| Original Article | ||
Open Vet J. 2023; 13(1): 42-47 Open Veterinary Journal, (2023), Vol. 13(1): 42–47 Original Research Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureusDhama Al-Sallami1, Amjed Alsultan2*, Kadhim Hassan Abbas3 and Simon R. Clarke41Department of Physiology, Pharmacology and Biochemistry, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah, Iraq 2Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah, Iraq 3Department of Veterinary Public Health, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah, Iraq 4School of Biological Sciences, University of Reading, Whiteknights, UK Submitted: 09/09/2022 Accepted: 11/12/2022 Published: 09/01/2023 *Corresponding Author: Amjed Alsultan. Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Al-Qadisiyah, Al- Diwaniyah, Iraq. Email: amjed.talib [at] qu.edu.iq © 2023 Open Veterinary Journal
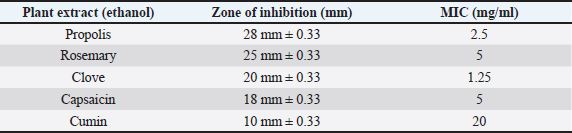
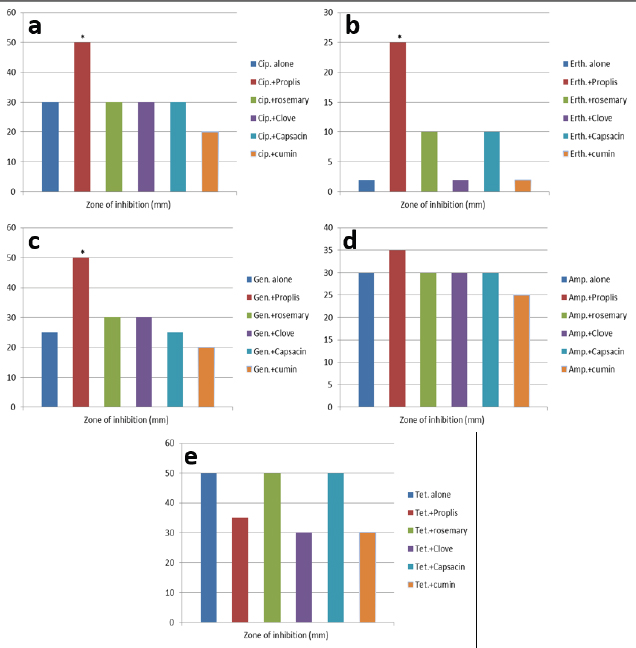
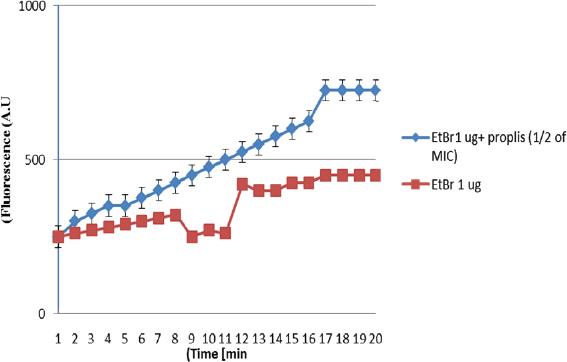
AbstractBackground: Antibiotic-resistant pathogens became a real global threat to human and animal health. This needs to concentrate the efforts to minimize and control these organisms. Efflux pumps are considered one of the important strategies used by bacteria to exclude harmful materials from the cell. Inhibition of these pumps can be an active strategy against multidrug resistance pathogens. There are two sources of efflux pump inhibitors that can be used, chemical and natural inhibitors. The chemical origin efflux pump inhibitors have many toxic side effects while the natural origin is characterized by a wide margin of safety for the host cell. Aim: In this study, the ability of some plant extracts like (propolis show rosemary, clove, capsaicin, and cumin) to potentiate the inhibitory activity of some antibiotics such as (ciprofloxacin, erythromycin, gentamycin, tetracycline, and ampicillin) against Staphylococcus aureus pathogen were tested. Methods: Efflux pump inhibitory activity of the selected plant extracts was tested using an ethidium bromide (EtBr) accumulation assay. Results: The results have shown that Propolis has a significant synergistic effect in combination with ciprofloxacin, erythromycin, and gentamycin. While it has no effect with tetracycline or ampicillin. Also, no synergic effect was noticed in a combination of the minimum inhibitory concentration for the selected plant extracts (rosemary, clove, capsaicin, and cumin) with any of the tested antibiotics. Interestingly, according to the results of the EtBr accumulation assay, Propolis has potent inhibitory activity against the S. aureus (MRS usa300) pump system. Conclusion: This study suggests that Propolis might act as a resistance breaker that is able to restore the activity of ciprofloxacin, erythromycin, and gentamycin against S. aureus strains, in case of the efflux-mediated antimicrobial resistance mechanisms. Keywords: Antibiotic resistance, Efflux pump inhibitors, Plant extracts, S. aureus. IntroductionSince the increase in the pathogenic bacteria that are able to resist different categories of antibiotics, multidrug-resistant (MDR) bacteria are turning into an actual warning to public health (Munita and Arias 2016; Rao et al., 2018; Mosolygó et al., 2019). Random use of antibiotics in medication increases the resistance to these antibiotics. For instance, methicillin resistance in Staphylococcus aureus, vancomycin resistance in Enterococcus faecalis, and fluoroquinolone resistance in Campylobacter jejuni. Also, bacteria may be naturally resistant to some antibiotics as specific genes are carried on the plasmid or on their own genomic DNA (Levy, 1998; Okeke et al., 2005; Kumar and Varela, 2013). In addition, horizontal transfer of the genetic material either plasmid or transposon between the bacteria is behind the MDR that has developed in many pathogens during the last years (Summers, 2006; Davies and Davies, 2010). Resistance to tetracycline by Escherichia coli was the first efflux pump identified in bacteria and later tetracycline resistance in C. jejuni has been identified (McMurry et al., 1980; Okeke et al., 2005; Iovine, 2013; Kumar and Varela, 2013). Pathogens improve their strategies to resist antibiotics through various mechanisms. One of the prominent and effective ways to resist the antibiotic is the extrusion of the antibiotic out of the bacterial cell by specific proteins known as efflux pump transporters (Livermore, 2003; Mosolygó et al., 2019; Sharma et al., 2019). Basically, the major facilitator superfamily (MFS) is important efflux pump transporters that are characterized by Gram-positive bacteria. NorA of S. aureus is considered a known and well-identified example of the MFS group. While the resistance-nodulation-division (RND) efflux pump family is the Gram-negative bacteria, the particular efflux pump family and the AcrAB in E. coli is the first transporter studied and identified in this group (Blanco et al., 2016; Alsultan and Alsallami, 2022). Inhibition of the efflux mechanisms in bacteria can be active to keep the antibiotic concentration intracellular enough to inhibit the pathogens’ activity and minimize the incidence and development of the disease (Mosolygó et al., 2019; Rao et al., 2018). The compounds that are able to decrease the resistance and inhibit efflux pumps inside the bacteria are known as efflux pump inhibitors EPIs (Piddock, 2006). These EPIs are either synthetic or natural in origin and basically, the plant source inhibitors have an effective inhibition role against various efflux pump families (Kumar and Pooja Patial, 2016). Plant EPIs are working to stop the efflux pump activity inside the bacterial cell and allow the antibiotic to be effective (Marquez, 2005). Also, plant-origin EPIs are discovered as antimicrobial compounds that affect different microorganisms a long time ago. Thus, analysis of the natural EPIs and purifying their component enable using of these products to design many EPIs that can be used successfully to inhibit different pathogens (Rao et al., 2018). There are many examples of plant-origin EPIs were used, such as alkaloid reserpine plant which was from the EPIs known as a Bmr efflux pump inhibitor in Bacillus subtilis, but their application in vitro is rare because of its toxic effect (Ahmed et al., 1993). Also, isoflavones which are extracted from Lupinus argenteus and have been discovered to enhance the antimicrobial effect of alpha-linoleic acid in addition to the berberine plant which potentiates the antibacterial effect of a fluoroquinolone (Morel et al., 2003). Molecular docking fosfomycin resistance protein (5WEW) of Klebsiella pneumoniae showed that bacopa monnieri can be a potent inhibitor of this protein and can be used for the treatment of urinary infections caused by bacteria (Mehta et al., 2022a). Interestingly, EPIs are able to decrease the minimum inhibitory concentrations (MICs) of antibiotics and minimize the probability of antibiotic resistance by bacteria (Kaatz, 2005; Mahmood et al., 2016). Therefore, studying the molecular interactions between the efflux pumps and EPIs is necessary to understand the mechanism of the antibacterial effect of EPIs and potentiate the antibacterial activity of antibiotics. Thus, EPIs are promising effective medications that act as a new antibiotic (Rao et al., 2018). Materials and MethodsBacteria and growth conditionStaphylococcus aureus strain USA300 was obtained from Biodefense and Emerging Infections Research Resources Repository (BEI Resources, NR-46070). The strain was routinely cultured in tryptone soya medium (TSB; Sigma Aldrich) at 37°C with shaking. Plants material extractionPlants were collected, rinsed in distilled water, dried, and ground by an electrical blender. Then, 50 g of the plant was extracted in 500 ml of ethanol using the Soxhlet apparatus (1:10 ratio) (Bhattacharya and Chandra, 2014). The extraction duration was about 8 hours daily at 72 hours in total time. After the extraction, the solvent was evaporated using a rotary evaporator and kept at 4°C. The process was performed at the physiology and pharmacology laboratory in the veterinary medicine college, University of Al-Qadisiyah. Assay of antibacterial activity using agar well diffusion methodAntimicrobial activity of the plant extracts was detected using the agar well diffusion method (Bhattacharya and Chandra, 2014). Briefly, 100 μl of standardized inoculate of each isolate was inoculated on nutrient agar plates by using sterile spreaders. A sterile gel puncher of 6 mm diameter was used to make wells over the agar plates. In the case of antibiotics, a commercial disc of the selected antibiotic was used instead of wells. One hundred microliters of each extract were poured into separate wells. Plates were incubated at 37°C for 18 hours. Then, the diameter of inhibitory zones around each well was measured in millimeters. The experiment was repeated three times. Minimum inhibitory concentrationThe MIC for each selected plant extract was detected and antimicrobial activity was noticed against S. aureus isolates. The broth dilution method was used following NCCLS (1993) and Ncube et al. (2008) with some modifications. Briefly, bacterial cultures were diluted in TSB broth. A density of 106 CFU/ml of the bacterial culture was used in the volume (0.5 ml) of each plant extract. A bacterial density of 0.5 was performed by serial dilutions from the stock solution ethanol extract mixed with sterile TSB broth. The final concentrations of the five plant extracts were in the range of 40, 35, 30, 25, 20, 10, 5, 2.5, 1.25, and 0.625 mg/ml. Exactly 0.1 ml of standardized inoculum (5 × 105 CFU/ml) was added to each tube of extract. Then, the tubes were overnight incubated, growth was determined, and MIC values were recorded. No growth was noticed in the lowest concentration of the extracts. Ethidium bromide (EtBr) accumulation assayThe inhibitory activity of the plant extract against antibiotics efflux capability in S. aureus was estimated using an EtBr accumulation assay. Briefly, this assay was performed by growing S. aureus USA 300 in TSB broth until the mid-log phase. Bacterial cells were washed with PBS, pellet resuspended with the same buffer, then 0.4% of glucose was added, and OD600 was adjusted to 0.6. EtBr (1 ug/ml) was added to 45 ul of the bacteria suspension, with or without the selected plant extract. The suspension was incubated for 30 minutes at 37°C. A microplate reader was used to measure the level of accumulation of EtBr using an excitation wavelength of λex=530 nm and an emission wavelength of λw=585 nm. The inhibitory activity of the selected plant extract against effluxing of EtBr was calculated using the following equation: RFF=RFtreated − RFuntreated where RFF is the fluorescence of the last time point (minute 20) of the EtBr accumulation, RFtreated corresponds to the relative fluorescence at the last time point of the EtBr retention curve in the presence of the plant extract tested, and RFuntreated is the relative fluorescence at the last time point of the EtBr retention in the absence of the plant extract tested. RFI=RFF/RFuntreated where RFI is a relative fluorescence index (RFI). Finally, the specific activity (SA) of the selected plant extracts on the accumulation of EtBr by S. aureus USA300 was calculated using the following equation: SA=RFI/ n (µmol), where n is the amount of the selected compound used in this assay. The experiment was repeated three independent times (Witek et al., 2020). ResultsDirect antibacterial activityThe antibacterial activity of the tested extract against S. aureus at a concentration of 25% (v/w) was summarized in Table 1. The results indicate that the tested extract exhibited a variable degree of inhibition zone (10–28 mm) against S. aureus USA300. Propolis extract had the highest inhibition zone (28 mm) against S. aureus USA300, followed by rosemary (25 mm), clove (20 mm), capsaicin (18 mm), and cumin (10 mm) extract. MICs of the tested plant extractThe MIC of the selected plant was estimated using double broth methods as described in the methods section. The results show that the MIC of propolis against S. aureus was 2.5 mg/ml, rosemary 5 mg/ml, clove 1.25 mg/ml, capsaicin 5 mg/ml, and cumin 20 mg/ml. The result is summarized in Table 1. Synergistic potential of both plant extracts with antibioticsSynergistic effects between certain antibiotics and the selected plant against S. aureus were tested using a well-diffusion assay. Among the selected plant extract, propolis showed a significant synergistic effect in combination with ciprofloxacin, erythromycin, and gentamycin and show no effect with tetracycline and ampicillin (Fig. 1). Where zone of inhibition in the case of ciprofloxacin alone (10 ug/disc) was 30.67 ± 0.33 mm diameter, combined MIC of propolis with ciprofloxacin led to a significant increase in the zone of inhibition to 50 ± 0.33 (p < 0.05). Also, the zone of inhibition in the case of erythromycin (10 ug/disc) and gentamycin (5 ug/disc) was 0.00 and 25 ± 0.33 mm in diameter respectively. A significant synergic effect was recorded when combining MIC of propolis with erythromycin (25 ± 0.33; p < 0.05) or gentamycin (50 ± 0.33; p < 0.05). There is no synergic effect was recorded in the combined MIC of the selected plant extracts (rosemary, clove, capsaicin, and cumin) with any of the tested antibiotics. Inhibitory effect on efflux pump in S. aureusEtBr accumulation assay was used to determine the efflux pump inhibitory activity of propolis in S. aureus. As described in the methods section, 1 ug/ml of EtBr with or without a sub-lethal dose of propolis (1/2 of MIC) was added to the S. aureus suspension. The level of accumulation of EtBr in the presence or absence of propolis was used to assess the efflux inhibitory action of the extract. As shown in Figure 1, the additions of propolis caused a significant increase in fluorescence intensity in S. aureus USA 300. As mentioned in methods section 2.5, the SA of inhibition of the efflux pump was calculated by dividing the RFI by the number of micromoles of propolis used in the experiment. The results show that using 1/2 of the MIC of propolis significantly increases the accumulation of EtBr (SA=20) (Fig. 2). Table 1. Zone of inhibition and MIC of the selected plant extracts.
Fig. 1. Antibacterial activity of combination of some antibiotics and selected plant extract against S. aureus usa300. (a–c): Combined the tested extract with ciprofloxacin or erythromycin or gentamycin respectively, only Propolis show significant synergetic antibacterial effect against the bacterium. (d and e): No synergistic effect was recorded combining ampicillin and tetracycline with all tested extracts including Propolis. DiscussionIn this study, the ability of some plant extracts to potentiate the inhibitory activity of some antibiotics against S. aureus was tested. Multidrug efflux pump is one of the important strategies used by bacteria to exclude noxious compounds from the cell and represents the first line of defense mechanism in bacteria (Schindler et al., 2013). It is well-documented that efflux pumps play a critical role in antibiotic resistance in S. aureus. Inhibition of these pumps can restore the activity of antibiotics against MDR pathogens including S. aureus. The combination of EPIs with antibiotics could increase the efficiency of the antibiotics by increasing the intracellular concentration of the drug and its bactericidal activity. Two types of inhibitors including natural and synthetic compound are existing. Several toxic side effects are usually associated with using of synthesized EPIs. Plant-based EPIs were evaluated in a wide range of bacteria, Mehta et al. (2016) have found that methanol extract of Phyllanthus emblica (Phyllanthaceae) has EPI activity against Salmonella enterica and Salmonella typhimurium AcrAB efflux pumps. AcrAB gene encoding to RND pump and these genes were regulated by RamA while RamR inhibits expression of RamA. In docking analysis with the 6EI9 (RamR) target protein of S. typhimurium. Also, it has been found that 11 phytol compounds that have a potent inhibitory activity for RND as compared with the standard drug (Mehta et al., 2021). In in vitro and in silico studies, Zingiber officinale Roscoe plant extract shows EPI activity against acrAB in S. typhimurium. In addition, docking results show that the plant extract is safe and can be used to treat clinical cases of S. typhimurium (Mehta et al., 2022b).
Fig. 2. Influence of Propolis tested at the concentrations corresponding to 1/2 of its MIC values on the accumulation of EtBr (1 ug/ml) in S. aureus strain MRS US300. Each data point expresses the mean and standard deviation from three replicates. In this study, the potentiation of the inhibitory activity of certain antibiotics against S. aureus was tested. Staphylococcus aureus NorA pump is responsible for the effluxion of a wide range of substances and antibiotics including ciprofloxacin and norfloxacin and resistance to these antibiotics are mediated by that pump. The previous study demonstrates that A. glauca has efflux pump inhibitory activity and increases the sensitivity of S. aureus to ciprofloxacin and norfloxacin (Jandaik et al., 2016). In this study, propolis was one of the selected plant extracts that potentiate the inhibitory activity of ciprofloxacin, erythromycin, and gentamycin. Furthermore, propolis has a potent inhibitory activity toward the S. aureus (MRS USA300) pump system. Further study is needed to find the specific pump protein that interacts with Propolis and the specific mechanism by which propolis is able to block the S. aureus pump system. Author contributionsAll authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication. FundingThis research received no external funding. Data availability statementAll data generated or analyzed during this study are available upon request to the authors. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAhmed, M., Borsch, C.M., Neyfakh, A.A. and Schuldiner, S. 1993. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. J. Biol. Chem. 268(15), 11086–11089. Alsultan, A. and Alsallami, D. 2022. Efflux-mediated bile resistance in Gram-positive pathogens. J. Pure Appl. Microbiol. 16(1), 10–18. Bhattacharya, K. and Chandra, G. 2014. Phagodeterrence, larvicidal and oviposition deterrence activity of Tragia involucrata L.(Euphorbiaceae) root extractives against vector of lymphatic filariasis Culex quinquefasciatus (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 4, S226-S232. Blanco, P., Hernando-Amado, S., Reales-Calderon, J.A., Corona, F., Lira, F., Alcalde-Rico, M. and Martinez, J.L. 2016. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms 4(1), 14. Davies, J. and Davies, D. 2010. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74(3), 417–433. Jandaik, S.U., Mehta, J. and Mohan, M. 2016. Synergistic and efflux pump inhibitory activity of plant extracts and antibiotics on Staphylococcus aureus strains. Asian J. Pharm. Clin. Res, 9, 277–282. Iovine, N.M. 2013. Resistance mechanisms in Campylobacter jejuni. Virulence 4(3), 230–240. Kaatz, G.W. 2005. Bacterial efflux pump inhibition. Curr. Opin. Investig. Drugs 6(2), 191–198. Kumar, R. and Pooja Patial, S.J. 2016. A review on efflux pump inhibitors of Gram-positive and gram-negative bacteria from plant sources. Int. J. Curr. Microbiol. Appl. Sci. 5(6), 834–855. Kumar, S. and Varela, M.F. 2013. Molecular mechanisms of bacterial resistance to antimicrobial agents. Chemotherapy 14, 18. Levy, S.B. 1998. The challenge of antibiotic resistance. Sci. Am. 278(3), 46–53. Livermore, D.M. 2003. Linezolid in vitro: mechanism and antibacterial spectrum. J. Antimicrob. Chemother. 51(suppl. 2), ii9–ii16. Mahmood, H.Y., Jamshidi, S., Sutton, J.M. and Rahman, K.M. 2016. Current Advances in Developing Inhibitors of Bacterial Multidrug Efflux Pumps. Curr. Med. Chem. 23(10), 1062–1081. Marquez, B. 2005. Bacterial efflux systems and efflux pumps inhibitors. Biochim 87(12), 1137–1147. McMurry, L., Petrucci Jr, R.E. and Levy, S.B. 1980. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc. Natl. Acad. Sci. 77(7), 3974–3977. Mehta, J.Y.O.T.I., Jandaik, S.U. and Urmila, S. 2016. Evaluation of phytochemicals and synergistic interaction between plant extracts and antibiotics for efflux pump inhibitory activity against Salmonella enterica serovar typhimurium strains. Int. J. Pharm. Pharm. Sci, 8, 217–223. Mehta, J., Rolta, R., Salaria, D., Awofisayo, O., Fadare, O.A., Sharma, P.P. and Kaushik, N. K. 2021. Phytocompounds from himalayan medicinal plants as potential drugs to treat multidrug-resistant Salmonella typhimurium: an in silico approach. Biomedicines 9(10), 1402. Mehta, J., Rolta, R. and Dev, K. 2022a. Role of medicinal plants from North Western Himalayas as an efflux pump inhibitor against MDR AcrAB-TolC Salmonella enterica serovar typhimurium: in vitro and in silico studies. J. Ethnopharmacol. 282, 114589. Mehta, J., Utkarsh, K., Fuloria, S., Singh, T., Sekar, M., Salaria, D. and Fuloria, N.K. 2022b. Antibacterial Potential of Bacopa monnieri (L.) Wettst. and its bioactive molecules against uropathogens—an in silico study to identify potential lead molecule (s) for the development of new drugs to treat urinary tract infections. Molecules 27(15), 4971. Morel, C., Stermitz, F.R., Tegos, G. and Lewis, K. 2003. Isoflavones as potentiators of antibacterial activity. J. Agric. Food Chem. 51(19), 5677–5679. Mosolygó, T., Kincses, A., Csonka, A., Tönki, Á.S., Witek, K., Sanmartín, C. and Spengler, G. 2019. Selenocompounds as novel antibacterial agents and bacterial efflux pump inhibitors. Molecules 24(8), 1487. Munita, J.M. and Arias, C.A. 2016. Mechanisms of antibiotic resistance. Microbiol. Spectr. 4(2), 10.1128/microbiolspec.VMBF-0016-2015. NCCLS. 1993. Performance Standards Antimicrobial Disc Susceptibility Tests. Approved Standard Fifth Edition. NCCLS Document M2-A5, Villanova, PA, USA. Ncube, N.S., Afolayan, A.J. and Okoh, A.I. 2008. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr. J. Biotechnol. 7(12), 1797-1806. Okeke, I.N., Laxminarayan, R., Bhutta, Z.A., Duse, A.G., Jenkins, P., O’Brien, T.F. and Klugman, K.P. 2005. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect. Dis. 5(8), 481–493. Piddock, L.J. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clinical Microbiol. Rev. 19(2), 382–402. Rao, M., Padyana, S., Dipin, K., Kumar, S., Nayak, B. and Varela, M.F. 2018. Antimicrobial compounds of plant origin as efflux pump inhibitors: new avenues for controlling multidrug resistant pathogens. J. Antimicrob. Agents 4, 1–6. Schindler, B.D., Jacinto, P. and Kaatz, G.W. 2013. Inhibition of drug efflux pumps in Staphylococcus aureus: current status of potentiating existing antibiotics. Future Microbiol. 8(4), 491–507. Sharma, A., Gupta, V.K. and Pathania, R. 2019. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 149(2), 129. Summers, A.O. 2006. Genetic linkage and horizontal gene transfer, the roots of the antibiotic multi-resistance problem. Anim. Biotechnol. 17(2), 125–135. Witek, K., Latacz, G., Kaczor, A., Czekajewska, J., Żesławska, E., Chudzik, A. and Handzlik, J. 2020. Phenylpiperazine 5, 5-dimethylhydantoin derivatives as first synthetic inhibitors of Msr (A) efflux pump in Staphylococcus epidermidis. Molecules 25(17), 3788. | ||
| How to Cite this Article |
| Pubmed Style Alsallami D, Alsultan A, Abbas KH, Clarke SR. Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. Open Vet J. 2023; 13(1): 42-47. doi:10.5455/OVJ.2023.v13.i1.5 Web Style Alsallami D, Alsultan A, Abbas KH, Clarke SR. Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. https://www.openveterinaryjournal.com/?mno=112763 [Access: April 25, 2024]. doi:10.5455/OVJ.2023.v13.i1.5 AMA (American Medical Association) Style Alsallami D, Alsultan A, Abbas KH, Clarke SR. Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. Open Vet J. 2023; 13(1): 42-47. doi:10.5455/OVJ.2023.v13.i1.5 Vancouver/ICMJE Style Alsallami D, Alsultan A, Abbas KH, Clarke SR. Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. Open Vet J. (2023), [cited April 25, 2024]; 13(1): 42-47. doi:10.5455/OVJ.2023.v13.i1.5 Harvard Style Alsallami, D., Alsultan, . A., Abbas, . K. H. & Clarke, . S. R. (2023) Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. Open Vet J, 13 (1), 42-47. doi:10.5455/OVJ.2023.v13.i1.5 Turabian Style Alsallami, Dhama, Amjed Alsultan, Kadhim Hassan Abbas, and Simon R. Clarke. 2023. Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. Open Veterinary Journal, 13 (1), 42-47. doi:10.5455/OVJ.2023.v13.i1.5 Chicago Style Alsallami, Dhama, Amjed Alsultan, Kadhim Hassan Abbas, and Simon R. Clarke. "Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus." Open Veterinary Journal 13 (2023), 42-47. doi:10.5455/OVJ.2023.v13.i1.5 MLA (The Modern Language Association) Style Alsallami, Dhama, Amjed Alsultan, Kadhim Hassan Abbas, and Simon R. Clarke. "Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus." Open Veterinary Journal 13.1 (2023), 42-47. Print. doi:10.5455/OVJ.2023.v13.i1.5 APA (American Psychological Association) Style Alsallami, D., Alsultan, . A., Abbas, . K. H. & Clarke, . S. R. (2023) Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. Open Veterinary Journal, 13 (1), 42-47. doi:10.5455/OVJ.2023.v13.i1.5 |