| Case Report | ||
Open Vet J. 2023; 13(1): 119-122 Open Veterinary Journal, (2023), Vol. 13(1): 119–122 Case Report Congenital porto-pulmonary shunt in a dogFederico Puccini Leoni1, Andrea Arcangeli1, Riccardo Di Puccio1 and Filippo Cinti2*1Vet Rad Firenze, Florence, Italy 2Surgery Department, San Marco Veterinary Clinic and Laboratory, Veggiano, Italy Submitted: 05/09/2022 Accepted: 27/12/2022 Published: 15/01/2023 *Corresponding Author: Filippo Cinti. Surgery Department, San Marco Veterinary Clinic and Laboratory, Veggiano, Italy. Email: filippocinti [at] icloud.com © 2023 Open Veterinary Journal
AbstractBackground: Congenital extra-hepatic porto-systemic shunts (CEPS) are a non-rare vascular anomaly observed in dogs, most commonly in small and toy pure breeds. Computed tomographic angiography (CTA) examination is considered the gold standard imaging modality for the diagnosis of anomalous vascular connections. Case Description: An anomalous congenital porto-pulmonary shunt was incidentally diagnosed in a 5-year-old French Bulldog. The anomalous vessel originated from the ventral aspect of the portal vein and went cranially towards the esophageal hiatus, entering the lobar vein of the caudal left pulmonary lobe. The dog did not show any significant clinical or computed tomography angiography-perceived hepatic abnormalities and no signs of portal hypertension were evidenced. No case of porto-pulmonary shunt in veterinary medicine have ever been reported, while in humans it was rarely described secondarily to portal hypertension, severe hepatopathies or complex cardiac malformations. Conclusion: CTA must be considered the best imaging modality for the diagnosis also of unusual CEPS and in the author’s opinion the congenital porto-pulmonary shunt described in the patient was of little or no clinical relevance. Keywords: Congenital extrahepatic shunt, Porto-pulmonary vascular connection, French Bulldog, Abdominal computed tomography angiography. IntroductionThe portal vein (PV) carries blood from the abdominal viscera to the hepatic parenchyma with a hepatopetal flow, collecting blood from the gastrointestinal tract, spleen and pancreas (Bertolini, 2017). The extrahepatic PV originates from the confluence of the cranial and the caudal mesenteric veins, and it cranially receives its major tributaries: the splenic and the gastroduodenal veins (Bertolini, 2017). In normal adult mammals, a vascular connection between the systemic venous circulation and the portal system should not exist and congenital disorders of the portal system reflect a variety of embryonic and fetal disturbances (Bertolini, 2017). Computed tomographic angiography (CTA) is considered the method of choice to diagnose porto-systemic shunts in humans (Henseler et al., 2001; Mankin, 2015). To date, CTA is also the best technique for the diagnosis of vascular anomalies in dogs, showing to have both sensitivity (96%) and specificity (89%) (Kim et al., 2013; Mankin, 2015). Porto-systemic shunts can be divided into congenital and acquired. The congenital ones may be further classified as extra-hepatic, intra-hepatic and arteriovenous hepatic malformations. A congenital extra-hepatic porto-systemic shunt (CEPS) is a developmental anomaly due to an anomalous vascular connection between the vitelline veins, forming the portal system, and the cardinal veins, that form the systemic veins (Bertolini, 2017). The presence of a CEPS allows portal blood to bypass the liver entering the systemic circulation, often causing significant clinical implications (Szatmari et al., 2013). Generally, a single CEPS is present, even though multiple CEPS have been reported (Mankin, 2015). The embryological development and the genetic basis of CEPS in dogs have not been established and remain poorly described in veterinary medicine (White et al., 2017); however, this condition occurs more frequently in small and toy pure breeds (Bertolini, 2017). In literature, many types of CEPS have been observed thanks to CTA (Bertolini, 2017; White et al., 2017). To our knowledge, no case of porto-pulmonary shunt in animals has ever been reported. Case DetailsA 5-year-old male French Bulldog of 13 kg was referred to our diagnostic center because of the presence of a voluminous swelling in correspondence of the intermandibular region, with a first suspect of a neoplastic lesion and less probably a severe sialocele. In the previous 2 weeks, the patient underwent a non-specified antimicrobial and anti-inflammatory therapy without significant improvements. The remote pathological anamnesis was silent. The anamnestic and clinical examination revealed depression, anorexia, severe breathing difficulty, abundant respiratory secretions and hyperthermia (39.8°C). Complete blood cell count (IDEXX ProCyte DxTM) indicated monocytosis (1.49 K/μl, range 0.16–1.12 K/μl), thrombocytopenia (64 K/μl, range 148–484 K/μl) and mild/moderate normocytic and normochromic non-regenerative anemia (RBC 4.53 M/μl, range 5.65–8.87 M/μl; HCT 28.0%, range 37.3%–61.7%, HGB 9.8 g/dl, range 13.1–20.5 g/dl), in first hypothesis indicating a chronic inflammatory disease. All biochemical parameters examined (IDEXX Catalyst OneTM), in particular alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), cholesterol, triglycerides, bilirubin, creatinine, urea and albumin were within normal limits, except for a mild increase in total proteins (10.0 g/dl, range 5.2–8.2 g/dl) and in globulin (7.7 g/dl, range 2.5–4.5 g/dl). The same day, the dog underwent a total-body CTA examination under general anesthesia, that was performed using a 16-slice scanner (GE Healthcare Optima CT520) with a slice thickness of 0.625 mm, 120 kW, 150 mA and a pitch < 1. The patient was positioned in sternal recumbency, and the image acquisition was carried out in the native scan phase and after about 40 seconds from the injection of 2 mg/kg iodinate contrast medium (Iopamiro, Iopamidolo 370 mgI/ml) followed by 1 mg/kg bolus of physiological solution (0.9% NaCl). Images were reformatted using bone and soft tissue algorithms. The CTA examination showed the presence of an anomalous vascular connection between the PV and the lobar vein of the caudal left pulmonary lobe. This anomalous vessel originated from the ventral aspect of the PV, cranially to the portal entering of the gastroduodenal vein. The vase went caudo-ventrally along the small gastric curvature, and subsequently dorso-cranially towards the esophageal hiatus, receiving two branches of the left gastric vein. At the hiatus, the shunt rotated dorsally around and in contact with the cardias, from the median line to the left side of the dog. Afterwards, the vessel entered the lobar vein of the caudal left pulmonary lobe, 1.1 cm before its entry into the left atrium (Figs. 1 and 2). The porto-pulmonary shunt showed an almost constant diameter, starting from 2.0 mm at its origin in correspondence of the PV, increasing to 2.6 mm at the level of the esophageal hiatus and 3.4 mm before entering the left lobar pulmonary vein. The diameter of the PV was 6.8 mm at the porta hepatis, while it was 7.8 mm before the origin of the anomalous vase. Neither hepatic lesions nor intraparenchymal portal vascular texture anomalies were observed, and the hepatic volume was within normal limits. No other shunts, varices, peritoneal effusion or CTA-perceived cardiac abnormalities were detected. No nephromegaly, based on renal length-to-aorta diameter (Hoey et al., 2016), was observed. The dog also underwent an echocardiographic and an abdominal ultrasound examination, performed by an experienced radiologist, without evidences of functional or morphological abnormalities. The final clinical diagnosis consisted of a severe infected sialocele sustained by Fusobacterium necrophorum isolated by cultural examination and the presence of the porto-pulmonary shunt was interpreted as an incidental finding. The patient died the following day because of respiratory failure and the owner did not allow the necropsy. DiscussionAll examined findings are compatible with a case of CEPS connecting the portal system to the pulmonary circulation in a French Bulldog. The abdominal ultrasound examination has not evidenced the porto-pulmonary vascular connection observed on the CTA examination, confirming that CTA is the best imaging modality for the diagnosis of CEPS (Kim et al., 2013; Mankin, 2015). It’s not clear if the mild/moderate normocytic and normochromic non-regenerative anemia was generated by the chronic inflammation or it was due to the anomalous vascular communication. At the same time, the increased total protein level could be related in first hypothesis to dehydration and inflammation, given that no other biochemical alterations were found. A decrease in hepatic volume was not detected, contrary to what usually happens in presence of CEPS (D’Anjou, 2008). On the other hand, the diameter of the PV, cranially to the origin of the porto-pulmonary shunt was mildly decreased, as it often occurs if a CEPS is present (D’Anjou et al., 2004; Szatmari et al., 2004). In veterinary literature, no porto-pulmonary shunt has ever been described and no significant similarities with our case have been found in human medicine. However, in humans spontaneous porto-pulmonary anastomoses were described in three patients with portal hypertension, caused by hepatic cirrhosis in two cases, while in the third one it was due to granulomatous hepatitis (Kumar et al., 2010). Moreover, all three patients showed other acquired vascular alterations consisting of paraesophageal or mediastinal varices. Another anomalous but congenital condition, called total anomalous pulmonary venous connection to the PV, is reported in humans, but it is associated with right atrial isomerism as part of the complex cardiac anomaly of heterotaxy syndrome (Boopathy et al., 2003). In the three cases observed in humans (Kumar et al., 2010), porto-pulmonary connections were acquired, and they were due to severe liver diseases, while in the total anomalous pulmonary venous connection to the PV severe cardiological abnormalities always coexisted (Boopathy et al., 2003). In our patient no clinical evidence of hepatopathy, portal hypertension or cardiological alteration were found. In conclusion, CTA must be considered the best imaging modality for the diagnosis also of unusual CEPS and based on the clinical evidences and the diagnostic imaging features, the described anomaly connection may be of little or no clinical relevance.
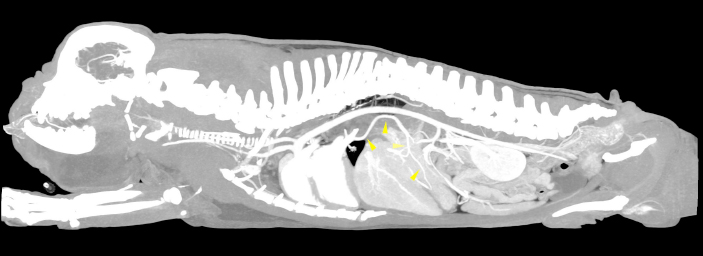
Fig. 1. Sagittal MIP view of the venous phase. The yellow arrowheads indicate the anomalous vessel connecting the portal system with the lobar vein of the caudal left pulmonary lobe.
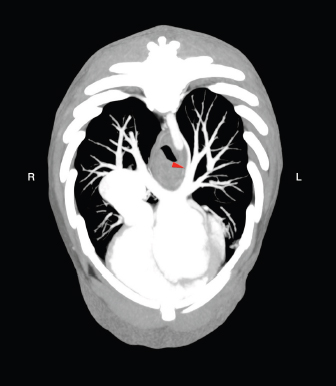
Fig. 2. Transverse MIP view of the venous phase. The red arrowhead indicates the site in which the anomalous vessel enters the lobar vein of the caudal left pulmonary lobe. Conflict of interestNone of the authors of this article has financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper. Authors’ contributionsFederico Puccini Leoni: Conception of the study, study design, collected the data, drafted, revised and approved the submitted manuscript; Andrea Arcangeli: collected the data and revised and approved the submitted manuscript; Riccardo Di Puccio: collected the data and revised and approved the submitted manuscript; Filippo Cinti: Conception of the study, study design, collected the data, drafted, revised and approved the submitted manuscript. ReferencesBertolini, G. 2017. The abdominal vasculature. The liver. In Body MDCT in small animals, 1st ed.. Eds., Bertolini, G. Cham, Switzerland: Springer, pp: 55–125. Boopathy Vijayaraghavan, S., Rao, A.R., Padmashree, G. and Raman, M.L. 2003. Prenatal diagnosis of total anomalous pulmonary venous connection to the portal vein associated with right atrial isomerism. Ultrasound. Obstet. Gynecol. 21, 393–396. D’Anjou, M.A. 2008. Liver. In Atlas of small animal ultrasonography, 1st ed. Eds., Pennick, D. and D’Anjou, M.A. Ames, IO: Blackwell, pp: 234–242. D’Anjou, M.A., Penninck, D., Cornejo, L. and Pibarot, P. 2004. Ultrasonographic diagnosis of portosystemic shunting in dogs and cat. Vet. Radiol. Ultrasound. 45, 424–437. Henseler, K.P., Pozniak, M.A., Lee, F.T. Jr. and Winter 3rd, T.C. 2001. Three-dimensional CT angiography of spontaneous portosystemic shunts. Radiographics. 21, 691–704. Hoey, S.E., Heder, B.L., Hetzel, S.J. and Waller 3rd, K.R. 2016. Use of computed tomography for measurement of kidneys in dogs without renal disease. J. Am. Vet. Med. Assoc. 248, 282–287. Kim, S.E., Giglio, R.F., Reese, D.J., Reese, S.L., Bacon, N.J. and Ellison, G.W. 2013. Comparison of computed tomographic angiography and ultrasonography for the detection and characterization of portosystemic shunts in dogs. Vet. Radiol. Ultrasound. 54, 569–574. Kumar, A., Gonzalez, G., Wilkinson, L., Mohammed T-L., Castro-Pavia, F., Glockner, J. and Kirsch, J. 2010. Computed tomography findings of spontaneous porto-pulmonary shunts in 3 patients with portal hypertension. J. Thorac. Imaging 25, W70–W74. Mankin, K.M. 2015. Current concepts in congenital portosystemic shunts. Vet. Clin. North Am. Small Anim. Pract. 4, 477–487. Szatmari, V. 2013. Vascular disorders. In Canine & feline gastroenterology, 1st ed. Eds., Washabau, R.J. and Day, M.J. St. Louis, MO: Elsevier Saunders, pp: 904–914. Szatmari, V., Rothuizen, J., van den Ingh, T.S., van Sluijs, F. and Voorhout, G. 2004. Ultrasonographic findings in dogs with hyperammonemia: 90 cases (200-2002). J. Am. Vet. Med. Assoc. 224, 717–727. White, R.N., Shales, C. and Parry, A.T. 2017. New perspectives on the development of extrahepatic portosystemic shunts. J. Small Anim. Pract. 58, 669–677. | ||
| How to Cite this Article |
| Pubmed Style Puccini-Leoni F, Arcangeli A, Di-Puccio R, Cinti F. Congenital porto-pulmonary shunt in a dog. Open Vet J. 2023; 13(1): 119-122. doi:10.5455/OVJ.2023.v13.i1.13 Web Style Puccini-Leoni F, Arcangeli A, Di-Puccio R, Cinti F. Congenital porto-pulmonary shunt in a dog. https://www.openveterinaryjournal.com/?mno=110743 [Access: April 25, 2024]. doi:10.5455/OVJ.2023.v13.i1.13 AMA (American Medical Association) Style Puccini-Leoni F, Arcangeli A, Di-Puccio R, Cinti F. Congenital porto-pulmonary shunt in a dog. Open Vet J. 2023; 13(1): 119-122. doi:10.5455/OVJ.2023.v13.i1.13 Vancouver/ICMJE Style Puccini-Leoni F, Arcangeli A, Di-Puccio R, Cinti F. Congenital porto-pulmonary shunt in a dog. Open Vet J. (2023), [cited April 25, 2024]; 13(1): 119-122. doi:10.5455/OVJ.2023.v13.i1.13 Harvard Style Puccini-Leoni, F., Arcangeli, . A., Di-Puccio, . R. & Cinti, . F. (2023) Congenital porto-pulmonary shunt in a dog. Open Vet J, 13 (1), 119-122. doi:10.5455/OVJ.2023.v13.i1.13 Turabian Style Puccini-Leoni, Federico, Andrea Arcangeli, Riccardo Di-Puccio, and Filippo Cinti. 2023. Congenital porto-pulmonary shunt in a dog. Open Veterinary Journal, 13 (1), 119-122. doi:10.5455/OVJ.2023.v13.i1.13 Chicago Style Puccini-Leoni, Federico, Andrea Arcangeli, Riccardo Di-Puccio, and Filippo Cinti. "Congenital porto-pulmonary shunt in a dog." Open Veterinary Journal 13 (2023), 119-122. doi:10.5455/OVJ.2023.v13.i1.13 MLA (The Modern Language Association) Style Puccini-Leoni, Federico, Andrea Arcangeli, Riccardo Di-Puccio, and Filippo Cinti. "Congenital porto-pulmonary shunt in a dog." Open Veterinary Journal 13.1 (2023), 119-122. Print. doi:10.5455/OVJ.2023.v13.i1.13 APA (American Psychological Association) Style Puccini-Leoni, F., Arcangeli, . A., Di-Puccio, . R. & Cinti, . F. (2023) Congenital porto-pulmonary shunt in a dog. Open Veterinary Journal, 13 (1), 119-122. doi:10.5455/OVJ.2023.v13.i1.13 |