| Review Article | ||
Open Vet J. 2022; 12(6): 1018-1026 Open Veterinary Journal, (2022), Vol. 12(6): 1018–1026 Review Article Innovative modern bio-preservation module of meat by lytic bacteriophages against emergent contaminantsManal Hadi Ghaffoori Kanaan1* and Ahmad M. Tarek21Department of Agriculture, Technical Institute of Suwaria, Middle Technical University, Baghdad, Iraq 2Department of Crime Evidence, Institute of Medical Technology Al-Mansour, Middle Technical University, Baghdad, Iraq Submitted: 06/10/2022 Accepted: 20/11/2022 Published: 20/12/2022 *Corresponding Author: Manal Hadi Ghaffoori Kanaan. Department of Agriculture, Technical Institute of Suwaria, Middle Technical University, Baghdad, Iraq. Email: manalhadi73 [at] yahoo.com © 2022 Open Veterinary Journal
AbstractMeat is a perishable product that has a short shelf life and can be ruined easily if the proper preservation measures are not employed. It is difficult to control all potential sources of microbial contamination due to the complexity of the habitats present during the pre-harvest, harvest, and post-harvest stages of the food supply chain. This is due to the fact that contamination can occur at any stage. As a consequence of this, the food industry is perpetually at risk of being tainted by microorganisms, notwithstanding the progress that has been made in contemporary technology. Antibiotic usage has exacerbated the problem, leading to the emergence of infections transmitted by antibiotic-resistant foods. It’s critical to work on novel ways to reduce microbial contamination in meat and in the meat processing environment. Therefore, to assure the wholesomeness of the finished product, several control procedures must be adopted throughout the food manufacturing and processing chain. Because of this, bacteriophages and the derivatives of these viruses have arisen as an innovative, significant, and risk-free option for the prevention, treatment, and/or elimination of such pollutants in a variety of foodstuff handling environments. So, the focus of this review was on the future potential of integrated phage, modified phage, and their derivatives as antimicrobials in the traditional farm-to-table setting, which encompasses areas like primary production, post-harvest processing, bio-sanitation, and bio-detection. In addition to presenting certain safety concerns. Also, this paper discusses how to assure the safe and successful use of bacteriophages in the future. Keywords: Bacterial food poisoning, Biocontrol, Meat, Phage. IntroductionFoodborne sickness (also known as food poisoning) refers to any ailment brought on by eating something that is tainted in some way, be it with pathogenic bacteria, virus, or parasite that infects food, or with a chemical or natural toxin like lethal mushrooms. Most cases of food poisoning can be traced back to mistakes made in the way meat was prepared, cooked, or stored (Kanaan and Abdullah, 2019). Furthermore, because meat contains nearly all of the needed nutritional components, it can be a good source and protective medium for certain bacteria, including possible infections that can cause a variety of health problems in consumers (Kanaan and Abdullah, 2019). As a result, meat may not only serve as a possible vehicle for disease transmission, but it may also allow infections to proliferate, reproduce, and develop specific harmful metabolites, making it an exceptionally sensitive commodity from the standpoint of public health (FDA, 2015; CDC, 2018). Various pathogenic organisms can enter meat and meat products from a variety of sources, causing a variety of foodborne diseases. Meat and meat products may transfer organisms or their poisonous metabolites (poisons) known as toxins to customers who are vulnerable (Kanaan and Al-Isawi, 2019). Poisoning syndromes are caused by ingesting toxins that have already been created in the food, i.e., pre-formed toxins (Kanaan and Abdullah, 2019). According to the Centers for Disease Control and Prevention (CDCs), 48 million cases of foodborne disease occur in the United States each year (CDC, 2021). At least 128,000 Americans have been admitted to hospitals, and 3,000 have died as a result of tainted food. While most foodborne illness cases go unreported to health departments, the CDC estimates that 31 known foodborne pathogens cause 9.4 million illnesses, with eight pathogens accounting for 90% of all illnesses due to known pathogens: Salmonella, Staphylococcus aureus, norovirus, Campylobacter, Toxoplasma, Escherichia coli O157:H7, Listeria, and Clostridium perfringens (CDC, 2018). The widespread recognition of meat’s health benefits may account for its widespread consumption. Meat has an “excellent” protein profile since it provides the body with every important amino acid. It has also been proven that plant sources cannot replace the protein as well as vitamins (particularly A and B12) found in flesh, demonstrating the latter’s nutritional relevance. During the slaughter, preparation, and preservation of meat, several biochemical changes (meat deterioration) and microorganisms (food illness) are linked with it (Olaoye, 2011). Meat deteriorates gradually from the time of slaughter till consumption due to its unique biological and chemical properties (Olaoye, 2011). Food preservation is a never-ending battle aimed at reducing or eliminating the risk of rotting and pathogenic bacteria in foods. Until now, methods for improving food safety have focused on the use of chemical preservatives, antibiotics, or more extreme physical treatments (e.g., high temperatures or refrigeration). These approaches, however, have a number of disadvantages. Due to the impairment/reduction of food nutritional content, episodes of unpleasant food responses, cardio-vascular illness, various carcinogenic and teratogenic qualities, as well as residual toxicity, there is currently a major debate concerning the safety aspects of chemical preservatives (Kanaan, 2019). Food’s organoleptic, nutritional, and physicochemical characteristics are all severely harmed by high-temperature processing. Refrigerators are either prohibitively expensive to maintain or lack the necessary infrastructure (electricity), making meat products vulnerable to microbiological and other causes of contamination (Singh, 2018). Consumers nowadays pay a lot of attention to the relationship between food and health for these reasons, especially as a result of public suspicion of chemical preservatives (Amin, 2012). The current review could be very beneficial as a source of information in many nations, particularly in developing countries, where meat production requires a higher level of sanitation and good manufacturing procedures, so this review focuses on understanding the impact of phage therapy on public health and bio-preservation of food, particularly meat and animal products (FAO, 1992). Individual education about food dangers and safety to protect customers from foodborne illnesses and toxins, as well as satisfaction with modern food net programs around the world in providing safe and affordable protected foods to all consumers. Meat consumptionAs a result of the global population increase, the demand for food sources is constantly increasing, especially animal sources because of their great nutritional importance to humans, where meat is one of the most important of these sources that demand is constantly increasing globally. Meat is well-known for its relevance in our diet since it is regarded as the best, optimal, and complete food. Despite that, in specific situations, meat can act as a possible route for the spread of certain diseases (Kanaan and Khashan, 2018; Kanaan, 2018, 2021; Kanaan and Abdulwahid, 2019; Kanaan and Mohammed, 2020; Kanaan et al., 2020, 2022; Kanaan and Tarek, 2020). Although still in their infancy in certain countries, processed meat products are growing in popularity and volume around the world (Heinz and Hautzinger, 2007; Lásztity, 2015). Many people rely on meat as their primary source of sustenance from animals, making it the most profitable product that cattle can produce. Meat can be eaten in one of two ways: either as a component of meals that are prepared at home or as processed meat products. The Food and Agriculture Organization predicts that by 2030, the average annual per capita intake of meat in developing countries will have risen from the low of 10 kg in the 1960s to 26 kg in 2000. This projection assumes that developing nations’ meat consumption will eventually equal that of developed nations within the next few decades. Meat consumption in developed nations has been largely steady (Heinz and Hautzinger, 2007). Burden of foodborne illnessesFood safety has been as a first priority, and reducing foodborne diseases is a vital goal for customers’ protection. Food contamination by bacteria or their toxins can occur in a variety of ways and at various places in the food chain, for example (WHO, 2007-2015; Annual Report of the Chief Scientist, 2013):
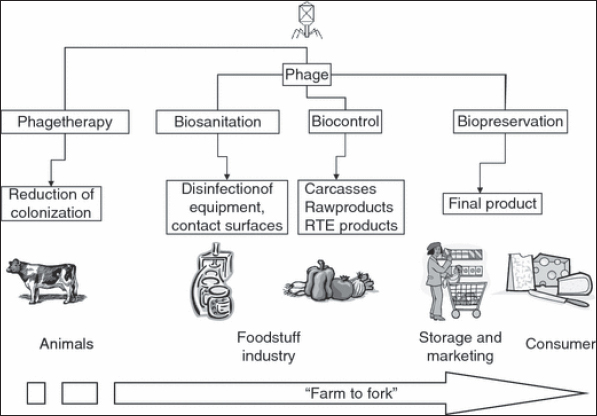
Food, especially meat, is often associated with disease outbreaks. When two or more persons, from more than one family, or residents of an institution, contract the same disease as a result of eating contaminated food, and the food was produced from the same contaminated source or became contaminated in the same way, it was called a foodborne outbreak. About 81% of the outbreaks reported in 2010 were linked to food, according to the public health groups involved. Poultry meat (29%), crustacean/shellfish (21%), and red meat were the most commonly detected foods in outbreaks (14%). According to pathogens, the most prevalent foods involved with foodborne outbreaks in 2010 were: Campylobacter is found in 80% of poultry meat, Norovirus is found in 100% of crustaceans and shellfish, and Campylobacter is found in 20% of red meat (20%) (CDC, 2015). Food bio-preservationBio-preservation is a food preservation technique that takes advantage of the antibacterial properties of naturally surviving beings as well as their metabolites. It can harmonize and rationalize essential safety standards with traditional preservation methods as well as modern food safety and quality needs (Singh, 2018). Meat is a protein-gorgeous, nutrient-dense foodstuff that is extremely consumable and has a short shelf life except it is preserved. Pathogenic germs that were present in the animal before it was slaughtered have a good chance of contaminating the meat. As a consequence of this, it is of the utmost importance to guarantee the stability, transportation, and storage of meat that is fit for human consumption. A number of interconnected factors, including holding temperature, influence the shelf-life and preservation of meat quality, resulting in observable variations in meat quality parameters. Spoilage caused by microbial development is the most critical element in maintaining meat quality (Salem, 2012; Kamarudheen et al., 2014; Nath et al., 2014). The effectiveness of biological antimicrobial structures like lactic acid bacteria (LAB) and/or their bacteriocins, bacteriophages, and bacteriophage-encoded enzymes is crucial to the bio-preservation procedures used for a wide variety of foods. They are utilized extensively in the food preparation industry to impart specific aromas, tastes, and textures to a wide range of items (Singh, 2018). Efforts to ensure the safety of food have, thus far, focused on either creating more potent chemical preservatives or subjecting food to more severe physical treatments (high temperatures). However, many disadvantages exist, comprising the known toxicity of numerous element preservatives (nitrites), the modification of organoleptic and nutritional possessions of foodstuffs, and especially recent purchaser purchasing and consumption styles, which demand safe but negligibly processed foodstuffs free of additives. Bio-preservation is gaining popularity as a natural way to extend the shelf life and ensure the safety of food goods. Bioprotective cultures can be employed to help ensure hygienic quality, but they should never replace rigorous attention to hygiene during production, processing, storage, and distribution (Salem, 2012; Kamarudheen et al., 2014; Nath et al., 2014). To be safe for human consumption, bio-preservatives must be generally recognized as safe and have no pathogenic or deleterious effects on food. There are two main categories of biological agents used in the food industry: starter cultures and protective cultures. Starter cultures are a type of microbe that is used to kick-start fermentation and aid in the creation of specific chemicals that give fermented foods their distinctive texture and flavor. Defensive probiotics is primarily utilized to manage antibacterial properties and minimize harmful microorganism endurance and growth in foods (Singh, 2018). One of the most common methods of food bio-preservation is fermentation, which involves the microbial development of foods. The most common type of these bacteria are LAB, and their byproducts include organic acids and other compounds that provide a unique flavor to food while also inhibiting the growth of harmful bacteria. Traditionally, natural fermentation processes have safeguarded a wide range of foods from spoilage. Fermented foods are becoming more common (60% of the diet in developed countries), and they are made by intentionally applying distinct microbial systems (starter/protective cultures) to raw foods to ensure homogeneity, quality, and safety. In addition, because traditional fermented foods have improved organoleptic qualities, a great deal of research has been conducted on the microbial biodiversity of these foods with the intention of recreating these characteristics, which are credited to the local microbiota, in an environment that is under stricter control (Muhialdin et al., 2022). Application of bacteriophages in the food industryDespite current technological advancements, the food business is constantly confronted with the potential of microbial contamination. Antibiotic abuse has exacerbated the situation, leading to an increase in the growth of antibiotic-resistant foodborne bacteria. It’s critical to work on novel strategies for preventing microbial contamination in food and the food processing environment. As a result, bacteriophage and derivatives have arisen as unique, sustainable, and nontoxic options for avoiding, treating, and/or eliminating these pollutants in an extensive variety of foodstuffs and food processing conditions. The existing and potential uses of complete phages, altered phages, and their products as antimicrobials in the outdated farmhouse-to-fork setting are investigated, including primary production, postharvest processing, bio sanitation, and bio-detection. The assessment also raises several safety issues in order to ensure that bacteriophages are used safely and effectively in the future (Ahmed et al., 2012; Brovko et al., 2012; Sillankorva et al., 2012; Chibeu, 2013; Endersen et al., 2021). Bacteriophages, often known as phages, are the most common germs on the planet and are widely distributed on foods of various origins (Hoyle and Kutter, 2021). These microorganisms have a well-known natural bio-therapeutic potential all around the world. In 2006, the authorization of the earliest phage-based product (ListShieldTM) to control L. monocytogenes in meat and poultry products was a watershed moment in Western phage history (Bren, 2007). Other phage products have recently received FDA approval for use as food biotherapeutics (Jassim and Limoges, 2014). The potential for phage biocontrol to manage other food diseases and spoilage organisms is highlighted by these advances in phage biocontrol, as well as confirming that the food business accepts the use of phages (Ahmed et al., 2012; Brovko et al., 2012; Sillankorva et al., 2012; Chibeu, 2013; Endersen et al., 2021). Selection of a proper phage in the food industryPhages intended for use in food must meet a number of criteria. Biocontrol applications should only look into highly lytic phages. The security of the phage usage should be thoroughly tested, and their host variety should insure all clinically related strains of the target bacterium. It is important to determine if their DNA contains genes that code for virulence factors like toxins and/or lysogenic features (Hagens and Loessner, 2010; Mahony et al., 2011). To completely assess the phage’s relevance in food systems as a biocontrol agent, the complete genomic sequence of the phage should be known. As a result, for biocontrol goals, only phages with low transduction frequencies should be employed. Furthermore, selected phages should infect a huge number of the board species and/or genera to have a broad host range (Hagens and Loessner, 2010). In general, host range criteria should be evaluated based on the intended use and desired result. Another crucial feature that should be defined in the chosen phage is its stability under various storage and application settings. Indeed, testing phages for endurance in the proposed-usage environment is critical, which necessitates capitalizing possessions in phage description (Gill and Hyman, 2010). On behalf of phages that are evaluated for biocontrol of pathogenic bacteria in foodstuff, the capacity to proliferate in surrogate nonpathogenic hosts for large-scale commercial production is critical from an economic standpoint (Hagens and Loessner, 2010). Bacteriophages as a biocontrol interventionBacteriophages in livestock farmingPrimary metabolites including amino acids, fatty acids, carbohydrates, and organic acids all depend on antibodies to function properly (Tian et al., 2018). Antibiotic resistance has led to the use of natural alternatives to prevent bacteria from sticking and proliferating, such as plant extracts, essential oils, bactericides, and isolated compounds (Budri et al., 2015). Even before the discovery of antibiotics, bacteriophages (phages), viruses that infect bacteria, were utilized and provided as therapeutic agents and are experiencing a revival. The bacteriophage is the most common and widespread type of virus, and it can be found in both natural and manufactured environments that are conducive to the growth of its bacterial host (de Melo et al., 2018). Phages, in contrast to traditional antibiotics, are highly selective antibacterial agents that have a negligible impact on the animal’s otherwise healthy microbial ecology (Petrovic Fabijan et al., 2020). Given the alarming rise in antimicrobial resistance around the world, phage therapy’s potential to serve as an antibiotic alternative in the treatment of a wide variety of bacterial diseases cannot be overstated (Nagel et al., 2016). Additionally, phages have been utilized to lessen the bacterial burden during the treatment of bacterial infections in animals (Adebayo et al., 2017). The time it takes for a phage particle to connect to a bacterial cell and then release other phage particles varies from 20 minutes to 12 hours (Żbikowska et al., 2020). The versatility of phages stems from their short half-lives, which have led to their employment in fields as diverse as disease control and pre-slaughter animal treatment (García et al., 2008). Bacteriophages in food industryThere is a lot of potentials for phages to be used as a natural antibacterial throughout the pre- and post-harvest stages of foodstuff production to combat food pathogens and spoilage organisms. Reduced microbial contamination in processed foods is crucial because both pathogenic and spoilage organisms have negative effects on the economy. Bacteriophages can be used from beginning to end in the food production process, including in animal decontamination, cleanliness of agricultural and industrial equipment and contact surfaces, biocontrol in raw meats and fresh produce, and even as natural preservatives in foods to lengthen their shelf life (Fig. 1). Phage biocontrol in food utilizing whole phages or their derivatives has been established in a number of experiments, with generally positive outcomes. Pre-harvest (in farm animals) and post-harvest (in meat, fresh, and packaged foods) studies have also been conducted to control for a variety of important and incipient foodborne pathogenic bacteria such as Salmonella, Listeria, Campylobacter, and E. coli (Atterbury et al., 2003; Goode et al., 2003; García et al., 2008; Tan et al., 2014). Bacteriophages in meat industryPhages have been utilized to manage Campylobacter jejuni contagion on the superficial layer of chicken skin as part of the investigation on phage biocontrol in the meat industry. When applied to C. jejuni, phages caused a 1-1.3 log drop in population size after just 24 hours. Together, phage therapy and 20°C freezing of chicken skin increased the effectiveness of biocontrol. Salmonella Enteritidis on chicken skin was reduced with the use of a bacteriophage cocktail of chemical agents, as demonstrated by the work of Hungaro et al. (2013). The number of S. Enteritidis CFU/cm2 was reduced by 1 log in both treatments. They concluded that in an industrial context, bacteriophages may be employed as an alternative bio-sanitizer for this infection on poultry carcasses. Hooton et al. (2011) tested anti-Salmonella phages on pig skin as well and found when administered to the skin at a diversity of contagion of 10 or higher that a phage mixture against S. Typhimurium reduces cell counts to undetectable levels. Those findings back up the use of phage to prevent Salmonella contagion of pig coating after slaughter. O’flynn et al. (2004) looked into using a three-phage combination to lower E. coli O157:H7 in cattle. They used 103 colony-forming units (CFU) of rifampicin-resistant E. coli O157:H7 to inoculate 18 cattle chops. Nine items were inoculated with the virus. All of the samples were incubated for an initial hour, then enhanced in brain-heart infusion broth for an additional 2 hours. On seven of the nine phage-cured samples, there were no measurable amounts of E. coli. Both positive samples had counts of less than 10 CFU/ml, while the control samples had counts of above 105 CFU/ml. Anany et al. (2011) showed that the use of phages immobilized on a modified cellulose membrane could eliminate L. monocytogenes and E. coli O157:H7 in both cooked and uncooked meat. The researchers found that the powerless phage mixture was effective at reducing titers by more than one log for both organisms across a wide temperature range. The heads of the phages (with a net negative charge) stick to the positively charged cellulose membranes, while the tails are free to seek out and destroy any invading microbes. The investigators believed that this method, which involved depositing the phage directly on the flesh’s surface, would help limit phage waste while simultaneously maximizing its potential.
Fig. 1. Phage application from farm to fork (García et al., 2008). Enzymes derived from phages as potential biocidal agents in food industryPhage-derivative enzymes have been studied for their potential as biocontrol agents in the food sector, and while this study has lagged behind that of their usage in human and veterinary medicine, it is garnering scholarly interest (Brovko et al., 2012; Chibeu et al., 2013; Chibeu and Balamurugan, 2019). In the final stage of their lytic life cycle, bacteriophages produce enzymes called endolysins (lysine) that degrade peptidoglycans (Young, 1992). Most effectively, they induce bacteriolysis in Gram-positive bacteria when applied exogenously (Heineman and Bull, 2007). Decontamination of food and controlling biofilm formation by phage therapyThe formation of biofilms poses a serious threat to the food industry since any product that comes into contact with these biofilms runs the risk of becoming contaminated and perhaps causing food poisoning or the spread of disease. The formation of biofilm allows germs to persist in the food environment for extended periods of time and to withstand antibacterial and sanitizing treatments (Folsom et al., 2006). It was found that a 3-log cycle drop in cell number may be achieved with phage treatment alone. During the same study, the phages’ resistance to inactivation by a quaternary ammonium compound (QUATAL) used for cleaning was also investigated, and it was shown that the phages were resistant to quantities of QUATAL up to 50 ppm. A 5-log reduction in Listeria attached to the surface was achieved using a combination of phage and 40 ppm QUATAL. Hibma et al. (1997) isolated a phage that was specific for Listeria L-forms, which have a defective or nonexistent cell wall structure, and used it to limit biofilm development by these bacteria. In other investigations, Listeria phage P100 was able to reduce L. monocytogenes biofilm formation on stainless steel surfaces by 5.29 log CFU/cm2 (Montañez-Izquierdo et al., 2012). Much promising research on the use of phages to suppress the production of biofilms by bacteria such as Pseudomonas spp., C. jejuni, and Staphylococcus epidermidis have been published (Brovko et al., 2012). On several food-processing surfaces, such as stainless steel and ceramic tiles, the phage combination BEC8 was explored to inhibit the growth of Enterohemorrhagic E. coli O157:H7 (Viazis et al., 2011). Following BEC8 phage combination treatment for 10 minutes at 37°C or 1 hour at 23°C, growth on both surfaces was below the detection limit. On hard and porous surfaces polluted with Enterococci, the Enterococcus faecalis-specific lytic phage jSUT1 resulted in a considerable reduction in the bacterial cell population (McLean et al., 2010). Future perspective of phage therapy in the foodstuff productionThe irrefutable antimicrobial possessions of phages, combined with decreasing antibiotic efficacy, and demand for pathogen-free and synthetic-chemical-free foods, has prompted a slew of businesses to participate in phage-based yields. With the invention of antibiotics, phage therapy went out of favor. It’s making a comeback, thanks to growing evidence of its worth as a natural, actual biotherapeutic as well as to combat antimicrobial resistance as this phenomenon creates a huge concern to global public health (Kanaan and Abdullah, 2021; Kanaan and Khashan, 2022; Mohammed et al., 2022). This is due to a better understanding of phage biology as well as a better awareness of their technical limits. For purposes of biocontrol, decontamination, sanitation, and detection, a rising number of food industry organizations are investing in the study and creation of products based on phages. Phages and similar variations have great potential as biopreservatives to combat foodstuff deteriorating organisms, as cleaning means on farms and in industrial settings, and as bio-recognition devices (biosensors) for detecting hazardous infections in food. An idea to reduce the financial burden of microbial pollution in foodstuffs and food handling surroundings is to investigate the commercial exploitation of bacteriophages. It’s crucial to note that phages aren’t a “magic bullet,” and they’re unlikely to take the place of chemical preservatives and cleaners. In combination with existing technologies or other natural antimicrobial agents, such as bacteriocins, phage use should be measured as one of the approaches in the so-called hurdle technology to improve food safety (Abedon et al., 2017; Fernández et al., 2018; Pires et al., 2020). ConclusionSince the use of chemical preservatives is becoming limited, biological control approaches, such as bio preservatives and biological control of bacteriophages, appear to be a potential alternative tool for controlling food contamination. The detailed knowledge of bacteriophage biology will influence the next step toward the production of extensive commercial applications, allowing consumers to feel secure about the safety of “edible viruses.” Authors’ contributionMHGK organized, wrote, and reviewed the manuscript in addition to gathering the data. The manuscript was edited by AMT. The final manuscript was read and approved by all writers. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbedon, S.T., García, P., Mullany, P. and Aminov, R. 2017. Phage therapy: past, present and future. Front. Microbiol. 8, 981. Adebayo, O.S., Gabriel-Ajobiewe, R.A.O.G., Taiwo, M.O. and Kayode, J.S. 2017. Phage therapy: a potential alternative in the treatment of multi-drug resistant bacterial infections. J. Microbiol. Exp. 5(5), 00173. Ahmed, K., Kaderbhai, N.N. and Kaderbhai, M.A. 2012. Bacteriophage therapy revisited. Afr. J. Microbiol. Res. 6(14), 3366–3379. Amin, R.A. 2012. Effect of bio preservation as a modern technology on quality aspects and microbial safety of minced beef. Glob. J. Biotechnol. Biochem. Res. 7(2), 38–49. Anany, H., Chen, W., Pelton, R. and Griffiths, M.W. 2011. Biocontrol of Listeria monocytogenes and Escherichia coli O157: H7 in meat by using phages immobilized on modified cellulose membranes. Appl. Environ. Microbiol. 77(18), 6379–6387. Annual Report of the Chief Scientist. 2012/2013. Petty France, UK: Food Standards Agency. Available via https://acss.food.gov.uk/sites/default/files/multimedia/pdfs/publication/cstar_2013. Atterbury, R.J., Connerton, P.L., Dodd, C.E., Rees, C.E. and Connerton, I.F. 2003. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69(10), 6302–6306. Bren, L. 2007. Bacteria-eating virus approved as food additive. Silver Spring, ML: FDA. 41(1), 20–22. Brovko, L.Y., Anany, H. and Griffiths, M.W. 2012. Bacteriophages for detection and control of bacterial pathogens in food and food-processing environment. Adv. Food. Nutr. Res. 67, 241–288. Budri, P.E., Silva, N.C., Bonsaglia, E.C., Júnior, A.F., Júnior, J.A., Doyama, J.T., Gonçalves, J.L., Santos, M.V., Fitzgerald-Hughes, D. and Rall, V.L. 2015. Effect of essential oils of Syzygium aromaticum and Cinnamomum zeylanicum and their major components on biofilm production in Staphylococcus aureus strains isolated from milk of cows with mastitis. J. Dairy. Sci. 98(9), 5899–5904. Centers for Disease Control and Prevention (CDCs). 2015. Diagnosis and management of foodborne illness. Available via https://www.aafp.org/afp/2015/0901/p358.html Centers for Disease Control and Prevention (CDCs). 2018. Available via https://www.cdc.gov/foodborneburden/index.html Centers for Disease Control and Prevention (CDCs). 2021. Centers for disease control and prevention (CDCs). Available via https://www.cdc.gov/foodsafety/cdc-and-food-safety.html Chibeu, A. 2013. Bacteriophages in food safety. In Microbial pathogens and strategies for combating them: science, technology and education. Ed., Méndez-Vilas, A. Badajoz, Spain: Formatex Research Center, pp: 1041–1052. Chibeu, A., Agius, L., Gao, A., Sabour, P.M., Kropinski, A.M. and Balamurugan, S. 2013. Efficacy of bacteriophage LISTEXTMP100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food. Microbiol. 167, 208–214. Chibeu, A. and Balamurugan, S. 2019. Quantitating phage efficacy in ready-to-eat meats. In Bacteriophages: methods and protocols. Eds., Clokie, M.R.J., Kropinski, A. and Lavigne, R. New York, NY: Humana Press, pp: 207–214. de Melo, A.G., Levesque, S. and Moineau, S. 2018. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 49, 185–190. Endersen, L., O’Mahony, J., Hill, C., Ross, R.P., McAuliffe, O. and Coffey, A. 2014. Phage therapy in the food industry. Annu. Rev. Food. Sci. Technol. 5, 327–349. Fernández, L., Gutiérrez, D., Rodríguez, A. and García, P. 2018. Application of bacteriophages in the agro-food sector: a long way toward approval. Front. Cell. Infect. Microbiol. 8, 296. Folsom, J.P., Siragusa, G.R. and Frank, J.F. 2006. Formation of biofilm at different nutrient levels by various genotypes of Listeria monocytogenes. J. Food. Protect. 69(4), 826–834. Food and Agriculture Organization (FAO). 1992. Meat and meat products in human nutrition in developing countries. P1-10. Available via https://www.fao.org/3/T0562E/T0562E05.htm Food and Drug Administration (FDA). 2015. Foodborne illnesses. Available via https://www.fda.gov/media/77727/. García, P., Martínez, B., Obeso, J.M. and Rodríguez, A. 2008. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 47, 479–485. Gill, J.J. and Hyman, P. 2010. Phage choice, isolation, and preparation for phage therapy. Curr. Pharmaceut. Biotechnol. 11(1), 2–14. Goode, D., Allen, V.M. and Barrow, P.A. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69(8), 5032–5036. Hagens, S. and Loessner, M.J. 2010. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr. Pharmaceut. Biotechnol. 11(1), 58–68. Heineman, R.H. and Bull, J.J. 2007. Testing optimality with experimental evolution: lysis time in a bacteriophage. Evolution 61(7), 1695–1709. Heinz, G. and Hautzinger, P. 2007. Meat processing technology for small- to medium-scale producers. Rome, Italy: FAO, pp:1–50. Hibma, A.M., Jassim, S.A. and Griffiths, M.W. 1997. Infection and removal of L-forms of Listeria monocytogenes with bred bacteriophage. Int. J. Food. Microbiol. 34(3), 197–207. Hooton, S.P., Atterbury, R.J. and Connerton, I.F. 2011. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food. Microbiol. 151(2), 157–163. Hoyle, N. and Kutter, E. 2021. Phage therapy: bacteriophages as natural, self-replicating antimicrobials. In Practical handbook of microbiology. Eds., Goldman, E. and Green, L.H. Boca Raton, FL: CRC Press, pp: 801–824. Hungaro, H.M., Mendonça, R.C.S., Gouvêa, D.M., Vanetti, M.C.D. and de Oliveira Pinto, C.L. 2013. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food. Res. Int. 52(1), 75–81. Jassim, S.A. and Limoges, R.G. 2014. Natural solution to antibiotic resistance: bacteriophages ‘The Living Drugs’. World. J. Microbiol. Biotechnol. 30(8), 2153–2170. Kamarudheen, N., George, C., Priya, C.L. and Rao, B.K.V. 2014. Biopreservation of meat by probiotic bacteria isolated from dairy products. Der. Pharm. Lett. 6(6), 266–271. Kanaan, M.H.G. 2018. Antibacterial effect of ozonated water against methicillin-resistant Staphylococcus aureus contaminating chicken meat in Wasit Province, Iraq. Vet. World. 11(10), 1445–1453. Kanaan, M.H.G. 2019. Meat bio preservation as a promising method in food technology. EC. Vet. Sci. 3(1). available at: https://www.researchgate.net/publication/332819911_Meat_Bio_Preservation_as_a_Promising_Method_in_Food_Technology. Kanaan, M.H.G. 2021. Prevalence, resistance to antimicrobials, and antibiotypes of Arcobacter species recovered from retail meat in Wasit marketplaces in Iraq. Int. J. One. Health. 7(1), 142–150. Kanaan, M.H.G. and Abdullah, S.S. 2019. Methicillin-resistant Staphylococcus Aureus as a superbug foodborne pathogen. Kanaan, M. and Abdullah, S. 2021. Evaluation of aqueous ozone as a method to combat multidrug-resistant Staphylococcus aureus tainting cattle meat sold in Wasit marketplaces. Mansoura. Vet. Med. J. 22(3), 117–123. Kanaan, M.H.G. and Abdulwahid, M.T. 2019. Prevalence rate, antibiotic resistance and biotyping of thermotolerant Campylobacter isolated from poultry products vended in Wasit markets. Curr. Res. Nutr. Food. Sci. 7(3), 905–917. Kanaan, M.H.G. and Al-Isawi, A.J.O. 2019. Prevalence of methicillin or multiple drug-resistant Staphylococcus aureus in cattle meat marketed in Wasit Province. Biochem. Cell. Arch. 19(1), 495–502. Kanaan, M.H.G., Al-Isawi, A.J.O. and Mohamme, A.F. 2022. Antimicrobial resistance and antibiogram of thermotolerant Campylobacter recovered from poultry meat in Baghdad Markets, Iraq. Arch. Razi. Inst. 77(1), 231–237. Kanaan, M.H.G., Al-Shadeedi, S.M., Al-Massody, A.J. and Ghasemian, A. 2020. Drug resistance and virulence traits of Acinetobacter baumannii from Turkey and chicken raw meat. Comp. Immunol. Microbiol. Infect. Dis. 70, 101451. Kanaan, M.H.G. and Khashan, H.T. 2018. Prevalence of multidrug resistant thermotolerant species of Campylobacter in Retail Frozen chicken meat in Baghdad Province. Curr. Res. Microbiol. Biotechnol. 6(1), 1431–1440. Kanaan, M.H.G. and Khashan, H.T. 2022. Molecular typing, virulence traits and risk factors of pandrug-resistant Acinetobacter baumannii spread in intensive care unit centers of Baghdad city, Iraq. Rev. Med. Microbiol. 33(1), 51–55. Kanaan, M.H.G. and Mohammed, F.A. 2020. Antimicrobial resistance of Campylobacter jejuni from poultry meat in local markets of Iraq. Plant. Arch. 20(1), 410–415. Kanaan, M.H.G. and Tarek, A.M. 2020. Clostridium botulinum, a foodborne pathogen and its impact on public health. Ann. Trop. Med. Publ. Health. 23, 49–62. Lásztity, R. 2015. Meat and meat products. In: Food quality and standards Encyclopedia of life support systems (EOLSS), pp: 1–6, vol. II. Mahony, J., McAuliffe, O., Ross, R.P. and Van Sinderen, D. 2011. Bacteriophages as biocontrol agents of food pathogens. Curr. Opin. Biotechnol. 22(2), 157–163. McLean, J.S., Wanger, G., Gorby, Y.A., Wainstein, M., McQuaid, J., Ishii, S.I., Bretschger, O., Beyenal, H. and Nealson, K.H. 2010. Quantification of electron transfer rates to a solid phase electron acceptor through the stages of biofilm formation from single cells to multicellular communities. Environ. Sci. Tech. 44(7), 2721–2727. Mohammed, F.A., Kanaan, M.H.G. and Tarek, A.M. 2022. Assessment of the effect of aqueous ozone treatment on the sensory attributes of retail meat in the Iraqi Wasit governorate. Rev. Electron. Vet. 23(3), 28–38. Montañez-Izquierdo, V.Y., Salas-Vázquez, D.I. and Rodríguez-Jerez, J.J. 2012. Use of epifluorescence microscopy to assess the effectiveness of phage P100 in controlling Listeria monocytogenes biofilms on stainless steel surfaces. Food. Contr. 23(2), 470–477. Muhialdin, B.J., Filimonau, V., Qasem, J.M., Ibrahim, S.A. and Algboory, H.L. 2022. Traditional fermented foods and beverages in Iraq and their potential for large-scale commercialization. J. Ethn. Foods. 9(1), 1–17. Nagel, T.E., Chan, B.K., De Vos, D., El-Shibiny, A., Kang’ethe, E.K., Makumi, A. and Pirnay, J.P. 2016. The developing world urgently needs phages to combat pathogenic bacteria. Front. Microbiol. 7, 882. Nath, S., Chowdhury, S., Dora, P.K.C. and Sarkar, S. 2014. Role of biopreservation in improving food safety and storage. Int. J. Eng. Res. Appl. 4(3), 26–32. O’flynn, G., Ross, R.P., Fitzgerald, G.F. and Coffey, A. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157: H7. Appl. Environ. Microbiol. 70(6), 3417–3424. Olaoye, O.A. 2011. Meat: an overview of its composition, biochemical changes and associated microbial agents. Int. Food. Res. J. 18(3), 877–885. Petrovic Fabijan, A., Khalid, A., Maddocks, S., Ho, J., Gilbey, T., Sandaradura, I., Lin, R.C., Ben Zakour, N., Venturini, C., Bowring, B. and Iredell, J.R. 2020. Phage therapy for severe bacterial infections: a narrative review. Med. J. Aust. 212(6), 279–285. Pires, D.P., Costa, A.R., Pinto, G., Meneses, L. and Azeredo, J. 2020. Current challenges and future opportunities of phage therapy. FEMS. Microbiol. Rev. 44(6), 684–700. Salem, M.A. 2012. Bio-preservation challenge for shelf life and safety improvement of minced beef. GJBBR 7(2), 50–60. Sillankorva, M.S. Oliveira, H. and Azeredo, J. 2012. Bacteriophages and their role in food safety. Int. J. Microbiol. 863945, 1–13. Singh, V.P. 2018. Recent approaches in food bio-preservation-a review. Open. Vet. J. 8(1), 104–111. Tan, T.L.H., Chan, K.G. and Lee, L.H. 2014. Application of bacteriophage in biocontrol of major foodborne bacterial pathogens. J. Mol. Biol. Mol. Imaging. 1(9), 4658187. Tian, M., Xu, X., Liu, F., Fan, X., and Pan, S. 2018. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food. Res. Int. 111, 205–211. Viazis, S., Akhtar, M., Feirtag, J. and Diez-Gonzalez, F. 2011. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food. Microbiol. 28, 149–157. World health organization (WHO). 2007-2015. WHO initiative to estimate the global burden of foodborne diseases. Available via https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng. Young, R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 56(3), 430–481. Żbikowska, K., Michalczuk, M. and Dolka, B. 2020. The use of bacteriophages in the poultry industry. Animals 10(5), 872. | ||
| How to Cite this Article |
| Pubmed Style Kanaan MHG, Tarek AM. Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants. Open Vet J. 2022; 12(6): 1018-1026. doi:10.5455/OVJ.2022.v12.i6.30 Web Style Kanaan MHG, Tarek AM. Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants. https://www.openveterinaryjournal.com/?mno=119671 [Access: April 25, 2024]. doi:10.5455/OVJ.2022.v12.i6.30 AMA (American Medical Association) Style Kanaan MHG, Tarek AM. Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants. Open Vet J. 2022; 12(6): 1018-1026. doi:10.5455/OVJ.2022.v12.i6.30 Vancouver/ICMJE Style Kanaan MHG, Tarek AM. Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants. Open Vet J. (2022), [cited April 25, 2024]; 12(6): 1018-1026. doi:10.5455/OVJ.2022.v12.i6.30 Harvard Style Kanaan, M. H. G. & Tarek, . A. M. (2022) Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants. Open Vet J, 12 (6), 1018-1026. doi:10.5455/OVJ.2022.v12.i6.30 Turabian Style Kanaan, Manal Hadi Ghaffoori, and Ahmad M. Tarek. 2022. Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants. Open Veterinary Journal, 12 (6), 1018-1026. doi:10.5455/OVJ.2022.v12.i6.30 Chicago Style Kanaan, Manal Hadi Ghaffoori, and Ahmad M. Tarek. "Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants." Open Veterinary Journal 12 (2022), 1018-1026. doi:10.5455/OVJ.2022.v12.i6.30 MLA (The Modern Language Association) Style Kanaan, Manal Hadi Ghaffoori, and Ahmad M. Tarek. "Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants." Open Veterinary Journal 12.6 (2022), 1018-1026. Print. doi:10.5455/OVJ.2022.v12.i6.30 APA (American Psychological Association) Style Kanaan, M. H. G. & Tarek, . A. M. (2022) Innovative Modern Bio-Preservation Module of Meat by Lytic Bacteriophages against Emergent Contaminants. Open Veterinary Journal, 12 (6), 1018-1026. doi:10.5455/OVJ.2022.v12.i6.30 |