| Original Article | ||
Open Vet J. 2022; 12(6): 919-928 Open Veterinary Journal, (2022), Vol. 12(6): 919–928 Original Research Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheepAsmaa R. F. Eweis1, Kodeir M. Hassan2, Sahar A. H. Shoman1, Hoda A. Taha1 and Ehab El-Sayed Mohamed2*1Faculty of Science, Ain Shams University, Cairo, Egypt2Agriculture Research Center (ARC), Veterinary Serum and Vaccine Research Institute (VSVRI), Cairo, Egypt Submitted: 25/08/2022 Accepted: 01/11/2022 Published: 01/12/2022 *Corresponding Author: Ehab El-Sayed Mohamed. Agriculture Research Center (ARC), Veterinary Serum and Vaccine Research Institute (VSVRI), Cairo, Egypt. Email: ehabelsayed [at] hotmail.com © 2022 Open Veterinary Journal
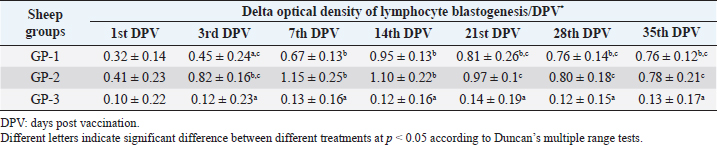
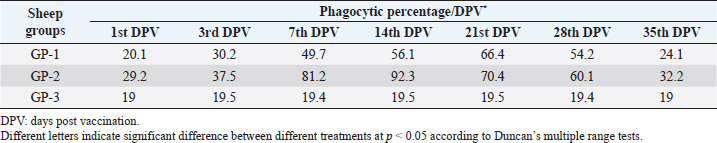
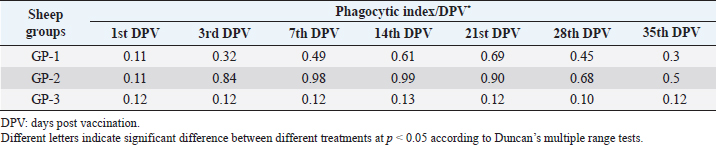
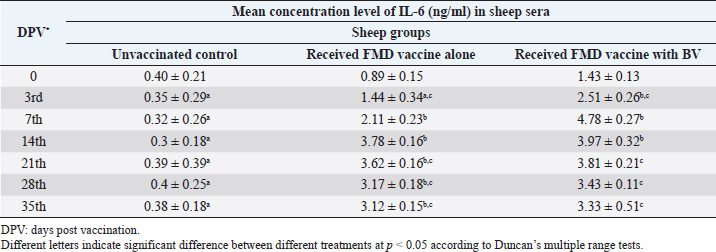
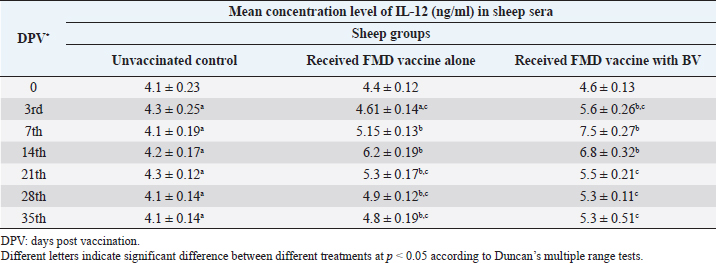
AbstractBackground: Foot-and-mouth disease (FMD) is one of the most highly contagious and economically significant diseases of cloven-hoofed animals worldwide. FMD virus (FMDV) is the cause of the disease. The virus has seven serological types, identified as; O, A, C, SAT1, SAT2, SAT3, and Asia1. The aim of this study enhancement of FMD vaccine immunogenicity is the unique way to control FMD in Egypt. Aim: Our research studied the effect of bee venom (BV) as simultaneously inoculated with the commercial vaccine on the immune response of experimentally vaccinated sheep in comparison with the inoculation of the vaccine alone through evaluation of the cellular and humoral immune response. Methods: Estimation of cellular immunity using phagocytic activity, phagocytic percentage, lymphocyte blastogenesis, interleukin-6 (IL-6), and interleukin-12 (IL-12) and estimation of humoral immunity using serum neutralization test (SNT) and enzyme-linked immunosorbent assay (ELISA). Result: Evaluation of the cellular immunity expressed in lymphocyte blastogenesis, phagocytic activity, phagocytic percentage, IL-6, and IL-12 showed higher levels in sheep vaccinated by the trivalent FMD vaccine (serotypes O pan Asia, A Iran O5, and SAT2/EGY/2012) with BV comparable to those induced by the vaccine alone. Following up the humoral immune response of vaccinated sheep revealed that FMDV antibodies serotypes O pan Asia, A Iran O5, and SAT2/EGY/2012 as measured by SNT and ELISA assay induced by FMD with BV were higher than those induced by inactivated FMD alone. Conclusion: The inoculation of BV with FMD vaccine simultaneously is of high benefit inducing high level of specific immunity which could be of long duration. Keywords: FMD, Bee venom, Cellular immunity, Humoral immunity. IntroductionFoot-and-mouth disease FMD is one of the most troubles viral diseases among livestock especially cloven-footed domestic and wild animals including cattle, buffaloes, sheep, goats, and pigs (Depa et al., 2012). It is a highly contagious viral disease among livestock in the world in the terms of economic impact and hindering on the trade of animals on national and international level and restriction of people movement which affect the tourism sector (Knight et al., 2017). FMD has the ability to cause great economic losses due to reduced milk yields, abortions, delayed conception, perinatal mortality, and premature culling and also due to hindering on the trade of animals both locally and internationally, and restrictions on the movement of people which affect the tourism sector (James and Rushton, 2002). During 2016–2019, different strains of FMD virus (FMDV) (O, A, SAT2) were circulated in Egypt that were detected from cattle and buffalo from various Egyptian governorates using enzyme-linked immunosorbent assay (ELISA) test. The three serotypes have different ratios for their expansion in Egypt where infected animals were infected with FMDV serotype O (79.3%), A (16.3%), and SAT2 (4.4%) (Ibrahim et al., 2018; El-Mayet et al., 2020). Controlling of FMD in susceptible animals such as cattle, sheep, and goats attained by vaccination through single vaccination is efficient in decreasing viral transmission between animals as a supplemental control measure (Orsel et al., 2007). In sheep, quadrivalent double emulsion (Montanide ISA206) vaccines were tested, and revealed that the oil adjuvant characterized by promotion of faster immune response than aluminum hydroxide gel vaccine. The neutralizing antibody titers in the animals were maintained at >3 log10 for 90 days (Patil et al., 2002). Serum neutralization test (SNT) and ELISA showed that the average protective FMD serum antibody titers in calves vaccinated with Montanide ISA 206 was begun at the 3rd week after vaccination and at the 10th week it achieved their higher level continuous protective level until 32nd week (Gamil, 2010). As a result of being humoral antibody titer does not give sufficient details about vaccine-stimulated immunity against FMD, a comparison among immunity conferred after vaccination and cell-mediated immune responses showed a positive relationship between viral neutralizing antibodies and cell-mediated immunity (Carr et al., 2013). Bee venom (BV) therapy (Apitherapy) has been used since ancient times (Kim et al., 2015). The venom has many scientific names as Apis Venenum Purum, Apitoxin, Bald-Faced Hornet, Bumblebee Venom, Honeybee Venom, Mixed Vespids, Pure BV, Wasp Venom, White-Faced Hornet, Yellow Hornet, and Yellow-Jacket Venom. Also, Apis mellifera (Honeybee); Bombus terrestis (Bumblebee); Vespula maculata (Hornet, Wasp) Family: Apidae; Vespidae (Meier and White, 1995). The major components of BV, melittin and phospholipase A2, acquire their predominance from their broad beneficial action. This work was designed to spot the light on honey BV as a natural material on the immune response of sheep to the trivalent FMD vaccine aiming to enhance the vaccine immunogenicity. Materials and MethodsFMD virusLocally isolated (FMDV) strains O PanAsia-2, A Iran O5, and SAT2/EGY/2012 of calves origin were typed and subtyped at the FMD Research Department (FMDRD), Veterinary Serum and Vaccine Research Institute (VSVRI), Abasia, Cairo and confirmed by the World Reference Laboratories, Pirbright, UK. These viral serotypes were in serological tests (SNT and ELISA). These viruses had the titer of 8.5, 8, and 7.0 TCID50/ml, respectively. FMD vaccineTrivalent FMD vaccine was supplied by VSVRI and used to vaccinate the experimental sheep under the effect of BV. Honey BVHoney BV of Apis mellifera lamarckii was obtained from Bee Keeping Department, Agriculture Research Center, Egypt. A stock solution of BV was prepared in sterile distilled water at 0.1% and sterilized by filtration through 0.2 μ pore-size filter as described previously (Kamal, 2016). Cell cultureBaby Hamster Kidney cell line (BHK21 clone13) was kindly supplied by the Animal Research Institute, Pirbright, UK and propagated at FMDRD using minimum essential medium (MEM) with Eagle’s salts supplemented with 10% new born calf serum according to Farag et al. (2006). These cells were maintained and propagated according to Macpherson and Stocker (1962); using MEM supplied by Gibco (G80 Gibco Limited, Parisley, UK) supplemented with 10% new born calf serum and antibiotics (100 μgs of streptomycin and 100 IU of penicillin-G sodium/ml). These cells were used for study the safety of BV and its antiviral activity and SNT. SheepThirty native breed sheep, of 1-year-old and 55 and 60 kg body weight, were divided into three groups (10 animals/group) as follow: -Group 1 (GP1) vaccinated with polyvalent FMD ISA 206 oil vaccine alone using a dose of 1 ml/animal inoculated subcutaneously. -Group 2 (GP2) vaccinated with polyvalent FMD ISA 206 using the same dose with 2 ml/animal as each 1 ml contains 3.9 µg of BV inoculated subcutaneously (Mansour et al., 2016). -Group 3 (GP3) was kept without any inoculation as negative control. SamplesHeparinized blood samples were obtained from all animals at 0-, 3-, 7-, 14-, 21-, and 28-days post-vaccination (DPV) for testing their cellular immune response using lymphocyte blastogenesis assay using cell proliferation kit (XTT kit), phagocytic activity and index. Serum samples for estimation of the interleukin-6 (IL-6) and IL-12 were obtained from all animals at the same time points shown above. Serum samples for monitoring of the humoral antibody response of the vaccinated sheep were collected weekly post-vaccination (WPV) for 1 month then every two WPV post-vaccination till the 4th month and finally every four WPV till the end of experiment (40th WPV). Evaluation of the cellular immune responseLymphocyte blastogenesis using XTT assay Lymphocyte blastogenesis assay was carried out using XTT assay according to EL-Naggar (2012) through separation of lymphocytes as described by Lucy (1977) and Lee (1984) and determination of viable cell number according to Mayer et al. (1974). Separation and cultivation of mononuclear cellsThe preparation of mononuclear cell suspension was separated by Ficollhypaque equilibrium centrifugation method (Antley and Hazen, 1988) from sheep peripheral blood cell suspension was adjusted to 107 viable mononuclear cells/ml RPMI medium containing 15% fetal calf serum and placed in cell culture six-wells plate. The monolayer cells were rinsed three times gently with RPMI medium to remove non-adherent cell. The adherent cells were then covered with RPMI medium containing 5% fetal calf serum (FCS) and incubated for 24 hours in CO2 incubator at 37 °C. Phagocytic activity of sheep macrophages by using Candida albicansThe monolayer of adherent mononuclear cells was washed gently three times with RPMI medium. Candida albicans cell suspension containing 105 cell/ml RPMI medium was incubated with the above monolayers in humidified CO2 incubator at 37 °C for 1 hour. After incubation, the monolayer cells were washed gently with cold RPMI medium and then fixed with methyl alcohol (0.3 ml/well) for 5 minutes. The alcohol was discarded and left to dry. The cells were stained with Giemsa stain for 3 minutes. Under the light microscope, using oil immersion lens, 10 fields were examined. The total number of phagocytic cells, the number of phagocytes ingested yeast cell, and the number of blastospores within individual phagocyte were determined. The percentage of phagocytes containing blastospores was determined by the method of Harmon and Glisson which was modified by Hussein (1989) and the mean number of blastospores (more than two blastospores) per infected phagocyte (phagocytic index) was calculated by Richardson and Smith (1981). Estimation of ILEstimation of the level of IL in the sera of experimental sheep including IL-6 and IL-12 was carried out using sheep IL-6 ELISA Kit Catalog No. EKE51028 supplied by Biomatik Company, Wilmington, DE, IL-12 ELISA Kit Catalog No. EKE925701 supplied by Biomatik Company, Wilmington, DE. Evaluation of sheep humoral immune responseSerum samples collected from sheep before and after vaccination were subjected to estimation their antibody titers against the three serotypes of FMDV (O pan Asia, A Iran O5, SAT2/EGY/2012 and SAT2/EGY/2018) by SNT using the micro titer technique described by Ferreira (1976) and indirect ELISA according to Voller et al. (1976). Statistical analysisThe obtained data were analyzed using analysis of variance in the Statistical Package for the Social Sciences version 12 statistical software package for PCS Multiple comparisons of means were made using Duncan’s multiple range tests at p < 0.05%. ResultsLymphocyte blastogenesis assay revealed that mean optical density of lymphocyte blastogenesis carried out on blood samples collected from vaccinated sheep reached its highest value by the 14th DPV showing higher levels in sheep received BV with FMD vaccine (1.10 ± 0.22b) than in sheep received the vaccine alone (0.95 ± 0.13b) and reached the lowest values (0.78 ± 0.21c) and (0.76 ± 0.12bc), respectively, by the 35th DPV as tabulated in Table 1. Phagocytic activity assay revealed that mean phagocytic percentage and index carried out on blood samples collected from vaccinated sheep reached its highest value by the 14th DPV that was higher in sheep received BV with FMD vaccine (92.3, 0.99) than that in sheep received the vaccine alone (66.4, 0.90) which was at 21st DPV showing the lowest values (32.2, 0.5) and (24.1, 0.3), respectively, by the 35th DPV as tabulated in Tables 2 and 3. Estimation of IL-6 and IL-12 in vaccinated sheep showed higher levels in sheep vaccinated with FMD vaccine with BV than in sheep vaccinated with FMD vaccine alone by the 14th DPV (3.97 ± 0.32b and 3.78 ± 0.16b for IL-6 and 6.8 ± 0.32b, and 6.2 ± 0.19b for IL-12, respectively. By the 35th DPV the lowest recorded levels of IL-6 and IL-12 were 3.33 ± 0.51c and 3.12 ± 0.15bc and 5.3 ± 0.51c and 4.8 ± 0.19bc, respectively, in sheep received BV with FMD vaccine and in sheep received the vaccine alone as shown in Tables 4 and 5. Table 1. Mean delta optical density of lymphocyte blastogenesis assay of vaccinated sheep.
Table 2. Phagocytic % of vaccinated sheep.
Table 3. Phagocytic index of vaccinated sheep.
Table 4. Mean concentration of IL-6 (ng/ml) in vaccinated sheep.
Table 5. Mean concentration of IL-12 (ng/ml) vaccinated sheep.
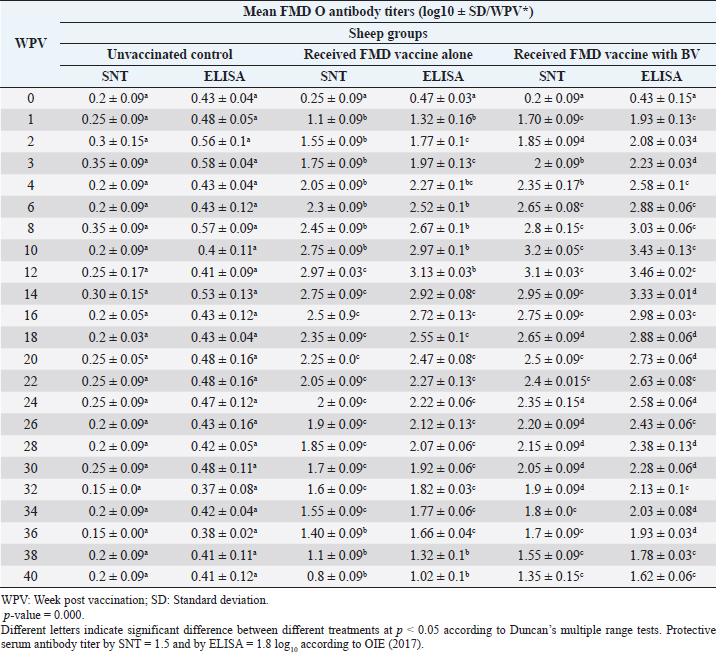
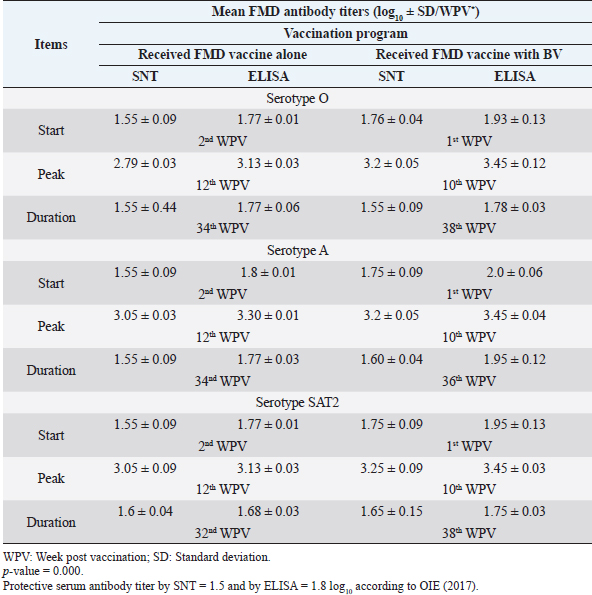
Monitoring of sheep immune response to FMD vaccine alone or with BV through application of SNT and solid phase indirect ELISA; revealed that vaccinated sheep exhibited FMD serotype O protective antibody titers (1.55 ± 0.09 and 1.77 ± 0.1 log10 by SNT and ELISA, respectively, by the second week post-vaccination with FMD vaccine alone. These values were higher with administration of BV (1.70 ± 0.09 and 1.93 ± 0.13 log10 by SNT and ELISA, respectively) by the first week post-vaccination. These titers recorded their peaks (2.97 ± 0.03 log10 by SNT and 3.13 ± 0.03 log10 by ELISA) on the 12th WPV and 3.2 ± 0.05 log10 by SNT and 3.43 ± 0.03 log10 by ELISA on the 10th WPV using the vaccine alone and with BV, respectively. FMD antibodies were gradually decreased to reach its lowest protective levels (1.55 ± 0.9 log10 by SNT and 1.77 ± 0.06 log10 by ELISA) on the 34th WPV with FMD vaccine alone while in the case of administration of BV these values were (1.55 ± 0.09 log10 by SNT and 1.78 ± 0.03 log10 by ELISA) on the 38th WPV. Non-protective FMD serotype O antibody titers (less than 1.5 and 1.8 log10 by SNT and ELISA, respectively) were recorded by the 36th and 40th WPV with FMD vaccine alone and with BV, respectively. These results are demonstrated in Table 6. Table 6. Mean FMD serotype O serum neutralizing antibody and ELISA titers in vaccinated sheep.
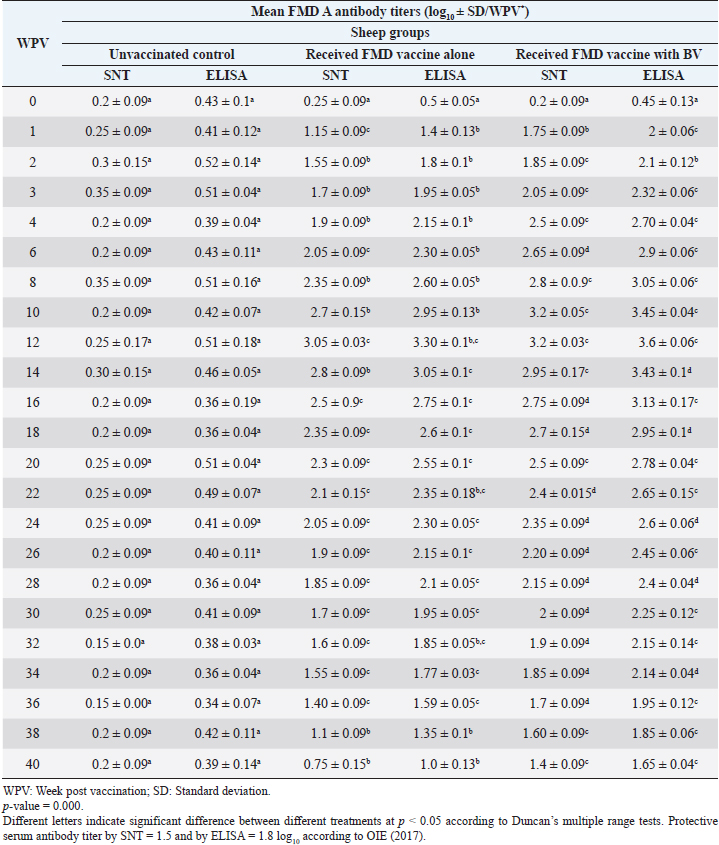
Table 7. Mean FMD serotype A serum neutralizing antibody and ELISA titers in vaccinated sheep.
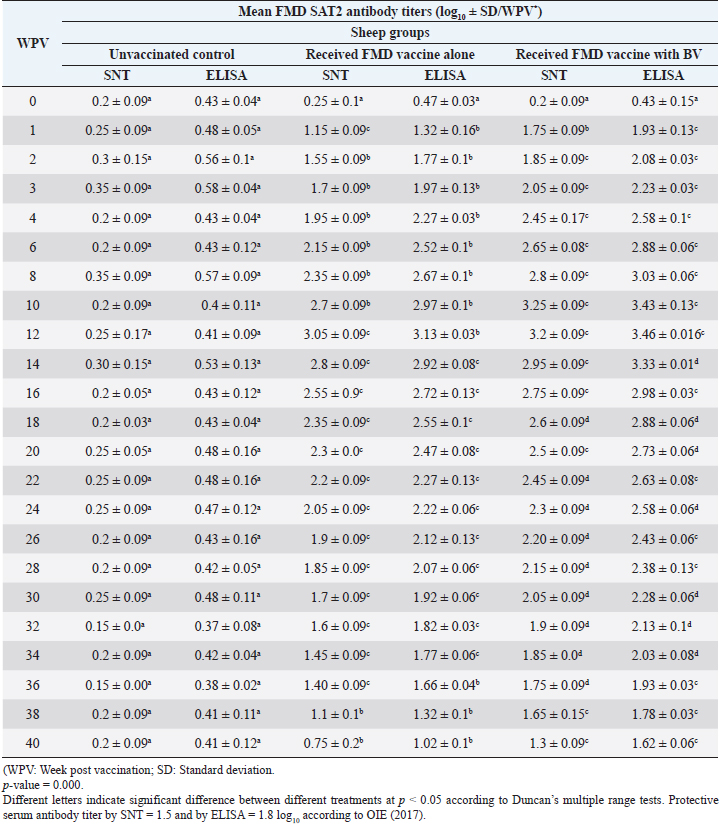
Administration of FMD vaccine alone and with BV resulted in induction of specific FMD serotype A antibodies as determined by application of SNT and solid phase indirect ELISA. These tests revealed that vaccinated sheep exhibited FMD serotype A protective antibody titers (1.55 ± 0.09 and 1.8 ± 0.1 log10 by SNT and ELISA, respectively, by the second week post-vaccination with FMD vaccine alone and (1.75 ± 0.09 log10 by SNT and 2 ± 0.06 log10 by ELISA) with administration of BV by the first week post-vaccination. These titers recorded their peaks (3.05 ± 0.03 log10 by SNT and 3.30 ± 0.01 log10 by ELISA) on the 12th WPV and 3.2 ± 0.05 log10 by SNT and 3.45 ± 0.04 log10 by ELISA on the 10th WPV using the vaccine alone and with BV, respectively. Such antibodies were gradually decreased to reach its lowest protective levels (1.55 ± 0.9 log10 by SNT and 1.77 ± 0.03 log10 by ELISA) on the 34th WPV with FMD vaccine alone while in the case of administration of BV these values were (1.60 ± 0.04 log10 by SNT and 1.95 ± 0.12 log10 by ELISA) on the 38th WPV. Non-protective FMD serotype O antibody titers (less than 1.5 and 1.8 log10 by SNT and ELISA, respectively) were recorded by the 36 and 40 WPV with FMD vaccine alone and with BV, respectively, as demonstrated in Table 7. Following up the levels of FMD serotype SAT2 antibodies in vaccinated sheep with FMD vaccine alone and with BV; it was found that such antibodies were detectable by application of SNT and solid phase indirect ELISA which showed that vaccinated sheep exhibited FMD serotype SAT2 protective antibody titers (1.55 ± 0.09 and 1.77 ± 0.1 log10 by SNT and ELISA, respectively, by the second week post-vaccination with FMD vaccine alone and (1.75 ± 0.09 log10 by SNT and 1.95 ± 0.13 log10 by ELISA) with administration of BV by the first week post-vaccination. These titers recorded their peaks (3.05 ± 0.03 log10 by SNT and 3.13 ± 0.03 log10 by ELISA) on the 12th WPV and 3.25 ± 0.09 log10 by SNT and 3.45 ± 0.04 log10 by ELISA on the 10th WPV using the vaccine alone and with BV, respectively. Such antibodies were gradually decreased to reach its lowest protective levels (1.60 ± 0.04 log10 by SNT and 1.82 ± 0.03 log10 by ELISA) on the 32nd WPV with FMD vaccine alone while in the case of administration of BV these values were (1.65 ± 0.15 log10 by SNT and 1.75 ± 0.03 log10 by ELISA) on the 38th WPV. Non-protective FMD serotype O antibody titers (less than 1.5 and 1.8 log10 by SNT and ELISA, respectively) were recorded by the 36 and 40 WPV with FMD vaccine alone and with BV, respectively as demonstrated in Table 8, and the immune modulator effect of BV is shown in Table 9 Table 8. Mean FMD serotype SAT2 serum neutralizing antibody and ELISA titers in vaccinated sheep.
Table 9. Cumulative table showing the immune modulator effect of BV on the immune response of sheep to the trivalent FMD vaccine.
DiscussionThe results of cellular immunity were coming as stated by Knudsen et al. (1979), who reported that cell-mediated immune response was a constitute of immune response against FMDV, and Garcia et al. (1996), Elwatany et al. (1999), Sonia et al. (2010), and Fakhry et al. (2012) mentioned that the delta optical density of lymphocyte blastogenesis assay and IL-6 at 0, 3, 7, 14, 21, and 28 -DPV showed that a significant difference between vaccinated and control groups started at 3rd DPV and increased gradually till 21st DPV using trivalent FMD Montanide inactivated vaccine. Also, our result come in parallel to El-Din et al. (2014) who concluded that the Egyptian trivalent FMD oil vaccine showed a maximum cellular immune response at 21 DPV. The results of humoral immunity came in parallel with the result obtained by Ibrahim et al. (2015) who indicated that vaccines emulsified using Montanide ISA 201 adjuvant elicited a protective humoral immune response from the 2nd WPV for ISA 201 oil by SNT and ELISA titers of (1.62 ± 0.047a and 1.8 ± 0.049a); (1.59 ± 0.076a and 1.836 ± 0.077a) and (1.71 ± 0.06b and 1.96 ± 0.074b) by SNT and ELISA for serotypes O, A, SAT2, respectively, and ISA 206 showed antibody titer by SNT and ELISA of (1.5 ± 0.082a and 1.84 ± 0.084a); (1.56 ± 0.037a and 1.818 ± 0.052a) and (1.5 ± 0.106a,b and 1.81 ± 0.104a,b) for FMDV serotypes O, A, and SAT2, respectively. Our results also were consistent with the statement of Hamblin et al. (1986), who explained that the SNT measures those antibodies which neutralize the infectivity of FMD virion, while ELISA probably measure all classes of antibodies even those produced against incomplete and non-infectious virus. In addition, these results agree with those obtained by Selim et al. (2010) who reported that the mean antibody titers against FMD vaccine strain O1/3/93 were detected in sheep sera vaccinated with Alumhydroxide gel vaccine following one WPV by SNT, whereas, the mean peak titers (1.9 log10) by SNT were detected by the 6th week post-vaccination. Our results also agreed with Mohamed et al. (2013) who used FMD ISA 206 oil bivalent vaccine alone and noticed that the specific FMD neutralizing antibody titer reached a protective level starting from the 4th WPV to record peak titer by the 16th WPV and then declined gradually afterward. El-Sayed et al. (2012) reported that vaccination of calves with the locally produced bivalent FMD adjuvant vaccine induced higher antibody titer than the recommended protective level (1.5 log10 for SNT and 1.8 log10 for ELISA) for type A and O estimated by SNT and ELISA. This antibody titer remained within the protective level up to 34 WPV. El-Din et al. (2014) concluded that the Egyptian trivalent FMD oil vaccine-induced protective humoral immune response extended for 32 WPV. The obtained high levels of FMD immune response in vaccinate sheep receiving BV could be attributed to effect of BV as immunomodulator which stimulates immune system to protect the body against infection by its stimulation of prostaglandin generation which have biological activities resulting in stimulation of IL-10, TNF alpha, and CD8 which result in regulation in IgE which responsible for histamine release (Rekka et al., 1990). Eiaka et al. (2016) found that following up rabies antibodies in vaccinated dogs using SNT revealed that BV induced the highest levels of antibodies (128) when inoculated before and simultaneously with vaccination by rabies vaccine. Rabies vaccine alone or before inoculation of BV induced lower titers of antibodies (32 and 64, respectively) by the fourth week post-vaccination. Also, ELISA confirming came parallel to those of SNT revealing that BV induced the highest levels of antibodies (7 and 6 log2, respectively) when inoculated before and simultaneously with vaccination by rabies vaccine and rabies vaccine alone or before inoculation of BV induced lower ELISA titers (5 log2) by the fourth week post-vaccination. Depending on the demonstrated data it could be concluded that BV enhances the immune response of vaccinated sheep to the trivalent FMD vaccine resulted in earlier induction of specific FMD serotypes antibodies than in the case of administration of the vaccine alone reaching their peak on earlier time with prolonged duration of immunity. ReferencesAntley, P.P. and Hazen, K.C. 1988. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect. Immun. 56(11), 2884–2890. Carr, B.V., Lefevre, E.A., Windsor, M.A., Inghese, C., Gubbins, S., Prentice, H., Juleff, N.D. and Charleston, B. 2013. CD4+ T-cell responses to foot-and-mouth disease virus in vaccinated cattle. J. Gen. Virol. 94(Pt 1), 97–107. Depa, P.M., Dimri, U., Sharma, M.C. and Tiwari, R. 2012. Update on epidemiology and control of foot and mouth disease—a menace to international trade and global animal enterprise. Vet. World 5(11), 694–704. Eiaka, A.M., El-Bagoury, G.F., El-Nahas, E.M. and Khodeir, M.H. 2016. Investigation of the effect of bee venom on the immune response of dogs to rabies vaccine. BVMJ 31(2), 254–257. El-Din, W.M.G., EES, I.H. and Ali, S.M. 2014. Humeral and cellular immune response of Egyptian trivalent foot and mouth disease oil vaccine in sheep. Res. Opin. Anim. Vet. Sci. 4(4), 178–185. El-Mayet, F.S., Sharawi, S.S.A., EL-Habbaa, A.S., Shahen, N.M. and Hagaa, N.M. 2020. Rapid detection and isolation of foot and mouth disease virus in samples from clinically suspected animals in Egypt during 2016–2019. BVMJ 39, 135–140. EL-Naggar, H. 2012. Preparation of inactivated lyophilized NDV vaccine. M.V.Sc. in Veterinary Science (Virology) Thesis, Cairo University, Cairo, Egypt. El-Sayed, E., Gamal El-Din, W.M., Rizk, S.A. and Abd El-Aty, M. 2012. Effect of different storage temperatures on the efficacy of the bivalent foot and mouth disease oil vaccine. J. Adv. Vet. Res. 2, 198–205. Elwatany, H., Shawky, M., Rhshdy, O.H. and El-Kelany, S. 1999. Relationship between cellular and humoral immunity responses in animal vaccinated with FMD vaccine. Zigzag Vet. Med. J. 27, 137–143. Fakhry, H.M., Rizk, S.A., Abu-Elnaga, H.I., Deghaidy, W., Talaat, A.A. and Hegazi, A.Z. 2012. Field application of bivalent foot and mouth disease vaccine adjuvanted with Montanide ISA (25, 50, 206) and IMS (1113-3015) as an alternative to aluminum hydroxide gel. Egypt. J. Virol. 9(1), 123–136. Farag, M.A., El-Kilany, S. and Abdel El-Rahman, A.O. The impact of live animal importation on the epizootiology of Foot and Mouth Disease in Egypt. In 8th Sci. Vet. Med. Zag. Conference, 2006 August 31–September 3, Hurghada, Egypt, 2006. Ferreira, M.E.V. Prubade microneutralization poraestudies de anticueropos de la fibre aftosa. In 13th Centropanamericano Fiebre Aftosa, (21/22), 1976, pp: 17–24. Gamil, M.A. 2010. Studies on the immune response of calves vaccinated inactivated bivalent FMD virus vaccine type O1 and A Egypt 2006. M.V.Sc. in Veterinary Science (Virology), Thesis, Benha University, Benha, Egypt. Garcia-Valcarcel, M., Doel, T., Collen, T., Ryan, M. and Parkhouse, R.M. 1996. Recognition of foot-and-mouth disease virus and its capsid protein VP1 by bovine peripheral T lymphocytes. J. Gen. Virol. 77(Pt 4), 727–735. Hamblin, C., Barnett, I.T. and Crowther, J.R. 1986. A new enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus. II. Application. J. Immunol. Methods 93(1), 123–129. Hussein, H.A. 1989. Immunosuppressive effect of MDV. B.V.SC, Virology Vet. Thesis, Cairo University, Cairo, Egypt. Ibrahim, M.S., Abd Algayed, M., Elgendy, E., Hagag, N. and Shahin, M. 2018. Molecular characterization of FMD virus during 2016-2017 in Egypt. Eur. J. Pharm. Med. Res. 5(12), 90–99. Ibrahim, E., Gamal, W.M., Hassan, A.I., Mahdy, S., Hegazy, A.Z. and Abdel-Atty, M.M. 2015. Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine. Vet. World 8(10), 1189–1198. James, A.D. and Rushton, J. 2002. The economics of foot and mouth disease. Rev. Sci. Tech. 21(3), 637–644. Kamal, S. 2016. In vitro study on the effect of bee venom on some cell lines and lumpy skin disease virus. J. Agri. Sci. Technol. 6, 124–135. Kim, Y.W., Chaturvedi, P.K., Chun, S.N., Lee, Y.G. and Ahn, W.S. 2015. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and E7 expression on cervical cancer cell line. Oncol. Rep. 33(4), 1675–1682. Knight-Jones, T.J.D., McLaws, M. and Rushton, J. 2017. Foot-and-mouth disease impact on smallholders—what do we know, what don’t we know and how can we find out more? Transbound. Emerg. Dis. 64(4), 1079–1094. Knudsen, R.C., Groocock, C.M. and Andersen, A.A. 1979. Immunity to foot-and-mouth disease virus in guinea pigs: clinical and immune responses. Infect. Immun. 24(3), 787–792. Lee, L.F. 1984. Proliferative response of chicken B and T lymphocyte to mitogen. Vet. Med. J. 15, 44–52. Lucy, F.L. 1977. Chicken Lymphocyte stimulation by mitogenes. A microassay with whole blood cultures. Avian Dis. J. 22, 296–307. Mansour, A.M., Elfiky, A.A., Fahmy, A. and Diab, A. 2016. Therapeutic effect of bee venom formulation in the treatment of FMD viral infection: preclinical and clinical evaluation. Int. J. Sci. Res. 6(6), 711–729. Macpherson, J.A. and Stocker, N.G. 1962. Polyoma transformation of hamster cell clones an investigation of genetic factors affecting all competence. Virology 16, 147–151. Mayer, S., Falkenrodt, A. and Tongio, M.M. 1974. Anomalous reactivity of sera containing cold lymphocytotoxins with chronic leukemic lymphocytes. Tissue Antigens 4(3), 266–267. Meier, J. and White, J. 1995. Clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press. Mohamed, E.M., Ibrahim, E.E. and Mohamed, F.S. 2013. Effect of administration of ultracorn with bivalent foot and mouth disease oil vaccine in calves. Vet. World 6, 486–492. OIE. 2017. Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: OIE. Available via www.oie.int/en/international-standardsetting/terrestrial-manual/access-online/ (Accessed 4 July 2022). Orsel, K., Dekker, A., Bouma, A., Stegeman, J.A. and de Jong, M.C. 2007. Quantification of foot and mouth disease virus excretion and transmission within groups of lambs with and without vaccination. Vaccine 25(14), 2673–2679. Patil, P.K., Bayry, J., Ramakrishna, C., Hugar, B., Misra, L.D. and Natarajan, C. 2002. Immune responses of goats against foot-and-mouth disease quadrivalent vaccine: comparison of double oil emulsion and aluminium hydroxide gel vaccines in eliciting immunity. Vaccine 20(21–22), 2781–2789. Rekka, E., Kourounakis, L. and Kourounakis, P. 1990. Antioxidant activity of and interleukin production affected by honey bee venom._Arzneimittelforschung 40(8), 912–913. Richardson, M.D. and Smith, H. 1981. Resistance of virulent and attenuated strains of Candida albicans to intracellular killing by human and mouse phagocytes. J. Infect. Dis. 144(6), 557–564. Selim, A.M.A., Abouzeid, N.Z., Aggour, A.M. and Sobhy, N.M. 2010. Comparative study for immune efficacy of two different adjuvants bivalent FMD vaccines in sheep. J. Am. Sci. 6, 1292–1298. Sonia, A., Rizk, H., Fakhry, M. and Abu-Elnaga, H.I. 2010. Comparative study of T cell proliferative response in cattle vaccinated with FMD vaccine using cell titre aqueous one solution non-radioactive assay (MTS). Zag. Vet. J. 38(4), 188–195. Voller, A., Bid Well, D. and Bartleha. 1976. Micro plate enzyme immuno assay for the immuno diagnosis of virus infection. Washington, DC: American Society for Microbiology, pp: 506–512. | ||
| How to Cite this Article |
| Pubmed Style Eweis AR, Hassan KM, Shoman SA, Taha HA, Mohamed EE. Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep. Open Vet J. 2022; 12(6): 919-928. doi:10.5455/OVJ.2022.v12.i6.18 Web Style Eweis AR, Hassan KM, Shoman SA, Taha HA, Mohamed EE. Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep. https://www.openveterinaryjournal.com/?mno=108625 [Access: April 20, 2024]. doi:10.5455/OVJ.2022.v12.i6.18 AMA (American Medical Association) Style Eweis AR, Hassan KM, Shoman SA, Taha HA, Mohamed EE. Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep. Open Vet J. 2022; 12(6): 919-928. doi:10.5455/OVJ.2022.v12.i6.18 Vancouver/ICMJE Style Eweis AR, Hassan KM, Shoman SA, Taha HA, Mohamed EE. Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep. Open Vet J. (2022), [cited April 20, 2024]; 12(6): 919-928. doi:10.5455/OVJ.2022.v12.i6.18 Harvard Style Eweis, A. R., Hassan, . K. M., Shoman, . S. A., Taha, . H. A. & Mohamed, . E. E. (2022) Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep. Open Vet J, 12 (6), 919-928. doi:10.5455/OVJ.2022.v12.i6.18 Turabian Style Eweis, Asmaa R.F., Kodeir M. Hassan, Sahar A.H. Shoman, Hoda A. Taha, and Ehab E. Mohamed. 2022. Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep. Open Veterinary Journal, 12 (6), 919-928. doi:10.5455/OVJ.2022.v12.i6.18 Chicago Style Eweis, Asmaa R.F., Kodeir M. Hassan, Sahar A.H. Shoman, Hoda A. Taha, and Ehab E. Mohamed. "Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep." Open Veterinary Journal 12 (2022), 919-928. doi:10.5455/OVJ.2022.v12.i6.18 MLA (The Modern Language Association) Style Eweis, Asmaa R.F., Kodeir M. Hassan, Sahar A.H. Shoman, Hoda A. Taha, and Ehab E. Mohamed. "Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep." Open Veterinary Journal 12.6 (2022), 919-928. Print. doi:10.5455/OVJ.2022.v12.i6.18 APA (American Psychological Association) Style Eweis, A. R., Hassan, . K. M., Shoman, . S. A., Taha, . H. A. & Mohamed, . E. E. (2022) Investigation of honey bee venom effect on the immunogenicity of foot-and-mouth disease vaccine in sheep. Open Veterinary Journal, 12 (6), 919-928. doi:10.5455/OVJ.2022.v12.i6.18 |