| Original Article | ||
Open Vet J. 2023; 13(4): 466-472 Open Veterinary Journal, (2023), Vol. 13(4): 466–472 Original Research Ellagic acid from whole pomegranate fruit reduces liver injury in a rat model of hepatic cholestasisRatna Widyawati1, Wiwik Misaco Yuniarti2*, and Bambang Sektiari Lukiswanto21Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia 2Division of Veterinary Clinic, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia *Corresponding Author: Wiwik Misaco Yuniarti. Division of Veterinary Clinic, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia. Email: wiwikmisaco [at] yahoo.com Submitted: 15/12/2022 Accepted: 20/03/2023 Published: 17/04/2023 © 2023 Open Veterinary Journal
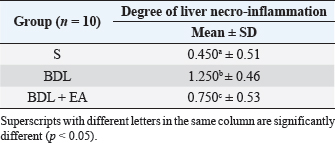
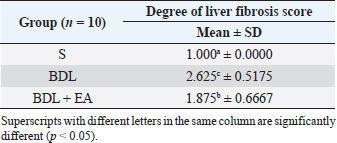
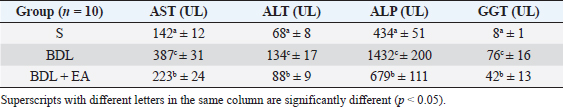
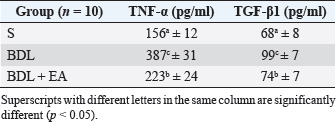
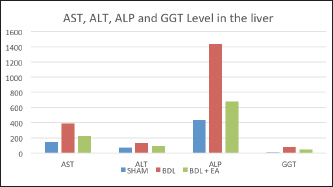
AbstractBackground: Cholestasis is a health problem, both in humans and animals, which in the disease's course involves oxidative stress, inflammation, and liver fibrosis. EA has been proven to have beneficial effects on various diseases. Aim: This study was conducted to determine the effect of EA in protecting liver damage because of cholestasis. In addition, to understand the underlying mechanism of liver damage in rats as a model animal by bile duct ligation (BDL) technique. Methods: In this study, male adult rats were used and randomly divided into three treatment groups. S is the sham-operated group, BDL is the group that is treated with BDL and the BDL-EA group is treated with BDL and given EA by gavage at a dose of 60 mg/kg bw/day, starting on the second day after BDL and given for 21 days. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT) were evaluated using spectrophotometer; tumor necrosis factor alpha (TNF-α) and transforming growth factor beta (TGF-β1) were evaluated using sandwich ELISA and histopathological examination using HE and Massion's Trichrome staining. Results: In this study, BDL significantly increased serum levels of AST, ALT, ALP, and hepatic GGT. In addition, BDL also increased levels of TNF-α, and TGF-β1 compared to sham-operated controls. Histological studies in the BDL group also showed that the BDL increased the degree of necro-inflammation and collagen deposition area in the liver compared to the sham-operated group. Administration of EA has been shown to significantly improve liver morpho-function of the liver. I attenuated these changes in the BDL-EA group, where all observed study variables appeared to have improved. Conclusion: EA has been shown to reduce cholestasis that causes liver injury and improves liver enzyme profiles, and is suspected to have occurred because of its activities as an antioxidant, anti-inflammatory, and anti-fibrotic. Keywords: BDL, Cholestasis, Ellagic acid, Liver fibrosis. IntroductionChronic cholestasis is one of the most common health problems nowadays (Aldana et al., 2020). This condition can be caused by impaired bile flow and/or secretion which will be followed by accumulation of bile acids that are toxic to hepatocytes and will cause severe liver injury and will progress to liver cirrhosis and liver failure (Liu et al., 2019). Chronic cholestasis, e.g., primary sclerosing cholangitis and primary biliary cirrhosis can cause this condition (Berry et al., 2014). Oxidative stress and inflammation play an important role in the development of liver injury during cholestasis (Chicoz-Lach and Michalak, 2014). Liver exposed to high concentrations of bile acids will start hepatocellular injury, followed by an infiltration phase of activated neutrophils in the injured area through the formation of reactive oxygen species (ROS) (Li et al., 2017). Excessive ROS production can induce cellular damage and enhance inflammatory reactions through increased tumor necrosis factor (TNF)-expression and IL-6 mRNA expression in chronic liver disease (Ramos-Tovar and Muriel, 2020). One of the proinflammatory and profibrogenic cytokines, namely transforming growth factor (TGF)-, is also activated by oxidative stress and consequently stimulates the transformation of hepatic stellate cells into myofibroblasts, which enhances extracellular matrix formation, and causes liver fibrosis. During the last few decades, the mechanism of liver cholestasis has been extensively investigated, but very few studies have been conducted to find an efficient therapeutic strategy to inhibit the progression of progressive liver injury caused by cholestasis (Dewidar et al., 2019). Ellagic acid has been shown to have the ability as an antioxidant agent, anti-inflammatory and able to prevent cancer cells from destroying the p53 gene (Rios et al., 2018). In addition, EA can also bind to cancer cells and form a complex molecule, so that cancer cells become inactive. Research on the hepatoprotective effect of EA has also shown that consumption of ellagic acid can increase the ability of liver tissue to detoxify reactive intermediates (Gupta et al., 2021). Ellagic acid can also inhibit the protein kinase tyrosine, a molecule associated with the ability of viruses to convert normal cells into cancer cells. Besides having chemoprotective activity against various chemicals that can induce cancer, EA also has antibacterial and antiviral activity. Another function of EA is to protect against cell damage due to free radicals (Mishra and Vinayak, 2014). Ellagic acid can reduce collagen levels, TGF-β1 expression, and the number of α-SMA in activated pancreatic stellate cells in rats suffering from chronic pancreatitis. Ellagic acid is also able to reduce the production of ROS. Based on these results, it is suspected that ellagic acid can be used as an alternative therapy in patients with chronic pancreatitis or pancreatic fibrosis and is thought to have an anti-fibrotic effect on other organs as well (Masamune et al., 2005). Therefore, there is a high possibility that EA may prevent oxidative stress-induced liver injury in chronic cholestasis. This study was done to find out the protective effect and potential mechanism of EA against cholestasis induced by the bile duct ligation (BDL) technique in rats. Materials and MethodsDrug and chemicalsEllagic acid was obtained from Xi'an Biof Bio Technology Co., Ltd.—China. The kits for all biochemical estimation were purchased from ERBA Diagnostic, Mannheim GmbH, Transasia Biomedical Ltd.—Germany. The other chemicals in this experiment were also of analytic grade. AnimalsThirty male rats, 2–3 months old, weighing 150–200 g, were used in this study. Rats were kept in animal cages and kept in good ventilation, temperature 22°C–25°C, 12 hours light and dark cycle per day and feed and drinking water are provided ad libitum. Rats were kept for 7 days for acclimatization before starting the experiment. All research was carried out under the ethical guidelines for the use of experimental animals from the Research Ethics Commission, Faculty of Veterinary Medicine, Universitas Airlangga. Experimental designRats were randomly assigned to three groups: the sham-operated control group (sham surgery, n=10): rats received all surgical procedures without BDL; BDL (n=10); rats in this group underwent BDL; BDL and EA (BDL+ EA, n=10): rats in this group underwent BDL then received EA. Surgical procedure and EA treatmentRats to be used as animal models of liver fibrosis were anesthetized using Ketamine HCl and Diazepam (100 mg/kg bb: 5 mg/kg bb intramuscular) in combination. The incision is made in the abdomen's midline about half the distance between the posterior abdomen and the xyphoid cartilage (Brandoni and Tores, 2009). In the bile duct, which is located 0.5–1 cm from the duodenal wall, two ligations were made with a distance of approximately 0.3 cm using prolene 7/0. The part that lies between the two ligations is cut to get a condition of complete obstruction of the bile duct. The bile ducts that have been bound and cut are returned to the abdominal cavity. The incised abdominal muscles and skin were closed again with interrupted sutures using 4/0 catgut and 4/0 prolene (Brandoni and Tores, 2009; Tag et al., 2015). With the BDL technique, animal models experienced fibrosis in the periportal area 2 days after BDL, and 2 weeks after BDL the area of fibrosis had expanded to the liver parenchyma (Beaussier et al., 2017). EA was given 2 days after BDL at a dose of 60 mg/kg bb/po/day (Devipriya et al., 2007), suspended with 0.3% sodium carboxymethyl cellulose and administered by gavage for 21 days. On day 22, all rats were subjected to liver excision and blood collection needed to examine the research variables. Histological examinationThe liver is excised for histopathological examination using Hematoxylin and Eosin (H&E) and Massion's Trichrome (MT) stain and is required to determine the levels of TNF-α and TGF-β1 in the liver. The degree of inflammatory activity was assessed on a 0–3 scale (0=none, 1=light activity, 2=moderate activity, 3=severe activity) (Mannan et al., 2017). The liver fibrosis stage was classified: 0=no fibrosis; 1=portal tract enlargement without septa, 2=portal tract enlargement with multiple septa, 3=multiple septa without cirrhosis, and 4=definite cirrhosis, using the METAVIR scoring system (Dhingra et al., 2016). Biochemical analysisAfter 21 days of treatment, rats were fasted overnight, anesthetized, and blood samples were collected intracardially and put into plastic tubes. The blood was left at room temperature to clot and then centrifuged at 3,000 rpm for 15 minutes to obtain serum. The serum obtained was collected and stored at −80°C until used. After that, the levels of alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT) in plasma were examined using a spectrophotometer (Roche 917 R Autoanalyzer) and a commercial kit. Measurement of TNF-α and TGF-β1 in liver tissueTNF-α levels were measured using the RayBio® Rat TNF-alpha ELISA Kit (RayBiotech, Inc., Norcross, GA) to conform to the instructions on the product label. TGF-β1 was quantitatively checked using the sandwich ELISA (Liu et al., 2020), using the PicoKine™ TGF-β1 Mice ELISA Kit (Pleasanton, CA). Statistical analysisThe effect of treatment on all research variables was analyzed using a one-way analysis of variance. If the F value is significantly different (p < 0.05), followed by the least significant difference test to see the effect of treatment on each treatment group. Analysis was performed using SPSS Windows version 20 (IBM, NY, USA). Ethical approvalThe Animal Care and Use Committee, Faculty of Veterinary Medicine, Universitas Airlangga Surabaya has approved experimental protocol that the research could be done with treatment that does not violate the medical ethic of animal welfare. ResultsLiver histological examinationExamination of liver necro-inflammation in the negative control group and BDL + EA showed that the degree of necro-inflammation was not significantly different (p > 0.05). However, the degree of liver necro-inflammation in the two groups was significantly different from the BDL group (p < 0.05) (Table 1 and Fig. 1). Evaluation of the degree of fibrosis in the three treatment groups showed significant differences from each other (p < 0.05). The highest degree of fibrosis was seen in the BDL group and followed by the BDL + EA and S groups, respectively (Table 2 and Fig. 1). Biochemical analysisAST, ALT, ALP, and GGT levels appeared to be significantly increased in the BDL group when compared to the sham group. All variables decreased significantly in the BDL-EA group when compared to the BDL group (p < 0.05) (Table 3 and Fig. 2). TNF-α and TGF-β1 levels were lower in the sham-operated group when compared to the BDL group. Administration of EA in the BDL-EA group significantly reduced TNF- and TGF-β1 levels when compared to the BDL group (p < 0.05) (Table 4 and Fig. 2). DiscussionThe main finding of this study is that EA treatment in bile duct-bound rats provides protection against cholestasis-induced liver injury and attenuates upregulation of hepatic oxidative stress, inflammation, and fibrosis, improving clinical liver function as well as histological structure. BDL technique significantly increases serum levels of AST, ALT, GGT, and ALP, which are biochemical markers of hepatocellular damage (Mannan et al., 2014). It is characterized by loss of hepatocyte membrane integrity and changes in hepatocyte membrane permeability leading to leakage of liver enzymes and is a sign of liver injury (Dhingra et al., 2016). BDL has been proven to cause an accumulation of hydrophobic bile acids, which are toxic and can cause hepatocyte cell death by means of its cytolytic detergent activity. This will cause membrane cell damage, and also increase the production of ROS from liver mitochondria that oxidatively damage lipids, proteins, and nucleic acids, ultimately resulting in hepatocytes apoptosis (Sporn et al., 1986). In this study, the administration of EA was thought to improve liver oxidative stress by increasing antioxidant capacity. This antioxidant effect attenuates cholestasis leading to liver injury, maintains hepatocyte membrane integrity, and improves liver function which was manifested by a significant decrease in ALT, AST, GGT, and ALP in the BDL-EA group compared to BDL rats, which levels were not significantly different from the sham operation group. This observation agrees with the findings of other researchers (Guicciardi et al., 2013; Moslemi et al., 2021). The hepatoprotective effect of EA on liver disease was reported by previous studies and is assumed to be mediated by its antioxidant properties, which depend mainly on its chemical structure (Perez and Briz, 2009). Table 1. Mean and standard deviation scores for the degree of liver necro-inflammation.
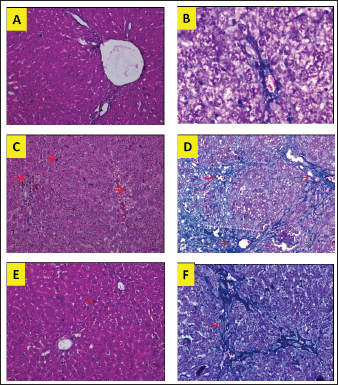
Fig. 1. Liver tissue section from group stained with HE and MT. In the S group, normal liver morphology was found (A and D); in BDL group, histological changes were found, characterized by high necro-inflammation and collagen deposition at the edge of the lobulus (B and E); and in BDL + EA group, the histopathological picture improved as a decrease in the degree of necro-inflammation and fibrosis (C and F). (H&E, 100×). In addition, the BDL rats in this study showed significantly higher levels of TNF- in the liver compared to the sham-operated controls. TNF is a key cytokine that starts various proinflammatory signaling pathways and activates local inflammatory responses. It is characterized by histopathological changes such as edema, congestion, cellular infiltration, and necrosis of the pancreas related to acute lung injury (Gheitasi et al., 2021). TNF-α has been recognized as a potent mediator of liver failure in several animal models of liver injury (Zandieh et al., 2011). TNF-α knockout in mice has been shown to inhibit liver damage caused by carbon tetrachloride hepatotoxin (Chen et al., 2018). EA treatment in this study attenuated cholestasis-induced liver inflammation as shown by the decrease of hepatic TNF- levels and a reduction in all histologic markers of inflammation in BDL-EA rats versus the BDL group. The results are similar to the results of similar earlier studies by other researchers (Sudo et al., 2005; Luan et al., 2013; Chastre et al., 2017). Ellagic acid has been reported to control the inflammatory process by modulating proinflammatory signaling pathways involving T cell proliferation, stimulation of B-lymphocytes and by inhibiting inflammatory reactions through inhibition of TNF-α (Umelsama and Sudhandiran, 2010). Therefore, it is reasonable to suspect that the anti-inflammatory activity of EA may occur through local inhibition of TNF release in liver tissue. In addition, the antioxidant effect of EA may occur through other indirect anti-inflammatory mechanisms that may occur. Oxidative stress is believed to activate Kupffer cells, which produce TNF-α (Abdekkader et al., 2022). Concurrent inhibition of pro-oxidant and anti-inflammatory markers after EA administration has been has been reported in many cases of liver injury with different inductions and in different animal models (Zhao et al., 2021). This suggests that EA may exert an anti-inflammatory effect, mainly through its radical scavenging activity. Thus, the EA in this study could improve cholestatic-induced liver inflammation directly through inhibition of the proinflammatory marker TNF-, and/or indirectly by reversing the oxidant/antioxidant imbalance. Table 2. Means and standard deviations of the degree of liver fibrosis score.
The results of this study showed that liver TGF-β1 levels were significantly higher in the BDL group compared to the sham-operated group. This condition is followed by enlargement of the liver area containing collagen fibers as a marker that liver fibrosis has occurred. Oxidative stress and inflammatory reactions are processes that go together and have a pivotal role in the process of liver fibrosis. This condition will activate stellate cells in the liver to differentiate into myofibroblast-like cells and produce TGF-β1. TGF-β1 play role in the process of liver fibrosis by regulating collagen formation (Shakoor et al., 2021). TGF-β1 is a pleiotropic cytokine potential that stimulates increased collagen production and inhibits metalloproteinase activity, leading to decreased extracellular matrix degradation and increased extracellular matrix accumulation (Yuan et al., 2017). Ellagic acid treatment in this study has been shown to significantly reduce TGF-β1 levels, and decreased the percentage of collagen fiber area in the BDL-EA group compared to the BDL group. This proves that EA has an anti-fibrotic effect, and this is under the results of previous research (Xu et al., 2016; Rios et al., 2019). It appears that controlling the inflammatory response with EA treatment can reduce hepatic TGF-β1 levels. Thus, the EA in this study could improve cholestatic-induced liver inflammation directly through inhibition of the proinflammatory marker TNF-, and/or indirectly by reversing the oxidant/antioxidant imbalance. The BDL group showed significantly higher liver TGF-β1 levels than the other groups. This was accompanied by a significantly increased percentage of the liver area containing collagen fibers. This is in line with the signs of a fibrotic liver. Various cells found in the injured liver will release various important mediators for the process of fibrogenesis. TNF-α and TGF-β are fibrogenic mediators produced by these cells. These various mediators play a role in the activation of HSCs and cause liver fibrosis. Besides the activity of these two cytokines, there is also an important role of matrix metalloproteinases and tissue inhibitor metalloproteinases (Devipriya et al., 2007; Roeb, 2018; Liu et al., 2020). Table 3. Mean and standard deviation of AST, ALT, ALP and GGT levels in liver.
Table 4. Mean and standard deviation of TNF-α and TGF-β1 levels in liver.
Fig. 2. Mean and standard deviation of AST, ALT, ALP and GGT levels in liver. Ellagic acid has been shown to have the ability to reduce collagen levels, TGF-β1 expression, and the number of -SMA in activated pancreatic stellate cells in white rats suffering from chronic pancreatitis. Ellagic acid can also reduce the production of ROS. Based on these results, it is suspected that ellagic acid can be used as an alternative therapy in patients with chronic pancreatitis or pancreatic fibrosis. ConclusionEllagic acid has been shown to reduce the effects of cholestasis leading to liver injury and increase the profile of liver enzymes (AST, ALT, ALP, and GGT) and proinflammatory cytokines (TNF- and TGF-). This is thought to occur through its activity as an antioxidant, anti-inflammatory, and anti-fibrotic. AcknowledgementsThe authors thank to Djoko Legowo, DVM., M. Kes., Staff of Division of Veterinary Pathology, Faculty of Veterinary Medicine, Airlangga University, Jl. Mulyorejo, Kampus C UNAIR, Surabaya, 60115, Indonesia, for his contribution in the histological studies. Conflict of interestAll authors have stated that there is no conflict of interest in carrying out this research. Authors contributionWMY was involved in conceptualization, data curation, validation, contributed to project administration, performed experiments, writing, and prepared the original draft of the manuscript. BSL did the research supervision performed the analysis and editing of the manuscript. RW was involved in the administration of research projects. All authors have reread, criticized, and approved the entire contents of the final manuscript. ReferencesAbdekkader, F., Elyamany, M., Gad, A.M., Assaf, N., Fawzy, H.M. and Elesawy, H. 2022. Ellagic acid attenuates liver toxicity induced by valproic acid in rats. J. Pharm. Sci. 143(1), 23–29. Aldana, A.J.G., Tapias, M. and Mindiola, A.L. 2020. Diagnostic and therapeutic approach for cholestasis in the adult. Rev. Colomb. Gastroenterol. 35(1), 69–78. Beaussier, M., Wendum, D., Schiffer, E., Dumont, S., Rey, C., Lienhart, A. and Housset, C. 2007. Prominent contribution of porta1iesenchimal cells to liver fibrosis in ischemic and obstructive cholestasis injury. J. Lab. Invest. 87(4), 3–14. Beery, R.M.M., Vaziri, H. and Forouhar, F. 2014. Primary biliary cirrhosis and primary sclerosing cholangitis: a review featuring a women's health perspective. J. Clin. Transl. Hepatol. 2(4), 266–284. Brandoni, A. and Tores, A.M. 2009. Extrahepatic cholestasis model. In Experimental surgical model in the laboratory rat, 1st ed. Eds., Rigalli, A. and Di Loreto, V.E. New York, NY: Taylor and Francis Group, pp: 139–141. Chastre, A., Belanger, M., Beauchesne, E., Nguyen, B.N., Desjardins, P. and Butterworth, R.F. 2017. Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. PLoS One 7(11), e49670. Chen, P., Chen, F. and Zhou, B. 2018. Antioxidative, anti-infammatory and anti-apoptotic efects of ellagic acid in liver and brain of rats treated by D-galactose. Nature 8(9), 1465–1674. Cichoz-Lach, H. and Michalak, A. 2014. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 20(25), 8082–8091. Devipriya, N., Sudheer, A.R. and Menon, V.P. 2007. Dose-response effect of ellagic acid on circulatory antioxidants and lipids during alcohol-induced toxicity in experimental rats. Fundam. Clin. Pharmacol. 21(6), 621–630. Devipriya, N., Sudheer, A.R., Srinivasan, M. and Menon, V.P. 2007. Effect of ellagic acid, a plant polyphenol, on fibrotic markers (mmps and timps) during alcohol-induced hepatotoxicity. Toxicol. Mech. Methods 17(6), 349–356. Dewidar, B., Meyer, C., Dooley, S. and Meindl-Beinker, N. 2019. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated. Cells 8(11), 1419–1421. Dhingra, S., Ward, S.C. and Thung, S.N. 2016. Liver pathology of hepatitis C, beyond grading and staging of the disease. World J. Gastroenterol. 22(4), 1357–1366. Gheitasi, I., Motaghi, N., Sadeghi, H., Sadeghi, H., Moslemi, Z., Eftekhari, M., Shakerinasab, N. and Doustimotlagh, A.H. 2021. Antioxidant and anti-inflammatory effects of origanum majoranal methanolic extract on bile duct ligation inmale rats. Evid. Based Complement Alternat. Med. 2021, 9927196. Guicciardi, M.E., Mahli, H., Mott, J.L. and Gores, G.J. 2013. Apoptosis and necrosis in the liver. Compr. Physiol. 3(2), 1–12. Gupta, A., Kumar, R., Ganguly, R., Singh, A.K., Rana, H.K. and Pandey, A.K. 2021. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol. Rep. 8(5), 44–52. Li, M., Cai, S.Y. and Boyer, J.L. 2017. Mechanisms of bile acid mediated inflammation in the liver. Mol. Aspect Med. 56(3), 45–53. Liu, N., Feng, J., Lu, Y., Liu, Q., Deng, J., Xia, Y., Guo, C. and Zhou, Y. 2019. Role of bile acids in the diagnosis and progression of liver cirrhosis: a prospective observational study. Exp. Ther. Med. 18(5), 4058–4066. Liu, X., Gao, X., Li, H., Li, Z., Wang, X., Zhang, L., Wang, B., Chen, X., Meng, X., Li, H., Li, Z., Wang, X., Zhang, L., Wang, B., Chen, X., Meng, X. and Yu, J. 2020. Ellagic acid exerts anti-fbrotic efects on hypertrophic scar fibroblasts via inhibition of TGF-β1/Smad2/3 pathway. Appl. Biol. Chem. 64(67), 1–10. Luan, Z.G., Zhang, J., Yin, X.H. and Guo, R.X. 2013. Ethyl pyruvate significantly inhibits tumour necrosis factor-α, interleukin-1β and high mobility group box 1 releasing and attenuates sodium taurocholate-induced severe acute pancreatitis associated with acute lung injury. Clin. Exp. Immunol. 172(3), 417–426. Mannan, R., Misra, V., Misra, S.P., Singh, P.A. and Dwivedi, M. 2014. A comparative evaluation of scoring systems for assessing necro-inflammatory activity and fibrosis in liver biopsies of patients with chronic viral hepatitis. J. Clin. Diagn. Res. 8(8), FC08–FC12. Masamune, A., Satoh, M., Kikuta, K., Suzuki, N., Satoh, K. and Shimosegawa, T. 2005. Ellagic acid blocks activation of pancreatic stellate cells. Biochem. Pharmacol. 70(6), 869–878. Mishra, S. and Vinayak, M. 2014. Ellagic acid inhibits PKC signaling by improving antioxidant defense system in murine T cell lymphoma. Mol. Biol. Rep. 41(7), 4187–4197. Moslemi, Z., Bharami, M., Hosseini, E., Mansourian, M., Daneshyar, Z., Eftekhari, M., Shakerinasab, N., Asfaram, A., Panahikhdan, E., Barmoudeh, Z. and Doustimotlagh, A.H. 2021. Portulaca oleracea methanolic extract attenuate bile duct ligation-induced acute liver injury through hepatoprotective and anti-inflammatory effects. Heliyon 1(7), 1–8. Perez, M.J. and Briz, O. 2009. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 15(14), 1677–1689. Ramos-Tovar, E. and Muriel, P. 2020. Review: molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants. 9(1279), 1–21. Rios, J.L., Giner, R.M., Marín, M. and Recio, M.C. 2018. A pharmacological update of ellagic acid. Planta Med. 84, 1068–1093. Roeb, E. 2018. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 68(69), 463–473. Shakoor, H., Feehan, J., Apostolopoulos, V., Platat, C., Aldhaheri, A.S., Ali, H.I., Ismail, L.C., Bosevski, M. and Stojanovskka, L. 2021. Immunomodulatory effects of dietary polyphenols. Nutrients 13(3), 728–739. Sporn, M.B., Wakefield, L.M. and Assoian, R.K. 1986. Transforming growth factor-beta: biological function and chemical structure. Science 233(4763), 532–534. Sudo, K., Yamada, Y., Moriwaki, H., Saito, K. and Seishima, M. 2005. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 29(3), 236–244. Tag, C.G., Sauer-Lehnen, S., Weiskirchen, S., Borkham-Kamphrost, F., Tolba, R.H., Tacke, F. and Weiskirchen, R. 2015. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J. Vis. Exp. 6(96), 52438. Umesalma, S. and Sudhandiran, G. 2010. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin. Pharmacol. Toxicol. 107(2), 650–655. Xu, F., Liu, C., Zhou, D. and Zhang, L. 2016. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 64(3), 157–167. Yuan, D., Huang, S., Berger, E., Zender, L., Haller, D. and Heikenwalder, M. 2017. Kupffer cell-derived tnf triggers cholangiocellular tumorigenesis through jnk due to chronic mitochondrial dysfunction and ROS. Cancer Cell 31(4), 771–789. Zandieh, A., Payabvash, S. and Pasalar, P. 2011. Gadolinium chloride, a Kupffer cell inhibitor, attenuates hepatic injury in a rat model of chronic cholestasis. Hum. Exp. Toxicol. 30(11), 1804–1810. Zhao, L., Mehmood, A., Soliman, M.M., Iftikhar, A., Iftikhar, M., Aboelenin, S.M. and Wang, C. 2021. Protective effects of ellagic acid against alcoholic liver disease in mice. Front. Nutr. 8(2), 744520. | ||
| How to Cite this Article |
| Pubmed Style Widyawati R, Yuniarti WM, Lukiswanto BS. Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis. Open Vet J. 2023; 13(4): 466-472. doi:10.5455/OVJ.2023.v13.i4.8 Web Style Widyawati R, Yuniarti WM, Lukiswanto BS. Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis. https://www.openveterinaryjournal.com/?mno=101344 [Access: April 16, 2024]. doi:10.5455/OVJ.2023.v13.i4.8 AMA (American Medical Association) Style Widyawati R, Yuniarti WM, Lukiswanto BS. Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis. Open Vet J. 2023; 13(4): 466-472. doi:10.5455/OVJ.2023.v13.i4.8 Vancouver/ICMJE Style Widyawati R, Yuniarti WM, Lukiswanto BS. Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis. Open Vet J. (2023), [cited April 16, 2024]; 13(4): 466-472. doi:10.5455/OVJ.2023.v13.i4.8 Harvard Style Widyawati, R., Yuniarti, . W. M. & Lukiswanto, . B. S. (2023) Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis. Open Vet J, 13 (4), 466-472. doi:10.5455/OVJ.2023.v13.i4.8 Turabian Style Widyawati, Ratna, Wiwik Misaco Yuniarti, and Bambang Sektiari Lukiswanto. 2023. Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis. Open Veterinary Journal, 13 (4), 466-472. doi:10.5455/OVJ.2023.v13.i4.8 Chicago Style Widyawati, Ratna, Wiwik Misaco Yuniarti, and Bambang Sektiari Lukiswanto. "Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis." Open Veterinary Journal 13 (2023), 466-472. doi:10.5455/OVJ.2023.v13.i4.8 MLA (The Modern Language Association) Style Widyawati, Ratna, Wiwik Misaco Yuniarti, and Bambang Sektiari Lukiswanto. "Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis." Open Veterinary Journal 13.4 (2023), 466-472. Print. doi:10.5455/OVJ.2023.v13.i4.8 APA (American Psychological Association) Style Widyawati, R., Yuniarti, . W. M. & Lukiswanto, . B. S. (2023) Ellagic acid from whole Pomegranate fruit reduces liver injury in a rat model of hepatic cholestasis. Open Veterinary Journal, 13 (4), 466-472. doi:10.5455/OVJ.2023.v13.i4.8 |