of the IOL. For all the eyes, 45º mini-flared Kelman tips were used, and phacoemulsification metrics were recorded (Table 3). The corneal incision was closed by 3–4 simple interrupted sutures (Dafilon 9/0®, B Braun, Melsungen, Germany), resulting in a watertight seal. Recovery from general anesthesia was uneventful. Immediate and short-term postoperative managementImmediate postoperative management included buprenorphine (15 μg/kg IV; Buprex®, Indivior Europ Limited, Dublin, Ireland), topical 0.1% nepafenac q4h (Nevanac 1 mg/ml®, Alcon Healthcare, Barcelona, Spain), ciprofloxacin q4h (Oftacilox®, Alcon Healthcare, Barcelona, Spain), carbomer gel q4h (Viscotears®, Bausch & Lomb, Laval, CA) and tonometry every 2 hours for the first 24 hours. IOP spikes were treated accordingly to the “VTH-UAB protocol for managing postoperative intraocular pressure spikes in dogs,” which includes topical carbonic anhydrase inhibitors, beta-blockers, synthetic prostaglandins, and anterior chamber decompression (ACD), depending on the IOP values (Table 4). Five out of the six eyes developed moderate-to-severe postoperative ocular hypertension (POH) (40–75 mmHg), requiring a combination of topical antiglaucoma drugs with ACD. The number of ACD needed to return the IOP to normal levels (≤20 mmHg) were as follows: case 1 (3 ACD OU), case 2 (2 ACD OU), and case 3 [1 ACD in the left eye (OS)]. Postoperative treatment at discharge consisted of topical 0.1% nepafenac q4h, ciprofloxacin q4h, carbomer gel q4h, and tropicamide q12h, as well as robenacoxib q24h (1 mg/kg, Onsior®, Elanco, IN). In addition, a combination of dorzolamide and timolol (Cosopt®, Santen Oy, Tampere, Finland) was added OU q6h in cases 1 and 2. At first recheck, 1 week postoperatively, dogs seemed to cope well with medication; nevertheless, cases 2 and 3 showed signs of unilateral discomfort in the right and left eye, respectively. At the examination, menace response was present OU in dogs 2 and 3 while diminished in dog one. Five out of the six eyes showed dazzle reflex but absent PLRs with unresponsive mydriatic pupils. At that time, mydriasis was classified as severe in three eyes (case 1 OU and case 3 OS) and moderate in 2 (case 2 OU) (Fig. 1). IOPs values were as follow: case 1 [55 mmHg right eye (OD); 42 mmHg OS], case 2 (72 mmHg OD; 12 mmHg OS) and case 3 (5 mmHg OD; 57 mmHg OS). Biomicroscopic examination revealed different degrees of bulbar conjunctival congestion and diffuse corneal edema; all the eyes showed mild aqueous flare, centered intraocular lens (IOL), and normal fundus examination. The hypertensive eyes (n=4) were treated with a drop of latanoprost (Xalatan®, Pfizer, New York, NY), and IOPs rechecked after 30 minutes. IOP dropped down to normal values; thus, latanoprost was added to the previous treatment, and tropicamide was discontinued. In the following rechecks, despite the use of latanoprost, mydriasis was still present in dogs one and two OU and in dog three OS. Based on the presence of the three classical signs (mydriasis, iris atrophy, and glaucoma), UZS was diagnosed in the three dogs, being initially classified as severe for case 1 OU and case 3 OS and moderate for case 2 OU. Table 1. Ocular findings of dogs affected by UZS after cataract surgery. Findings at first presentation and last recheck are shown.
Table 2. Abnormalities of the preoperative complete blood count and serum biochemistry studies for three dogs affected by UZS.
Table 3. Metrics of the peristaltic phacoemulsification procedures of three dogs postoperatively affected by UZS.
Table 4. VTH-UAB protocol for managing postoperative IOP spikes in dogs.
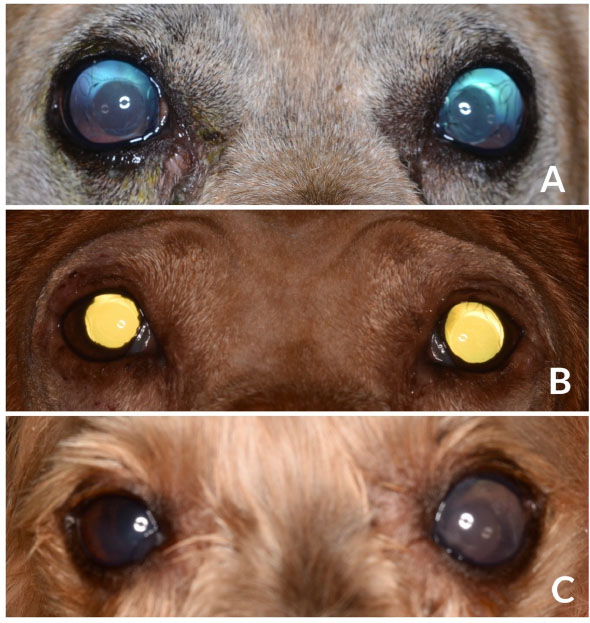
Fig. 1. Images of diabetic dogs affected by UZS. (A): Fixed, severely dilated pupils in a 9-year-old mixbreed dog, 1-week after bilateral cataract surgery. (B): Fixed moderately dilated pupils (OS>OD) in a IWH, 2-week after bilateral cataract surgery. (C): Fixed severely dilated left pupil in a YT dog, 1-week after bilateral cataract surgery. Dogs from figures A and B showed preoperative mild iris atrophy but normal PLRs. Long-term postoperative treatment and outcomeTwo weeks after the surgery, case 1 was rechecked due to a sudden episode of blindness. Menace response and dazzle reflex were absent, and pupils rested at the same severe mydriatic status. IOPs were 70 mmHg OD and 56 mmHg OS. Dynamic gonioscopy (17 mm Koeppe goniolens®, Ocular Instruments Inc., Washington, DC) confirmed the closure of the ICA due to a complete peripheral anterior synechia. Options were discussed with the owners, and an intraocular surgery was performed for breaking synechiae. Surgery occurred uneventfully, but although intracameral acetylcholine was injected in both eyes (Acetilcolina 10 mg/ml cusí®, Alcon Healthcare, Barcelona, Spain), no pupil movement was observed. Along with postoperative rechecks, pupils remained dilated, and IOP spikes in both eyes required further management with mannitol iv (Mannitol Mein 20%®, Fresenius SE&Co, Bad Homburg v.d.H., Germany) and topical combination of brinzolamide and brimonidine (Simbrinza®, Alcon Healthcare, Barcelona, Spain). At the time of writing this paper, menace response was only present OS with both pupils severely mydriatic and unresponsive to light. IOPs at that moment were 7 mmHg OU, and the dog was under topical medication with brinzolamide/brimonidine q6h and latanoprost q12h, as well as with systemic citicoline (15 mg/kg PO; Neuro-CPD®, Vetilea SL, Barcelona, Spain). Similarly, case 2 was reviewed 3 weeks after the surgery due to severe discomfort in the right eye. No menace response, dazzle reflex, nor PLRs were observed. OD and IOP were 65 mmHg. Due to the uncomplete response to the systemic medication with mannitol (2 g/kg iv) and the topical medication with latanoprost, brinzolamide, and brimonidine, persistent IOP spikes lead to a comfortable but blind eye OD. At last recheck before writing this manuscript, positive menace response and dazzle reflexes were present OS and absent OD, although the pupils remained moderately mydriatic and unresponsive to light. IOP was16 mmHg OD and 6 mmHg OS, and the dog were under a topical combination of brinzolamide and brimonidine OU q6h (Simbrinza®, Alcon Healthcare, Barcelona, Spain) and systemic citicoline. At last recheck, case 3 showed menace response and dazzle reflex OU, had a moderately mydriatic and unresponsive left pupil, and the IOPs were 7 mmHg OD and 22 mmHg OS. The dog was under topical antiglaucoma medication OS (dorzolamide q8h and latanoprost q24h) and systemic citicoline. Based on the outcome and the partial pupillary recovery of the left eye of case 3, severity was reestablished, being considered as severe in two eyes (case 1) and moderate in three eyes (case 2 and case 3). DiscussionPhacoemulsification is considered as the gold-standard treatment for cataracts, being routinely performed by veterinary ophthalmologists (Michau, 2021). In the hands of experienced surgeons, surgical outcomes mirror the ones described in human´s ophthalmology, with a low incidence of complications reported (17.3%) (Klein et al., 2011). Postoperative complications have decreased significantly over time due to factors such as improvement in the patient selection process, tissue handling, use of nondepolarizing neuromuscular blocking agents, adequate preoperative mydriasis, shortened surgical times, type of IOL, and use of ophthalmic viscosurgical devices (OVDs), among others. Immediate postoperative complications described in dogs so far include corneal incision dehiscence and infection, corneal ulceration, corneal endothelial decompensation and edema, intraocular hemorrhage, anterior uveitis, fibrin, toxic anterior chamber syndrome, and POH (Michau, 2021). Similarly, the most common long-term postoperative complications described are corneal lipidosis, degenerations and ulcerations, endophthalmitis, posterior and anterior capsule opacification, IOL decentration or luxation, glaucoma, retinal detachment, and peripheral ocular neuropathies (Michau, 2021). In addition, conversely to what has been widely described in human literature (Galiani and Aminlari, 2002), there are only two descriptions of the atonic pupil as a postoperative complication of phacoemulsification (Bilotta and Busse, 2021; Michau, 2021). Nevertheless, none of the above were associated with high IOPs. UZS, although by no means frequent, has been a well-recognized, postoperative ocular complication in humans since 1963 (Urrets-Zavalia, 1963). Along the last decades, the syndrome, initially described by a fixed dilated pupil with iris atrophy and increased IOP, has englobed other clinical presentations, keeping fixed dilated pupils the main clinical sign (Spierer and Lazar, 2014). Nowadays, UZS has two well-known presentations with varying severity. The milder form is characterized by fixed mydriasis seen as a sole finding. The pupil dilation may diminish gradually in the next month or so until it returns to its normal size. As a rule, the slow process of recovery occurs spontaneously, leaving as a sequela diffuse atrophy of the anterior layers of the iris (Spierer and Lazar, 2014; Isac et al., 2019). This form may easily go unnoticed based on the milder clinical findings and the mandatory use of postoperative topical mydriatics. A more severe form of UZS can be seen in less fortunate cases, showing marked fixed mydriasis is accompanied with iris atrophy and increased IOP. In most cases, mydriasis is irreversible and even exaggerated in parts, producing at the same time an ectropion of the pigment epithelium at the pupillary border and secondary dyscoria. Invariably, there is also a marked tendency toward the formation of synechiae. The clinical picture is much reminiscent of that encountered after a severe attack of high IOP (Magalhães et al., 2016). Despite the five affected eyes of this case series showing the clinical appearance of iris atrophy and glaucoma, three were initially classified as severe forms of UZS based on their fixed severe mydriasis, and two as moderate forms. Along the postoperative period, one eye showed partial pupillary improvement, showing more moderate mydriasis, thus being re-classified as a moderate form. Although UZS has shown no predilection for age, gender, or systemic concomitant diseases in humans (Magalhães et al., 2016), the three dogs reported in the present study were diabetic. The role diabetes mellitus may have played in the development of the condition is uncertain; however, considering that vasculitis is a relatively common complication in diabetic patients (Rask-Madsen and King, 2013), it could have helped in its development. Without a doubt, the relationship between diabetes mellitus and UZS requires further investigation. In human literature, the clinical signs of UZS have been reported to appear between 1 and 21 days after the surgery (Totuk et al., 2018; Kurtz and Fradkin, 2021). The exact time for the clinical signs to appear in the present study is unknown, as our first post-phacoemulsification recheck was performed 7 days after the surgery in all the cases. Even though the clinical signs were evidenced at the first recheck, they could have shown before and gone unnoticed by the owner. Although the exact mechanism of UZS is still unknown, two main etiologies have been historically considered overtime. The first, observed after intraocular or non-penetrating surgery, seems to be caused by an IOP spike and impairment of the vascular supply to the iris constrictor muscle, leading to iris atrophy with subsequent persistent pupil dilation (Tuft and Buckley, 1995). The IOP spike, if short, could induce the milder form of the presentation (with mydriasis as the sole clinical sign), while if maintained over time, it could induce the more severe form of the syndrome (mydriasis, iris atrophy, and glaucoma). The second etiology, observed after 360-degree laser procedures, would seem to be caused by impairment of the parasympathetic pathway to the iris constrictor muscle, related to direct laser damage to the short ciliary nerves that run radially from the posterior pole toward the iris sphincter muscle (Vieira et al., 2017). This form does not involve iris atrophy or secondary glaucoma, and thus we believed we should avoid grouping all the cases of fixed and dilated pupils under the same syndrome, as they do not have neither the same signs nor the same etiology. Thus, we suggest another syndrome should be defined to cover the cases in which the fixed and dilated pupil was not caused by iris ischemia precipitated by an IOP spike but by the direct impairment of the parasympathetic pathway to the iris constrictor muscle. The five eyes reported in the present study showed moderate to severe immediate POH which could have triggered the syndrome. In fact, the eye with milder clinical signs of UZS was the one with lower POH values, needing just one ACD for reestablishing normal IOP. Different mechanisms could induce IOP spikes during the intraoperative or immediate postoperative period. Intraoperatively, the IOP could inadvertently rise as a result of OVDs. Studies comparing the effect of various viscoelastic formulations on postoperative pressure in humans undergoing cataract surgery abound in the literature with various conclusions (Henry and Olander, 1996). Although significant pupil atrophy has not been reported after the widespread use of OVDs in cataract extraction surgery, the IOP increase related to the use of OVDs during intraocular surgery has been postulated as a reason for UZS (Tan and Humphry, 1993; Bourcier et al., 2001). Apart from the mechanical vascular compression, OVDs may cause a toxic effect, resulting in ischemia and, consequently, fixed and dilated pupils. Another critical point for intraoperative IOP increase is the phacoemulsification fluidics. Studies have reported that the different phacoemulsification approaches, the influx of irrigation fluid into the eye, and the efflux of the fluid through the side port and corneal incision site influence the IOP (Wilbrandt and Wilbrandt, 1993; Kang et al., 2015). In fact, it is generally believed that maintenance of some incision leakage during the procedure acts as a fluidics buffer, thus reducing the intraoperative IOP. In the five eyes here reported, a clear corneal incision of 2.8 mm width was performed, corresponding to the width required by the phacoemulsification needle and infusion sleeve. All the eyes underwent a one-handed divide-and-conquer technique with no significant difference regarding the phacoemulsification time, power, and volume of fluids used when compared to other cataracts of the same degree of maturity in the same institution. Similarly, immediate postoperative spikes have been associated with different factors, such as OVDs retention in the anterior chamber angle, preexisting compromise of outflow facility, surgical trauma, hyphema, inflammation, and the skillfulness of the surgeon (Michau, 2021). In the present study, besides cohesive OVDs were routinely removed from the anterior chamber and lenticular bag, moderate-to-severe IOP spikes were detected in all the affected eyes throughout the first 24 postoperative hours. Surprisingly, those spikes were higher in the most severely affected eyes, requiring repeated ACD to return to normal IOP values. Postoperative IOP spikes have been previously associated with ocular blood flow dysregulation and tissue ischemia (Tranos et al., 2013). Elevated and maintained IOP combined with a low systemic blood pressure has been shown to reduce the ocular perfusion pressure, thus inducing tissue ischemia (Costa et al., 2014). Unfortunately, no blood pressure measurements were taken during the immediate postoperative period; thus, this hypothesis could not be confirmed. The five ocular surgeries were uneventfully performed by experienced surgeons, and no preexisting compromise of outflow facility was detected by gonioscopy in none of the eyes. Several studies compare the outcomes of cataract surgery in relation to POH. A study on the use of ultrasound biomicroscopy to investigate angle and globe morphology before and after phacoemulsification in dogs concluded that dogs with larger ICA and AOD measurements before surgery were at greater risk of developing POH (Rose et al., 2008). Another recent study revealed that eyes with abnormal gonioscopy findings are at an increased risk of postoperative glaucoma compared with eyes with normal gonioscopy findings (Sanders et al., 2021). Therefore, gonioscopy is recommended as a part of the presurgical assessment in all dogs before phacoemulsification. Although TASS has been reported to be a risk factor for developing UZS in humans (Nizamani et al., 2013), the degree of postoperative intraocular inflammation seen in those eyes was considered as normal, with mild to moderate degree of aqueous flare that improved with medical treatment along the postoperative period. Although the use of mydriatics, more specifically, atropine, has been classically postulated as an etiology for UZS, none of the dogs of this study were treated with topical atropine nor with intracameral phenylephrine. Mydriatic preoperative medication consisted of topical tropicamide and phenylephrine (1%). Nowadays, there is no curative treatment for this condition. The use of topical parasympathetic (pilocarpine) and sympatholytic agents (dapiprazole and guanethidine) has been investigated. Although the majority of studies on the use of topical pilocarpine and guanethidine showed no improvement of the fixed and dilated pupils (Bonnet et al., 1969; Bourcier et al., 2001; Espana et al., 2007; Kaeser and Kawasaki, 2010), when both combined, they were able to partially treat sympathetic spasm and induce miosis (Lagoutte et al., 1983). Similarly, dapiprazole was successfully used for restoring pupil size in a patient with UZS after a keratoplasty procedure (Spadea et al., 2008). In parallel, several reconstructive surgical treatments have been described in humans for symptomatic permanent mydriasis. These include keratopigmentation (Reed, 1994; Alio et al., 2012), a black diaphragm intraocular lens (Sundmacher et al., 1994), and pupilloplasties (Ogawa, 1998; Narang et al., 2017). At the time of writing this report, three eyes remained still visual, all under topical antiglaucoma drugs. None of them responded to topical pilocarpine or latanoprost during the study period, maintaining mydriasis. No overt signs of photophobia were observed. To summarize, this is the first published description of UZS in dogs, occurring after phacoemulsification surgery. Even though no exact, demonstrable causative element could be determined, we believe that the use of ocular viscoelastic devices or topical tropicamide/phenylephrine was not directly associated with the onset of UZS. Conversely, POH should be considered a triggering condition for this syndrome, as it directly affects the blood flow autoregulation and intrinsic uveal tissue integrity. Until the contrary is proved, diabetes mellitus might be considered a risk factor for developing this syndrome after cataract surgery in dogs. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsMarta Leiva, Francisco Cantero, and Laura Gaztelu did the diagnosis, provide key information and analyzed the data. Francisco Cantero and Marta Leiva designed the manuscript, wrote the original draft, and revised it critically for important intellectual content. All the authors had direct patient contact, revised, edited the manuscript, and gave their final approval for the version to be published. ReferencesAlio, J.L., Rodriguez, A.E., Toffaha, B.T. and Aswad, A.E. 2012. Femtosecond-assisted keratopigmentation double tunnel technique in the management of a case of Urrets-Zavalia syndrome. Cornea 31, 1071–1074. Anwar, D.S., Chu, C.Y., Prasher, P., Bowman, R.W. and Mootha, V.V. 2012. Features of Urrets-Zavalia syndrome after descemet stripping automated endothelial keratoplasty. Cornea 31, 1330–1334. Aralikatti, A.K., Tomlins, P.J. and Shah, S. 2008. Urrets–Zavalia syndrome following intracameral C3F8 injection for acute corneal hydrops. Clin. Exp. Ophthalmol. 36, 198–199. Bilotta, T. and Busse, C. 2021. Urrets-Zavalia syndrome in a dog following cataract surgery. ECVO 2021 Annual conference meeting. Bonnet, M., Lemarchands, H. and Martin, J. 1969. Prevention and treatment of the “irreducible mydriasis-progressive atrophy of the iris” syndrome following perforating keratoplasty for keratoconus. Ann. Ocul. 202, 1139–1146. Bourcier, T., Laplace, O., Touzeau, O., Moldovan, S.M., Borderie, V. and LarochE, L. 2001. Urrets-Zavalia syndrome. J. Fr. Ophtalmol. 24, 303–308. Bozkurt, K.T., Acar, B.T. and Acar, S. 2012. Fixed dilated pupil as a common complication of deep anterior lamellar keratoplasty complicated with descemet membrane perforation. Eur. J. Ophthalmol. 23, 164–170. Chelnis, J.G., Sieminski, S.F. and Reynolds, J.D. 2012. Urrets-Zavalia syndrome following goniotomy in a child. JAAPOS 16, 312–313. Costa, V.P., Harris, A., Anderson, D., Stodtmeister, R., Cremasco, F., Kergoat, H., Lovasik, J., Stalmans, I., Zeitz, O., Lanzl, I., Gugleta, K. and Schmetterer, L. 2014. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 92, 252–266. Espana, E.M., Ioannidis, A., Tello, C., Liebmann, J.M., Foster, P. and Ritch, R. 2007. Urrets-Zavalia syndrome as a complication of argon laser peripheral iridoplasty. Br. J. Ophthalmol. 91, 427–429. Foroutan, A., Tabatabaei, S.A., Soleimani, M. and Nekoozadeh, S. 2016. Urrets-Zavalia syndrome in different methods of keratoplasty. Int. J. Ophthalmol. 9, 1358–1360. Fournié, P., Ponchel, C., Malecaze, F. and Arné, J.L. 2009. Fixed dilated pupil (Urrets-Zavalia syndrome) and anterior subcapsular cataract formation after descemet stripping endothelial keratoplasty. Cornea 28, 1184–1186. Galiani, D.L. and Aminlari, A. 2002. Atonic pupil following cataract extraction: incidence over a ten-year period. ARVO Ann. Meet. Abs. 43, 386–386. Henry, J.C. and Olander, K. 1996. Comparison of the effect of four viscoelastic agents on early postoperative intraocular pressure. J. Cataract Refract. Surg. 22, 960–966. Hildebrand, G.D., Wickremasinghe, S.S., Tranos, P.G., Harris, M.L. and Little, B.C. 2003. Efficacy of anterior chamber decompression in controlling early intraocular pressure spikes after uneventful phacoemulsification. J. Cataract Refract. Surg. 29(6), 1087–1092. Isac, M.M.S., Ting, D.S.J. and Patel, T. 2019. Spontaneous pupillary recovery of Urrets-Zavalia syndrome following descemet’s membrane endothelial keratoplasty. Med. Hypothesis Discov. Innov. Ophthalmol. 8, 7–10. Jain, R., Assi, A. and Murdoch, I.E. 2000. Urrets-Zavalia syndrome following trabeculectomy. Br. J. Ophthalmol. 84, 338–339. Kaeser, P.F. and Kawasaki, A. 2010. Disorders of pupillary structure and function. Neurol. Clin. 28, 657–677. Kang, S., Park, S., Noh, H. and Seo, K. 2015. Fluid dynamics and intraocular pressure using venturi phacoemulsification machine in dogs ex vivo. Vet. Ophthalmol. 18, 309–316. Klein, H.E., Krohne, S.G., Moore, G.E. and Stiles, J. 2011. Postoperative complications and visual outcomes of phacoemulsification in 103 dogs (179 eyes): 2006–2008. Vet. Ophthalmol. 14, 114–120. Kurtz, S. and Fradkin, M. 2021. Urrets-Zavalia syndrome following cataract surgery. Case Rep. Ophthalmol. 12, 659–663. Lagoutte, F., Thienpont, P. and Comte, P. 1983. Proposed treatment of the Urrets-Zavalia syndrome. A propose of one reversible case. J. Fr. Ophtalmol. 6, 291–294. Lifshitz, T. and Yassur, Y. 1988. Accommodative weakness and mydriasis following laser treatment at the peripheral retina. Ophthalmologica 197, 65–68. Magalhães, O.A., Kronbauer, C.L., Müller, E.G. and Sanvicente, C.T. 2016. Update and review of Urrets-Zavalia syndrome. Arq. Bras. Oftalmol. 79, 202–204. Maurino, V., Allan, B.D.S., StevenS, J.D. and Tuft, S.J. 2002. Fixed dilated pupil (Urrets-Zavalia syndrome) after air/gas injection after deep lamellar keratoplasty for keratoconus. Am. J. Ophthalmol. 133, 266–268. Michau, T.M. 2021. Surgery of the lens. In Veterinary Ophthalmology, 6th ed. Eds., Gelatt, K.N., Ben-Shlomo, G., Gilger, B.C., Hendrix, D.V.H., Kern, T.J. and Plummer, C.E. Hoboken, NJ: Willey and Sons, pp: 1479–1546. Minasian, M. and Ayliffe, W. 2002. Fixed dilated pupil following deep lamellar keratoplasty (Urrets-Zavalia syndrome). Br. J. Ophthalmol. 86, 114–123. Monson, M.C., Mamalis, N. and Olson, R.J. 1992. Toxic anterior segment inflammation following cataract surgery. J. Cataract Refract. Surg. 18, 184–189. Narang, P., Agarwal, A. and Kumar, D.A. 2017. Single-pass four-throw pupilloplasty for Urrets-Zavalia syndrome. Eur. J. Ophthalmol. 28, 552–558. Nizamani, N.B., Bhutto, I.A. and Talpur, K.I. 2013. Cluster of Urrets-Zavalia syndrome: a sequel of toxic anterior segment syndrome. Br. J. Ophthalmol. 97, 953. Ogawa, G.S. 1998. The iris cerclage suture for permanent mydriasis: a running suture technique. Ophthalmic Surg. Lasers Imaging Retina 29, 1001–1009. Park, S.H., Kim, S.Y., Kim, H.I. and Yang, S.W. 2008. Urrets-Zavalia syndrome following iris-claw phakic intraocular lens implantation. J. Refract. Surg. 24, 959–961. Pérez-Cambrodí, R.J., Piñero-Llorens, D.P., Ruiz-Fortes, J.P., Blanes-Mompó, F.J. and Cerviño-Expósito, A. 2013. Fixed mydriatic pupil associated with an intraocular pressure rise as a complication of the implant of a phakic refractive lens (PRL). Semin. Ophthalmol. 29, 205–209. Rask-Madsen, C. and King, G.L. 2013. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metabolism 17, 20–33. Reed, J.W. 1994. Corneal tattooing to reduce glare in cases of traumatic iris loss. Cornea 13, 401–405. Rose, M.D., Mattoon, J.S., Gemensky-Metzler, A.J., Wilkie, D.A. and Rajala-Schultz, P.J. 2008. Ultrasound biomicroscopy of the iridocorneal angle of the eye before and after phacoemulsification and intraocular lens implantation in dogs. Am. J. Vet. Res. 69, 279–288. Sanders, M.T., Morton, J.M., Kaese, H.J., Ford, M. and Stanley, R.G. 2021. Association between preoperative gonioscopic status and postoperative glaucoma after phacoemulsification in dogs: a retrospective cohort study of 505 eyes. Vet. Ophthalmol. 24, 39–49. Spadea, L., Viola, M. and Viola, G. 2008. Regression of Urrets-Zavalia syndrome after deep lamellar keratoplasty for keratoconus: s case study. Open J. Ophthalmol. 2, 130–131. Spierer, O. and Lazar, M. 2014. Urrets-Zavalia syndrome (fixed and dilated pupil following penetrating keratoplasty for keratoconus) and its variants. Surv. Ophthalmol. 59, 304–310. Srinivasan, M. and Patnaik, L. 2004. Fixed dilated pupil (Urrets-Zavalia syndrome) in corneal dystrophies. Cornea 23, 81–83. Sundmacher, R., Reinhard, T. and Althaus, C. 1994. Black-diaphragm intraocular lens for correction of aniridia. Ophthalmic Surg. 25, 180–185. Tan, A.K. and Humphry, R.C. 1993. The fixed dilated pupil after cataract surgery. Is it related to intraocular use of hypromellose? Br. J. Ophthalmol. 77, 639–641. Totuk, O.M.G., Bouveret, B. and Orucoglu, F. 2018. Urrets-Zavalia syndrome after foreign-body removal from the anterior chamber. J. Cataract Refract. Surg. 44, 1046. Tranos, P., Nasr, M.B., Asteriades, S., Vakalis, A. and Georgalas, I. 2013. Bilateral diffuse iris atrophy after the use of oral clarithromycin. Cutan. Ocul. Toxicol. 33, 79–81. Tuft, S.J. and BuckleY, R.J. 1995. Iris ischemia following penetrating keratoplasty for keratoconus (Urrets-Zavalia syndrome). Cornea 14, 618–622. Urrets-Zavalia, A. 1963. Fixed, dilated pupil, iris atrophy and secondary glaucoma. A distinct clinical entity following penetrating keratoplasty in keratoconus. Am. J. Ophthalmol. 56, 257–265. Vieira, G.M., Vieira, F.J. and Ritch, R. 2017. Urrets-Zavalia syndrome after diode laser transscleral cyclophotocoagulation. J. Glaucoma 26, 678–682. Walton, D.S. 2013. Urrets-Zavalia syndrome following goniotomy in a child. J AAPOS 17, 114–115. Wilbrandt, H.R. and Wilbrandt, T.H. 1993. Evaluation of intraocular pressure fluctuations with differing phacoemulsification approaches. J. Cataract Refract. Surg. 19, 223–231. Yuzbasioglu, E., Helvacioglu, F. and Sencan, S. 2006. Fixed, dilated pupil after phakic intraocular lens implantation. J. Cataract Refract. Surg. 32, 174–176. | ||
| How to Cite this Article |
| Pubmed Style FC, Leiva M, Gaztelu L, Cerrada I, Vilao R, Pena T. Urrets-Zavalia syndrome following cataract surgery in dogs: A case series. Open Vet J. 2022; 12(1): 138-147. doi:10.5455/OVJ.2022.v12.i1.17 Web Style FC, Leiva M, Gaztelu L, Cerrada I, Vilao R, Pena T. Urrets-Zavalia syndrome following cataract surgery in dogs: A case series. https://www.openveterinaryjournal.com/?mno=134620 [Access: April 24, 2024]. doi:10.5455/OVJ.2022.v12.i1.17 AMA (American Medical Association) Style FC, Leiva M, Gaztelu L, Cerrada I, Vilao R, Pena T. Urrets-Zavalia syndrome following cataract surgery in dogs: A case series. Open Vet J. 2022; 12(1): 138-147. doi:10.5455/OVJ.2022.v12.i1.17 Vancouver/ICMJE Style FC, Leiva M, Gaztelu L, Cerrada I, Vilao R, Pena T. Urrets-Zavalia syndrome following cataract surgery in dogs: A case series. Open Vet J. (2022), [cited April 24, 2024]; 12(1): 138-147. doi:10.5455/OVJ.2022.v12.i1.17 Harvard Style , F. C., Leiva, . M., Gaztelu, . L., Cerrada, . I., Vilao, . R. & Pena, . T. (2022) Urrets-Zavalia syndrome following cataract surgery in dogs: A case series. Open Vet J, 12 (1), 138-147. doi:10.5455/OVJ.2022.v12.i1.17 Turabian Style , Francisco Cantero, Marta Leiva, Laura Gaztelu, Irene Cerrada, Rita Vilao, and Teresa Pena. 2022. Urrets-Zavalia syndrome following cataract surgery in dogs: A case series. Open Veterinary Journal, 12 (1), 138-147. doi:10.5455/OVJ.2022.v12.i1.17 Chicago Style , Francisco Cantero, Marta Leiva, Laura Gaztelu, Irene Cerrada, Rita Vilao, and Teresa Pena. "Urrets-Zavalia syndrome following cataract surgery in dogs: A case series." Open Veterinary Journal 12 (2022), 138-147. doi:10.5455/OVJ.2022.v12.i1.17 MLA (The Modern Language Association) Style , Francisco Cantero, Marta Leiva, Laura Gaztelu, Irene Cerrada, Rita Vilao, and Teresa Pena. "Urrets-Zavalia syndrome following cataract surgery in dogs: A case series." Open Veterinary Journal 12.1 (2022), 138-147. Print. doi:10.5455/OVJ.2022.v12.i1.17 APA (American Psychological Association) Style , F. C., Leiva, . M., Gaztelu, . L., Cerrada, . I., Vilao, . R. & Pena, . T. (2022) Urrets-Zavalia syndrome following cataract surgery in dogs: A case series. Open Veterinary Journal, 12 (1), 138-147. doi:10.5455/OVJ.2022.v12.i1.17 |