| Original Article | ||
Open Vet J. 2022; 12(2): 171-181 Open Veterinary Journal, (2022), Vol. 12(2): 171–181 Original Research Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implantLuise Prestel1, Jessica Joerling2, Klaus Failing3, Henrik Wagner1 and Axel Wehrend1*1Clinic for Obstetrics, Gynaecology, and Andrology of Large and Small Animals, Justus Liebig University Giessen, Giessen, Germany 2Clinic for Ruminants, Justus Liebig University Giessen, Giessen, Germany 3Biomathematics and Data Processing Group, Justus Liebig University Giessen, Giessen, Germany *Corresponding Author: Axel Wehrend. Clinic for Obstetrics, Gynaecology, and Andrology of Large and Small Animals, Justus Liebig University Giessen, Giessen, Germany. Email: Axel.Wehrend [at] vetmed.uni-giessen.de Submitted: 27/10/2021 Accepted: 24/02/2022 Published: 11/03/2022 © 2022 Open Veterinary Journal
AbstractBackground: Regarding animal welfare and reversible suppression of reproduction the need for alternatives to surgical castration, like slow-release gonadotropin-releasing hormone (GnRH) implants, is increasing. Aim: In this study, we evaluated whether the onset of puberty can be suppressed by implantation of a slow-release GnRH implant (4.7 mg deslorelin) in juvenile rams. Methods: Seven juvenile rams (3–5.5 months) were treated with the GnRH analog deslorelin to analyze the effects on testicular development, sonographic findings of testicular tissue, testosterone concentration in the blood, spermatogenesis, and sperm parameters from the epididymis after castration. Seven rams of the same age without an implant served as controls. Results: Follow-up examinations were conducted over 5 months, after which four rams per group were castrated. No significant group differences were found in the andrological parameters on the group level, but testicular development was suppressed in three rams in the treated group. Histological examination revealed spermatogenesis in the testicular tissue in three of four animals treated with a GnRH analog. Conclusion: The onset of puberty in juvenile rams cannot be reliably suppressed by using the slow-release GnRH implant Suprelorin®. Keywords: Castration, Male, Hormonal downregulation, Puberty, Sheep. IntroductionSuppression of sexual behavior and reproduction of small male ruminants represents an important aspect for the management of herds and in hobby husbandry. Besides surgical castration, low-risk and reversible alternatives (Mihsler et al., 2016), such as vaccination against gonadotropin-releasing hormone (GnRH) (Needham et al., 2019a) and the application of GnRH analogs (Tilbrook et al., 1993), are being applied. Apart from enriching animal welfare by avoiding stress and pain, chemical castration does not negatively affect carcass quality and therefore should be given preference over surgical castration (Gökdal et al., 2010; Needham et al., 2019a, 2019b). As another pharmaceutical, the use of altrenogest to suppress reproduction was recently investigated in goats, but reliable suppression of reproductive function was not successful (Mihsler-Kirsch et al., 2021). In 1982, the first successful immunization trials against GnRH were conducted in small ruminants (Jeffcoate et al., 1982) and subsequent trials have confirmed the efficacy of GnRH vaccines (Godfrey et al., 1996; Kiyma et al., 2000; Thompson, 2000; Ulker et al., 2005; Han et al., 2015; Aponte et al., 2018; Rocha et al., 2021). However, in one study it was observed that although motile sperm were produced after the GnRH vaccination had worn off, fertility was limited, suggesting that immunization may not be fully reversible (Godfrey et al., 1996). In another study, a significant reduction of testicular volume was achieved through immunization, but in 40% of the immunocastrated rams, spermatozoa were detectable in the seminiferous tubes negating a 100% success of immunization (Aponte et al., 2018). On the other hand, Hanet al. (2015) showed that immunization against GnRH resulted in reduced diameter and weight of testes and epididymides, undetectable androstenone concentration in adipose tissue, and spermatozoa were completely absent in seminiferous tubes. GnRH vaccines must be administered at least twice for effective antibody formation, which makes it impractical in large herds, and the duration as well as the completeness of the effect cannot be predicted. Thus, the present study aimed to evaluate whether the single implantation of a slow-release GnRH implant is fundamentally suitable for reproductive suppression in juvenile rams. Previous studies on the use of GnRH agonists have shown that they are basically appropriate. To the best of our knowledge, the veterinary pharmaceutical Suprelorin® implant with the active ingredient deslorelin has not been tested so far in juvenile rams. In adult rams, the application of GnRH analogs with a prolonged duration of action leads to a paradoxical reaction. In the first few hours (stimulation phase), an increased release of follicle-stimulating hormone and luteinizing hormone is followed by a marked increase in testosterone concentration (Jiménez-Severiano et al., 2007), subsequently resulting in a reduced responsiveness of the pituitary gland to GnRH (Adams, 2005). According to the manufacturer, the Suprelorin® implant is licensed for reproduction suppression in male dogs and ferrets (Vinke et al., 2008; Driancourt and Briggs, 2020), takes effect 6 weeks postimplantation, and has guaranteed activity for 6 months at the 4.7 mg dosage. Compared to natural GnRH, deslorelin (D-Trp(6)-N-Et-D-GlyNH2(10)-GnRH) contains chemical modifications of the amino acid sequence at position 6 (amino acid D-tryptophan instead of glycine), which protects against proteolysis, and at position 10 (N-EtNH2 instead of Gly-NH2), which improves receptor binding affinity (PubChem CID25077495). In adult Soay rams, the intravenous application of GnRH agonist for eight consecutive days resulted in significantly decreased plasma concentration of testosterone, but did not affect the testicular diameter compared to the control group (Fraser and Lincoln, 1980). The implantation of the GnRH agonist goserelin every 4 weeks in juvenile male sheep could effectively suppress puberty, which was reflected by significantly lower testicular weights in the treated group (Robinson et al., 2014). In addition, other modified GnRH analogs have also been used in two previous studies, but these were applied as repeated injections rather than as implants and led to contrary results (Tilbrook et al., 1993; Chandolia et al., 1997). Until today, no research has been conducted on the slow-release implant containing deslorelin in rams, but slow-release GnRH implants have been used with varying degrees of success in rabbits, rats, cats, boars, goats, and marsupials (Herbert et al., 2007; Kauffold et al., 2010; Edwards et al., 2013; Goericke-Pesch et al., 2014; Fontaine, 2015; Goericke-Pesch et al., 2015; Giriboni et al., 2020). Research in the field of chemical suppression of reproduction has already been going on for a long time and has made many advances noting species-specific differences in endocrine response (Thau et al., 1985). In animal husbandry, there is great interest in the reversible suppression of testicular function in order to a) enable mixed-sex pasture management and b) preserve breeding process. The present study aimed to determine if single implantation of the slow-release GnRH implant Suprelorin® can reduce testicular development and thereby suppress the onset of puberty in juvenile rams. Materials and MethodsAnimals, feeding, and housingFourteen rams from three different breeds (8 Merino Landschafe, 4 Bentheimer Landschafe, and 2 Scottish Blackface) were included in this study and were equally distributed between an experimental group (group 1, Suprelorin® implant) and a control group (group 2, no Suprelorin® implant). The breeds were chosen to include seasonal (Scottish Blackface and Bentheimer Landschaf) and aseasonal rams (Merino Landschaf). In analogy to the application in dogs, sheep from the experimental group were implanted with one 4.7 mg deslorelin implant (Suprelorin®, Virbac Arzneimittel GmbH, Bad Oldesloe, Germany) subcutaneously at the side of the navel at 3–5.5 months of age, i.e., before the onset of puberty in summer (June–July). The sheep were housed in a communal loose housing system measuring 5 × 10 m with water and hay freely available, while concentrate was fed once daily in pellet form. Clinical examinationThe first examination was carried out 14 days postimplantation. Ten additional examinations took place at 14-day intervals including the determination of heart and respiratory rate, internal body temperature, body size, body weight, and fecal consistency. Besides, in the experimental group, the implantation site was examined. Manual and sonographic andrological examinationThe andrological examination included the documentation of testicular parameters such as length, width, thickness (height) (using a testimeter according to Podany), and testicular consistency (by manual palpation performed by an experienced veterinarian), which was classified into five levels (level 1=soft elastic to level 5=coarse). Sonographic examination of the testes was performed using a Honda HS-1500VET ultrasound scanner (Honda Electronics Co., Ltd., Tokyo, Japan). A 50-mm multifrequency transrectal ultrasound probe (HLV-375M; 7.5 MHz) was used, following previous studies by Pugh et al. (1990) and Gouletsou et al. (2003). For better coupling of the ultrasound probe, the scrotum was sheared and shaved. Five images were obtained per testicular side (1× longitudinal section, 2× transverse section, 1× epididymal head, and 1× epididymal tail). In the longitudinal section and the two cross-sections, the region of interest (ROIs) for gray scale analysis [gray scale normal distribution, mean of gray scale distribution (Lmean), standard deviation, modal value for the most frequently found gray pixels (Nmost), and maximum value (N-all)] were analyzed. Two ROIs, each 0.25 cm² in size, were defined per image, each located between the skin line and on either side of the rete testis according to the protocol of Kauffold et al. (2011) (Fig. 1). Blood collection and determination of serum testosterone concentrationsBlood was drawn from the jugular vein every 14 days to determine the testosterone concentration. Blood was collected in serum tubes (Kabe, Nürmbrecht, Germany), centrifuged at 2,576 g for 5 minutes, and serum was frozen at −30°C until further analysis. Testosterone was measured in duplicate (including quality controls), using established radioimmunological measurement methods (Hoffmann and Landeck, 1999). The detection limit of testosterone was 0.1 ng/ml. Both intra- and interassay coefficients of variation were 7.8%–9.0%.
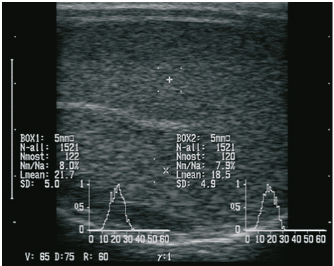
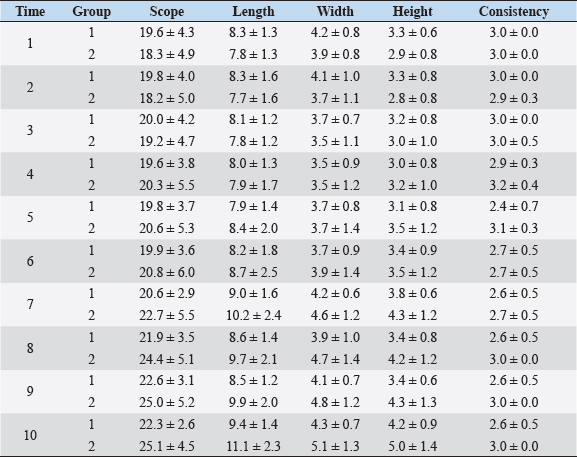
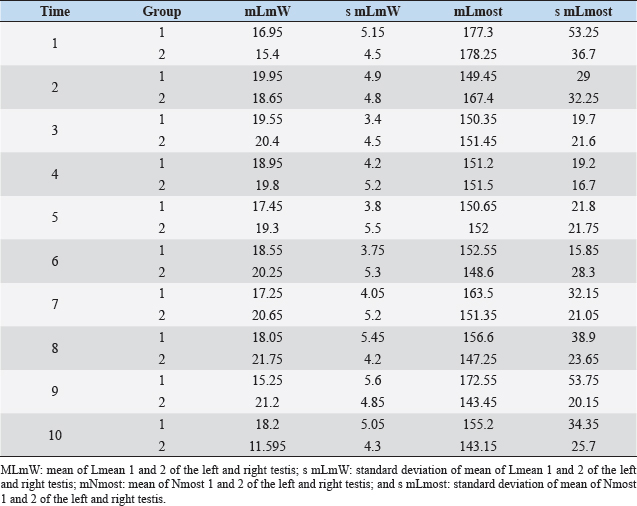
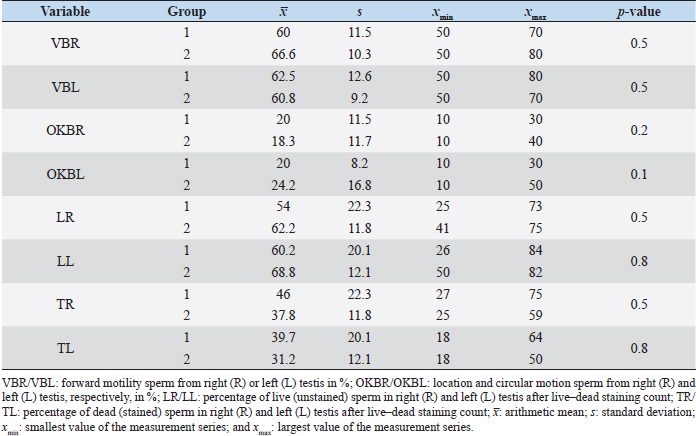
Fig. 1. Sonographic image of the testicular tissue (Honda HS- 1500 VET, 7.5 MHz, transrectal probe) presenting moderate echogenicity, and the mediastinum testis presenting as a hyperechogenic structure. The ROIs were located on both sides of the mediastinum testis with a basal area of 0.25 cm2. Surgical castration, histological and spermatological examinationAfter 140 days, 8 animals (4 rams per group) were surgically castrated according to standard procedures. The testicular tissue was examined histologically for signs of spermatogenesis. Pieces of tissue from the testis, epididymal head, body, and tail were removed with a scalpel and fixed in Formol. After embedding in paraffin, tissue sections with a thickness of 5 µm were prepared and stained with hematoxylin and eosin. Spermatogenesis is defined as the presence of all stages of spermatocytogenesis where evident spermatozoa could be seen in the lumen of the seminiferous tubules. In addition, sperm had to be detected in the epididymal tissue. Furthermore, seminal fluid was transferred from the epididymis tail to a microscope slide, and a spermatological examination was performed. Examination criteria included mass movement, single movements (forward, circular, and local movement), and the detection of dead spermatozoa after live–dead staining with eosin-nigrosin (Tanghe et al ., 2002). In the present study, 200 spermatozoa were counted and the results were quantitatively expressed as percentage. Sperm motion parameters were evaluated by computer-assisted semen analysis (CASA, AndroVision®-System, Fa. Minitube, Tiefenbach), which is described in detail by Hahn et al. (2019). Statistical analysis The qualitative traits of mass movement, testicular consistency, and the presence of spermatogenesis are presented as frequency tables generated using the program BMDP4F (Statistical Solutions Ltd., Cork, Ireland); potential differences between the two groups were evaluated using Fisher’s generalized test. Histological results were evaluated using a scoring key with “1” indicating that spermatogenesis was present in the tissue and “2” representing no spermatogenesis. The group and time influence were analyzed using a two-factor analysis of variance with measurement repetitions of the time parameter; the BMDP2V program was used for the analysis of approximately normally distributed characteristics. For comparing the semiquantitative characteristic mass movement, the Wilcoxon–Mann–Whitney exact test was applied using the program “StatXact.” Differences in the individual parameters were compared using the t-test. This was preceded in each case by Levene’s test to test the characteristics for group-dependent scatter differences, using the BMDP3D program. Ethical approvalAll experimental procedures were approved by the ethics committee of Regierungspräsidium Giessen (Regional Council of Giessen), Germany (Approval number V 54-19c 20 15 H 01 GI 18/14 No. 24/2011). All methods were carried out in accordance with relevant guidelines and regulations and in compliance with the ARRIVE guidelines. All rams belonged and were maintained and managed at the Clinic for Obstetrics, Gynecology, and Andrology of Large and Small Animals of the Justus Liebig University Giessen. ResultsThe clinical examination parameters did not differ significantly between groups (p > 0.05), but there was a highly significant effect of time (p < 0.001). Neither signs of inflammation at the implantation site could be detected, nor statistically significant group differences were found during the andrological examination (p > 0.05, Table 1), but there was a highly significant effect of time in both groups (p < 0.001). Nevertheless, the rams in the experimental group did not show uniform testicular development, with three of seven rams showing a reduced testicular development (Additional File 1). On average, the testicular diameter before surgical castration was 21.6 cm in the experimental group and 25.9 cm in the control group (Fig. 2). In the sonographic examinations, the testicular tissue was found to be homogeneous with medium echogenicity in all cases. The mediastinum testis was hyperechogenic (Fig. 1), while the epididymal tissue was clearly hypoechogenic and heterogeneous. The results of the gray scale analysis, presented in Table 2, did not show a significant effect of treatment (p > 0.05). In three of the four treated rams, sperm could be detected in the epididymis tail after castration. In the control group, no sperm could be obtained from the epididymis tail in one of the four rams. The results of the spermatological examination are given in Table 3. No significant group differences could be determined (p > 0.05). Table 1. Arithmetic mean and standard deviation of the clinical findings of the andrological examination with respect to testis circumference, length, width, and height in cm, and consistency (Con) of both testis of rams from treated group (1, n=7) and control group (2, n=7).
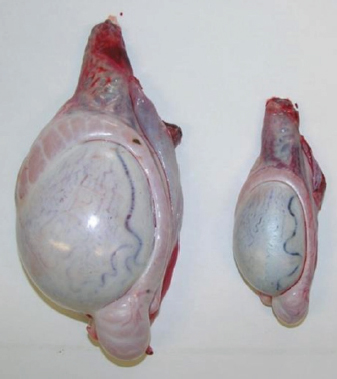
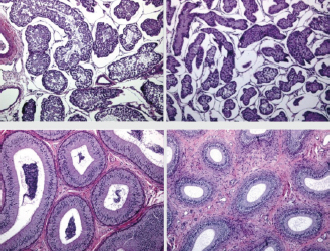
Fig. 2. Different testicular convolute sizes of a ram after treatment with deslorelin (right) and without treatment (left). Histological examination of the testicular and epididymal tissue revealed no evidence of ongoing spermatogenesis in the rams in which no sperm was collected from the epididymis (Fig. 3). In the control group, active spermatogenesis was detected in the testicular tissue of three of the four rams. Of the four treated rams, one did not show spermatogenesis, but results were not statistically significant (p > 0.05). The results of the testosterone determination did not yield significant group differences (p > 0.05, Table 4); nevertheless, some individuals of the treated group displayed testosterone concentrations above the basal level. Overall, in experimental group 1, the highest concentrations of testosterone were measured at the first examination and no further peaks were detected during the subsequent course of the trial. DiscussionIf the aim of a treatment is to achieve suppression of reproduction, then it must be absolute in order to apply it in practice. Thus, a relatively small number of 14 animals were used in this study, since data from a few animals can also indicate if reproductive suppression is possible. The seasonality of the animals was also considered; as different breeds of sheep were included in this study. Different methods were used to evaluate the effects of the implant in order not to rely solely on clinical andrological parameters. Castration followed by spermatological examination of the epididymal semen and histological examination of the testicular tissue was used as the gold standard to verify whether reproductive suppression was present. Gray scale analysis of testicular tissue was chosen because it is more sensitive than the simple B-mode method (Gärtner et al., 1998; Kauffold et al., 2011). The human eye distinguishes only 20–30 different shades of gray, whereas 256 different gray values can be differentiated using sonographic gray value analysis (Oberholzer et al., 1996; Lieu, 2010). This method has also been established for various animal species (Chandolia et al., 1997; Aravindakshan et al., 2000; Kauffold et al., 2011). Table 2. Results of quantitative gray scale analysis by sonography (Honda HS-1500 VET, 7.5 MHz, transrectal probe) with the corresponding arithmetic mean of Nmost (modal value for the most frequently found gray pixels) and Lmean (mean of gray scale distribution) values of rams from treated group (1) and control group (2).
Fig. 3. Testis and epididymal body (from top to bottom). Each from a sheep with normal (left) and completely suppressed spermatogenesis (right). H&E staining, 100× magnification. Repeated examination of clinical parameters, body weight, and implantation site revealed no adverse side effects of implantation, which is in accordance with other studies that have performed implantation in boars and queens and male rabbits (Kauffold et al., 2010; Goericke-Pesch et al., 2013, 2015). Table 3. Comparison of spermatological findings of rams from the treated group (1) and control group (2) on the 10th test date of the experimental period.
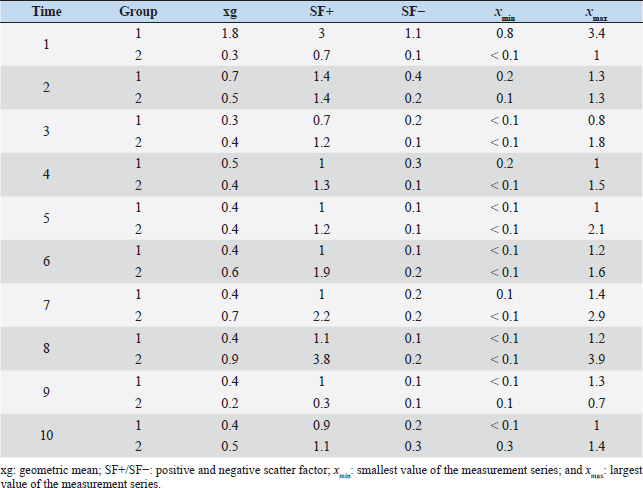
It is noteworthy that no reliable reproductive suppression was possible with the GnRH implant. Although more animals in the experimental group than in the control group showed suppressed testicular development and spermatogenesis, a 100% effect could not be achieved and additionally no statistically significant group differences could be detected. Evidence that the onset of puberty occurred during the present study can be concluded from the significant growth of testicular dimensions over time in the experimental phase. According to Belibasaki (2000), sexual maturity in rams occurs on average after 180 days. Overall, there are four studies in the literature where GnRH analogs were used to suppress reproduction in juvenile rams. The repeated implantation (with 4-week-intervals) of Zoladex (GnRH agonist: goserelin) in prepubertal 8-week-old male sheep inhibited the pubertal increase in testosterone release and the increase in scrotal volume (Robinson et al., 2014). In the study of Tilbrook et al. (1993), the efficacy of implants and mini-pumps with GnRH agonists in prepubertal rams was evaluated. The application or renewal of the mini-pump was performed every 4 weeks. At the end of the trial (16 weeks), 100% suppression of reproduction was not achieved in either group. While 9 of 10 rams in the control group had mounted and/or ejaculated when presented with estrous ewes, sexual behavior was suppressed in 7 of 10 rams treated with the implant. Lincoln et al. (1986) implanted a mini-pump and used 50 μg buserelin/animal/day for treating rams, thus achieving a reduction in testicular size in all rams. Whether there was also an abrogation of spermatogenesis was not described. This is in contrast to the results of a study by Chandolia et al. (1997), who administered a long-acting formulation of the GnRH agonist leuprolide to rams at 1.5 mg/kg intramuscularly at 3 and 7 weeks of age, and following-up with for 24 weeks. At the end of the study, semen could be obtained from all rams by electroejaculation, and the parameters of mass and single movement were normal. Furthermore, no significant differences in testicular circumference and weight development were observed between the two groups. These results agree with the present study, where the GnRH implant does not provide absolute suppression of reproduction. The observed differences between our study and some studies mentioned above may be due to the application interval and the chosen agonist. The use of an implant with a long duration of action is important for the use in practice. With respect to Suprelorin®, the recommended dosage is one implant per dog, regardless of the size of the dog. However, the application of the implant in dogs over 40 kg should be performed after benefit/risk analysis. As an example, in a Boerboel dog with a body weight of about 70 kg, the maintenance of downregulation of the hypothalamo–pituitary–gonadal axis was insufficient with Gonazon® (GnRH agonist: azagly-nafarelin) (Goericke-Pesch et al., 2010). The weight of the sheep in our study was 30–60 kg at the end of the experimental phase, so further studies with double the dosage of deslorelin may be helpful in answering the question whether the 4.7 mg preparation is underdosed for the sheep. The extent to which the release of deslorelin occurred over the entire 140-day study period cannot be conclusively determined and requires further research. Table 4. Comparison of geometric mean (xg), positive and negative scatter factor (SF+/SF-) and the largest (xmax) and smallest (xmin) values of the measurement series of testosterone concentration in ng/ml of treated group (1) and control group (2).
Further possible causes for the inconsistent effect of the Suprelorin® implant are species differences and differences in gonadal GnRH receptor number (Thau et al., 1985); for example, it has been shown that rats react more sensitively than mice, monkeys, or rabbits (Thau et al., 1985). In stallion, a reliable suppression of reproduction by GnRH agonists is also not feasible, due to a postulated species-specific conformation of the GnRH receptor (Brinsko et al., 1998). Furthermore, animals with a low pulsatile release of GnRH are thought to be less responsive to GnRH agonist treatment (Goericke-Pesch et al., 2015). In humans and dogs, gonadotropins are subject to high-frequency pulsatile release (4.5 peaks/6 hours), whereas the frequency is lower in bulls, stallions, and rabbits (4–6 peaks/24 hours). In rams, depending on the season, 3–7 pulses can be measured in 24 hours (Sanford et al., 1974), suggesting that these species may be less sensitive to GnRH agonists. At the current time, there is no conclusive explanation about the individual effect of GnRH treatment, which was also visible in the present trial. Further studies are necessary to investigate differences in the gonadotropin secretion pathways (Jiménez-Severiano et al., 2007). Conclusively, the present study evaluated the possibility of downregulating gonadal function in prepubertal rams by the implantation of a slow-release GnRH agonist implant. No statistically significant differences between the groups were found in the andrological examination. Nevertheless, the parameters of testicular examination in the experimental group suggest a varying response to the treatment. Since there were no significant group differences in the histological and spermatological examination, the singular application of the slow-release GnRH implant, including deslorelin at 4.7 mg, is not suitable for reproductive suppression in juvenile rams. Further studies with a higher dosage and different application intervals could provide information on whether the GnRH implant is indeed unsuitable in rams. In summary, it can be stated that the goal, namely the reduction of testicular development and the suppression of the onset of puberty, could not be achieved with the single implantation of the GnRH agonist deslorelin. Concretely, the negative outcome of this study could have been the result of a very low dosage or release of the GnRH implant. In few cases, a reduced testicular diameter, as well as the absence of spermatogenesis, could be observed; however, the implant cannot be used reliably in rams in the described application. Moreover, further studies could include estrous ewes to evaluate the reduction of sexual behavior. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo funding was obtained for this study. Authors’ contributionsInvestigation was carried out by LP. AW designed the methodology with the help of HW. AW was responsible for conceptualization, resources, and supervision. JJ wrote the original draft. KF performed the formal analysis of the results. All authors read and approved the final manuscript. ReferencesAdams, T.E. 2005. Using gonadotropin-releasing hormone (GnRH) and GnRH analogs to modulate testis function and enhance the productivity of domestic animals. Anim. Reprod. Sci. 88, 127–139. Aponte, P.M., Gutierrez-Reinoso, M.A., Sanchez-Cepeda, E.G. and Garcia-Herreros, M. 2018. Active immunization against GnRH in pre-pubertal domestic mammals: testicular morphometry, histopathology and endocrine responses in rabbits, guinea pigs and ram lambs. Animal 12, 784–793. Aravindakshan, J.P., Honaramooz, A., Bartlewski, P.M., Beard, A.P., Pierson, R.A. and Rawlings, N.C. 2000. Pattern of gonadotropin secretion and ultrasonographic evaluation of developmental changes in the testis of early and late maturing bull calves. Theriogenology 54, 339–354. Belibasaki, K. 2000. Sexual activity and body and testis growth in prepubertal ram lambs of Friesland, Chios, Karagouniki and Serres dairy sheep in Greece. Small Rumin. Res. 37, 109–113. Brinsko, S.P., Squires, E.L., Pickett, B.W. and Nett, T.M. 1998. Gonadal and pituitary responsiveness of stallions is not down-regulated by prolonged pulsatile administration of GnRH. J. Androl. 19, 100–109. Chandolia, R.K., Bartlewski, P.M., Omeke, B.C., Beard, A.P., Rawlings, N.C. and Pierson, R.A. 1997. Ultrasonography of the developing reproductive tract in ram lambs: effects of a GnRH agonist. Theriogenology 48, 99–117. Driancourt, M.A. and Briggs, J.R. 2020. Gonadotropin-releasing hormone (GnRH) agonist implants for male dog fertility suppression: a review of mode of action, efficacy, safety, and uses. Front. Vet. Sci. 7, 483. Edwards, B., Smith, A. and Skinner, D.C. 2013. Dose and durational effects of the gonadotropin-releasing hormone agonist, deslorelin: the male rat (Rattus norvegicus) as a model. J. Zoo. Wildl. Med. 44, S97–S101. Fontaine, C. 2015. Long-term contraception in a small implant: a review of Suprelorin (deslorelin) studies in cats. J. Feline Med. Surg. 17, 766–771. Fraser, H.M. and Lincoln, G.A. 1980. Effects of chronic treatment with an LHRH agonist on the secretion of LH, FSH and testosterone in the ram. Biol. Reprod. 22, 269–276. Gärtner, T., Zacharias, M., Jenderka, K.V., Heynemann, H. and Cobet, U. 1998. Equipment-independent ultrasound tissue characterization of testis and prostate. Radiologe 38, 424–433. Giriboni, J., Lacuesta, L., Santiago-Moreno, J. and Ungerfeld, R. 2020. Chronic use of a GnRH agonist (deslorelin) or immunization against GnRH: effects on testicular function and sperm quality of bucks. Domest. Anim. Endocrinol. 71, 106395. Godfrey, S.I., Walkden-Brown, S.W., Martin, G.B. and Speijers, E.J. 1996. Immunisation of goat bucks against GnRH to prevent seasonal reproductive and agonistic behaviour. Anim. Reprod. Sci. 44, 41–54. Goericke-Pesch, S., Georgiev, P., Antonov, A., Vodenicharov, A., Navarro, C. and Wehrend, A. 2014. Reversibility of germinative and endocrine testicular function after long-term contraception with a GnRH-agonist implant in the tom-a follow-up study. Theriogenology 81, 941–946. Goericke-Pesch, S., Georgiev, P., Atanasov, A., Albouy, M., Navarro, C. and Wehrend, A. 2013. Treatment of queens in estrus and after estrus with a GnRH-agonist implant containing 4.7 mg deslorelin; hormonal response, duration of efficacy, and reversibility. Theriogenology 79, 640–646. Goericke-Pesch, S., Groeger, G. and Wehrend, A. 2015. The effects of a slow release GnRH agonist implant on male rabbits. Anim. Reprod. Sci. 152, 83–89. Goericke-Pesch, S., Wilhelm, E., Ludwig, C., Desmoulins, P.O., Driancourt, M.A. and Hoffmann, B. 2010. Evaluation of the clinical efficacy of Gonazon implants in the treatment of reproductive pathologies, behavioral problems, and suppression of reproductive function in the male dog. Theriogenology 73, 920–926. Gökdal, O., Atay, O., Ulker, H., Kayaardi, S., Kanter, M., DeAvila, M.D. and Reeves, J.J. 2010. The effects of immunological castration against GnRH with recombinant OL protein (Ovalbumin-LHRH-7) on carcass and meat quality characteristics, histological appearance of testes and pituitary gland in Kıvırcık male lambs. Meat Sci. 86, 692–698. Gouletsou, P.G., Amiridis, G.S., Cripps, P.J., Lainas, T., Deligiannis, K., Saratsis, P. and Fthenakis, G.C. 2003. Ultrasonographic appearance of clinically healthy testicles and epididymides of rams. Theriogenology 59, 1959–1972. Hahn, K., Failing, K. and Wehrend, A. 2019. Effect of temperature and time after collection of buck sperm quality. BMC Vet. Res. 15, 355. Han, X., Gu, L., Xia, C., Feng, J., Cao, X., Du, X., Zeng, X. and Song, T. 2015. Effect of immunization against GnRH on hypothalamic and testicular function in rams. Theriogenology 83, 642–649. Herbert, C.A., Eckery, D.C., Trigg, T.E. and Cooper, D.W. 2007. Chronic treatment of male tammar wallabies with deslorelin implants during pouch life: effects on development, puberty, and reproduction in adulthood. Biol. Reprod. 76, 1054–1061. Hoffmann, B. and Landeck, A. 1999. Testicular endocrine function, seasonality and semen quality of the stallion. Anim. Reprod. Sci. 57, 89–98. Jeffcoate, I.A., Lucas, J.M. and Crighton, D.B. 1982. Effects of active immunization of ram lambs and bull calves against synthetic luteinizing hormone releasing hormone. Theriogenology 18, 65–77. Jiménez-Severiano, H., D’Occhio, M.J., Lunstra, D.D., Mussard, M.L., Davis, T.L., Enright, W.J. and Kinder, J.E. 2007. Comparative response of rams and bulls to long-term treatment with gonadotropin-releasing hormone analogs. Anim. Reprod. Sci. 98, 204–224. Kauffold, J., Kessler, M., Richter, A., Beynon, N. and Wehrend, A. 2011. B-mode ultrasound and grey-scale analysis of the epididymis in boars, and the relationship to semen parameters. Reprod. Domest. Anim. 46, 108–113. Kauffold, J., Rohrmann, H., Boehm, J. and Wehrend, A. 2010. Effects of long-term treatment with the GnrH agonist deslorelin (Suprelorin) on sexual function in boars. Theriogenology 74, 733–740. Kiyma, Z., Adams, T.E., Hess, B.W., Riley, M.L., Murdoch, W.J. and Moss, G.E. 2000. Gonadal function, sexual behavior, feedlot performance, and carcass traits of ram lambs actively immunized against GnRH. J. Anim. Sci. 78, 2237–2243. Lieu, D. 2010. Ultrasound physics and instrumentation for pathologists. Arch. Pathol. Lab. Med. 134, 1541–1556. Lincoln, G.A., Fraser, H.M. and Abbott, M.P. 1986. Blockade of pulsatile LH, FSH and testosterone secretion in rams by constant infusion of an LHRH agonist. J. Reprod. Fertil. 77, 587–597. Mihsler, L., Wagner, H. and Wehrend, A. 2016. Suppression of sexual activity and reproduction in male small ruminants. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 44, 171–178. Mihsler-Kirsch, L., Wagner, H., Failing, K. and Wehrend, A. 2021. Downregulation of testicular function in the goat by altrenogest. BMC Vet. Res. 17, 183. Needham, T., Lambrechts, H. and Hoffman, L.C. 2019a. Extending the interval between second vaccination and slaughter: I. Effects on growth, scrotal size and stress responses of immunocastrated ram lambs. Animal 13, 1952–1961. Needham, T., Lambrechts, H. and Hoffman, L.C. 2019b. Extending the interval between second vaccination and slaughter: II. Changes in the reproductive capacity of immunocastrated ram lambs. Animal 13, 1962–1971. Oberholzer, M., Ostreicher, M., Christen, H. and Brühlmann, M. 1996. Methods in quantitative image analysis. Histochem. Cell. Biol. 105, 333–355. Pugh, C.R., Konde, L.J. and Park, R.D. 1990. Testicular ultrasound in the normal dog. Vet. Radiol. 31, 195–199. Robinson, J.E., Evans, N.P., Dumbell, R., Solbakk, A.-K., Ropstad, E. and Haraldsen, I.R.H. 2014. Effects of inhibition of gonadotropin releasing hormone secretion on the response to novel objects in young male and female sheep. Psychoneuroendocrinology 40, 130–139. Rocha, L.F., Souza, R.S., Santana, A.L.A., Macedo, D.S., Santana, A.M.S., da Silva, R.C., Bezerra, P.A., de Jesus, R.D.L. and Barbosa, L.P. 2021. Reproductive parameters of lambs immunocastrated with anti-GnRH vaccine. Anim. Reprod. 18, e20200237. Sanford, L.M., Winter, J.S., Palmer, W.M. and Howland, B.E. 1974. The profile of LH and testosterone secretion in the ram. Endocrinol. 95, 627–631. Tanghe, S., van Soom, A., Sterckx, V., Maes, D. and Kruif, A. 2002. Assessment of different sperm quality parameters to predict in vitro fertility of bulls. Reprod. Domest. Anim. 37, 127–132. Thau, R.B., Limonta, P., Schmidt, F. and Sundaram, K. 1985. Species differences in the sensitivity to GnRH analogs. J. Steroid Biochem. 23, 811–817. Thompson, D.L. 2000. Immunization against GnRH in male species (comparative aspects). Anim. Reprod. Sci. 60–61, 459–469. Tilbrook, A.J., Galloway, D.B., Williams, A.H. and Clarke, I.J. 1993. Treatment of young rams with an agonist of GnRH delays reproductive development. Horm. Behav. 27, 5–28. Ulker, H., Kanter, M., Gökdal, O., Aygün, T., Karakuş, F., Sakarya, M.E., deAvila, D.M. and Reeves, J.J. 2005. Testicular development, ultrasonographic and histological appearance of the testis in ram lambs immunized against recombinant LHRH fusion proteins. Anim. Reprod. Sci. 86, 205–219. Vinke, C.M., van Deijk, R., Houx, B.B. and Schoemaker, N.J. 2008. The effects of surgical and chemical castration on intermale aggression, sexual behaviour and play behaviour in the male ferret (Mustela putorius furo). Appl. Anim. Behav. Sci. 115, 104–121. | ||
| How to Cite this Article |
| Pubmed Style LP, Joerling J, Failing K, Wagner H, Wehrend A, . Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. Open Vet J. 2022; 12(2): 171-181. doi:10.5455/OVJ.2022.v12.i2.3 Web Style LP, Joerling J, Failing K, Wagner H, Wehrend A, . Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. https://www.openveterinaryjournal.com/?mno=132613 [Access: April 19, 2024]. doi:10.5455/OVJ.2022.v12.i2.3 AMA (American Medical Association) Style LP, Joerling J, Failing K, Wagner H, Wehrend A, . Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. Open Vet J. 2022; 12(2): 171-181. doi:10.5455/OVJ.2022.v12.i2.3 Vancouver/ICMJE Style LP, Joerling J, Failing K, Wagner H, Wehrend A, . Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. Open Vet J. (2022), [cited April 19, 2024]; 12(2): 171-181. doi:10.5455/OVJ.2022.v12.i2.3 Harvard Style , L. P., Joerling, J., Failing, K., Wagner, H., Wehrend, A. & (2022) Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. Open Vet J, 12 (2), 171-181. doi:10.5455/OVJ.2022.v12.i2.3 Turabian Style , Luise Prestel, Jessica Joerling, Klaus Failing, Henrik Wagner, Axel Wehrend, and . 2022. Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. Open Veterinary Journal, 12 (2), 171-181. doi:10.5455/OVJ.2022.v12.i2.3 Chicago Style , Luise Prestel, Jessica Joerling, Klaus Failing, Henrik Wagner, Axel Wehrend, and . "Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant." Open Veterinary Journal 12 (2022), 171-181. doi:10.5455/OVJ.2022.v12.i2.3 MLA (The Modern Language Association) Style , Luise Prestel, Jessica Joerling, Klaus Failing, Henrik Wagner, Axel Wehrend, and . "Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant." Open Veterinary Journal 12.2 (2022), 171-181. Print. doi:10.5455/OVJ.2022.v12.i2.3 APA (American Psychological Association) Style , L. P., Joerling, J., Failing, K., Wagner, H., Wehrend, A. & (2022) Suppression of reproductive function in juvenile rams by a slow-release gonadotropin-releasing hormone implant. Open Veterinary Journal, 12 (2), 171-181. doi:10.5455/OVJ.2022.v12.i2.3 |